Green Extraction Approach for Isolation of Bioactive Compounds in Wild Thyme (Thymus serpyllum L.) Herbal Dust—Chemical Profile, Antioxidant and Antimicrobial Activity and Comparison with Conventional Techniques
Abstract
:1. Introduction
2. Results and Discussion
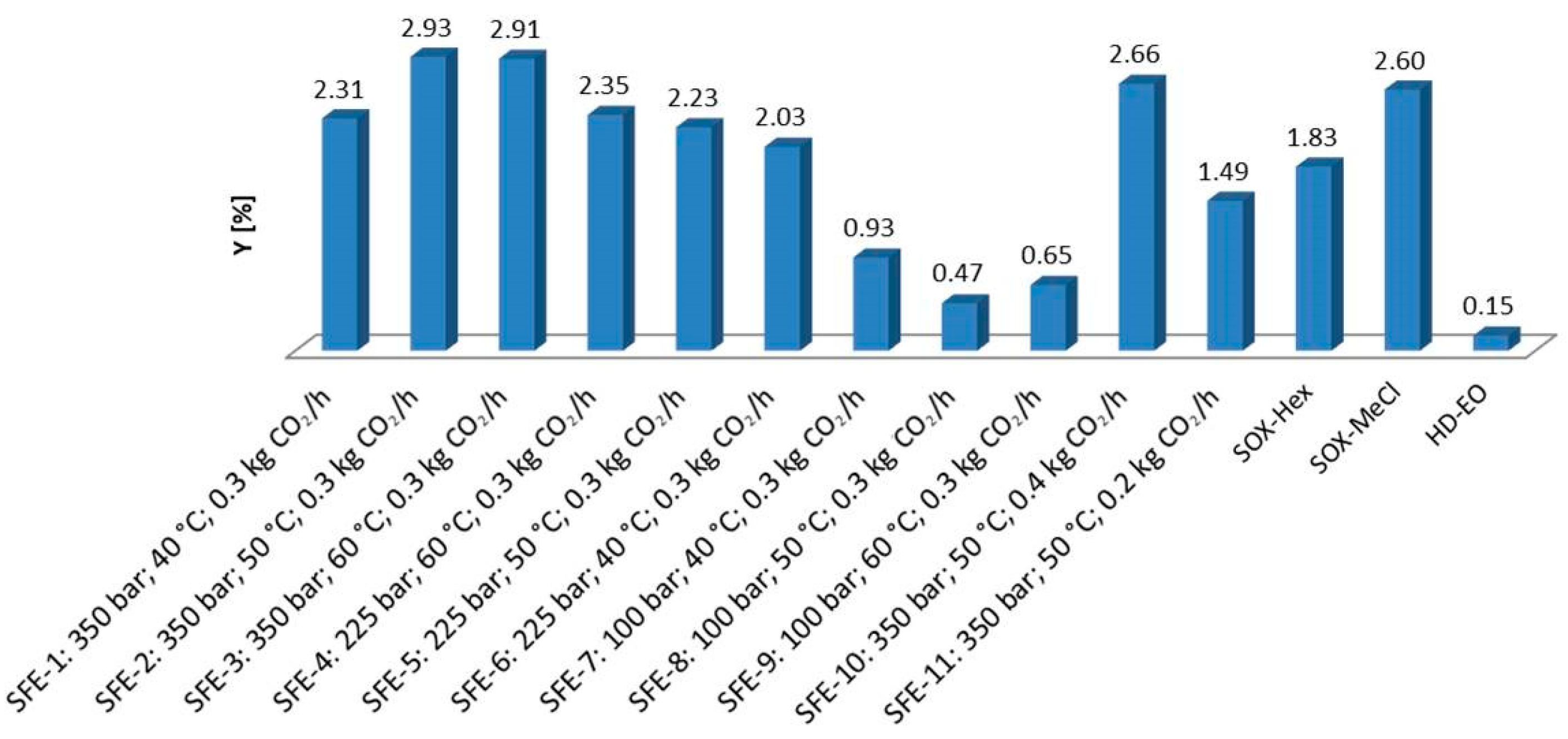
2.1. Total Extraction Yield (Y) and Chemical Composition
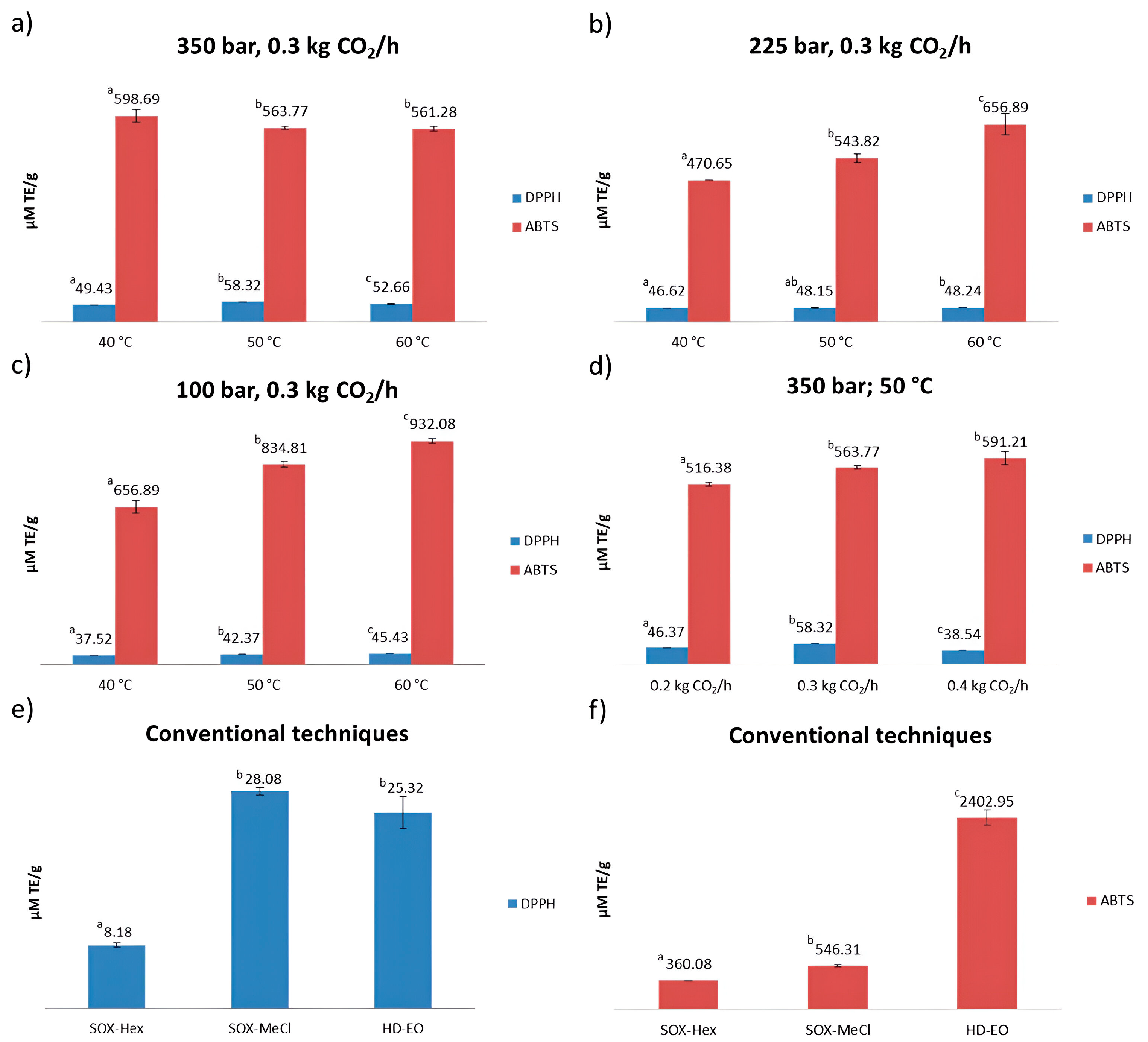
2.2. In Vitro Antioxidant Activity
2.3. Antimicrobial Activity
3. Materials and Methods
3.1. Sample
3.2. Chemicals
3.3. Supercritical Fluid Extraction (SFE)
3.4. Soxhlet Extraction (SOX)
3.5. Hydrodistillation (HD)
3.6. Analysis of Chemical Composition via GC-MS Techniques
3.6.1. HS-GC-MS
3.6.2. GC×GC-TOF/MS
3.7. Antioxidant Activity
3.8. Antimicrobial Activity
3.8.1. Bacterial Strain and Culture Conditions
3.8.2. Broth Microdilution Method
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Casiglia, S.; Bruno, M.; Scandolera, E.; Senatore, F.; Senatore, F. Influence of harvesting time on composition of the essential oil of Thymus capitatus (L.) Hoffmanns. & Link. growing wild in northern Sicily and its activity on microorganisms affecting historical art crafts. Arab. J. Chem. 2019, 12, 2704–2712. [Google Scholar] [CrossRef]
- Salehi, B.; Abu-Darwish, M.S.; Tarawneh, A.H.; Cabral, C.; Gadetskaya, A.V.; Salgueiro, L.; Hosseinabadi, T.; Rajabi, S.; Chanda, W.; Sharifi-Rad, M. Thymus spp. plants-Food applications and phytopharmacy properties. Trends Food Sci. Technol. 2019, 85, 287–306. [Google Scholar] [CrossRef]
- Zarzuelo, A.; Crespo, E. The medicinal and non-medicinal uses of thyme. In Thyme; CRC Press: Boca Raton, FL, USA, 2002; pp. 277–306. ISBN 0429218656. [Google Scholar] [CrossRef]
- Hosseinzadeh, S.; Jafarikukhdan, A.; Hosseini, A.; Armand, R. The application of medicinal plants in traditional and modern medicine: A review of Thymus vulgaris. Int. J. Clin. Med. 2015, 6, 635–642. [Google Scholar] [CrossRef]
- Jarić, S.; Mitrović, M.; Pavlović, P. Review of ethnobotanical, phytochemical, and pharmacological study of Thymus serpyllum L. Evid.-Based Complement. Altern. Med. 2015, 2015, 101978. [Google Scholar] [CrossRef] [PubMed]
- Madouni, N.; Meddah, B.; Aicha, T.T.; Bensouici, C.; Cakmak, Y.; Piras, A.; Falconieri, D.; Sonnet, P. Chemical profile, antioxidant and photoprotective activities of essential oil and crude extracts of Algerian Thymus serpyllum. Nov. Biotechnol. Chim. 2021, 20, e916. [Google Scholar] [CrossRef]
- Šojić, B.; Tomović, V.; Kocić-Tanackov, S.; Kovačević, D.B.; Putnik, P.; Mrkonjić, Ž.; Đurović, S.; Jokanović, M.; Ivić, M.; Škaljac, S.; et al. Supercritical extracts of wild thyme (Thymus serpyllum L.) by-product as natural antioxidants in ground pork patties. LWT 2020, 130, 109661. [Google Scholar] [CrossRef]
- Jovanović, A.A.; Đorđević, V.B.; Zdunić, G.M.; Pljevljakušić, D.S.; Šavikin, K.P.; Gođevac, D.M.; Bugarski, B.M. Optimization of the extraction process of polyphenols from Thymus serpyllum L. herb using maceration, heat- and ultrasound-assisted techniques. Sep. Purif. Technol. 2017, 179, 369–380. [Google Scholar] [CrossRef]
- Mrkonjić, Ž.; Rakić, D.; Olgun, E.O.; Canli, O.; Kaplan, M.; Teslić, N.; Zeković, Z.; Pavlić, B. Optimization of antioxidants recovery from wild thyme (Thymus serpyllum L.) by ultrasound-assisted extraction: Multi-response approach. J. Appl. Res. Med. Aromat. Plants 2021, 24, 100333. [Google Scholar] [CrossRef]
- Mrkonjić, Ž.; Rakić, D.; Takači, A.; Kaplan, M.; Teslić, N.; Zeković, Z.; Lazarević, I.; Pavlić, B. Polyphenols Recovery from Thymus serpyllum Industrial Waste Using Microwave-Assisted Extraction—Comparative RSM and ANN Approach for Process Optimization. Foods 2022, 11, 1184. [Google Scholar] [CrossRef] [PubMed]
- Carpentieri, S.; Mazza, L.; Nutrizio, M.; Jambrak, A.R.; Ferrari, G.; Pataro, G. Pulsed electric fields-and ultrasound-assisted green extraction of valuable compounds from Origanumv ulgare L. and Thymus serpyllum L. Int. J. Food Sci. Technol. 2021, 56, 4834–4842. [Google Scholar] [CrossRef]
- Mrkonjić, Ž.; Rakić, D.; Kaplan, M.; Teslić, N.; Zeković, Z.; Pavlić, B. Pressurized-liquid extraction as an efficient method for valorization of Thymus serpyllum herbal dust towards sustainable production of antioxidants. Molecules 2021, 26, 2548. [Google Scholar] [CrossRef]
- Pavlić, B.; Mrkonjić, Ž.; Teslić, N.; Cvetanović Kljakić, A.; Pojić, M.; Mandić, A.; Stupar, A.; Santos, F.; Rita, A.; Duarte, C.; et al. Natural Deep Eutectic Solvent (NADES) Extraction Improves Polyphenol Yield and Antioxidant Activity of Wild Thyme (Thymus serpyllum L.) Extracts. Molecules 2022, 27, 1508. [Google Scholar] [CrossRef]
- Mrkonjić, Ž.; Pezo, L.; Brdar, M.; Rakić, D.; Mrkonjić, I.L.; Teslić, N.; Zeković, Z.; Pavlić, B. Valorization of wild thyme (Thymus serpyllum L.) herbal dust by supercritical fluid extraction–Experiments and modeling. J. Appl. Res. Med. Aromat. Plants 2024, 100529. [Google Scholar] [CrossRef]
- Shen, C.; Cai, Y.; Wu, X.; Gai, S.; Wang, B.; Liu, D. Characterization of selected commercially available grilled lamb shashliks based on flavor profiles using GC-MS, GC× GC-TOF-MS, GC-IMS, E-nose and E-tongue combined with chemometrics. Food Chem. 2023, 423, 136257. [Google Scholar] [CrossRef]
- Bączek, K.; Pióro-Jabrucka, E.; Kosakowska, O.; Węglarz, Z.; Goyal, S.; Pathak, R.; Pandey, H.K.; Kumari, A.; Tewari, G.; Bhandari, N.S.; et al. Intraspecific variability of wild thyme (Thymus serpyllum L.) occurring in Poland. J. Appl. Res. Med. Aromat. Plants 2020, 2, 30–35. [Google Scholar] [CrossRef]
- Aćimović, M.; Lončar, B.; Jeremić, J.S.; Cvetković, M.; Pezo, L.; Pezo, M.; Todosijević, M.; Tešević, V. Weather Conditions Influence on Lavandin Essential Oil and Hydrolate Quality. Horticulturae 2022, 8, 281. [Google Scholar] [CrossRef]
- Hussain, A.I.; Anwar, F.; Chatha, S.A.S.; Latif, S.; Sherazi, S.T.H.; Ahmad, A.; Worthington, J.; Sarker, S.D. Chemical composition and bioactivity studies of the essential oils from two Thymus species from the Pakistani flora. LWT-Food Sci. Technol. 2013, 50, 185–192. [Google Scholar] [CrossRef]
- Goyal, S.; Pathak, R.; Pandey, H.K.; Kumari, A.; Tewari, G.; Bhandari, N.S.; Bala, M. Comparative study of the volatile constituents of Thymus serpyllum L. grown at different altitudes of Western Himalayas. SN Appl. Sci. 2020, 2, 1208. [Google Scholar] [CrossRef]
- Sfaei-Ghomi, J.; Meshkatalsadat, M.H.; Shamai, S.; Hasheminejad, M.; Hassani, A. Chemical characterization of bioactive volatile molecules of four Thymus species using nanoscale injection method. Dig. J. Nanomater. Biostruct. 2009, 4, 835–841. [Google Scholar]
- Bendif, H.; Adouni, K.; Miara, M.D.; Baranauskienė, R.; Kraujalis, P.; Venskutonis, P.R.; Nabavi, S.M.; Maggi, F. Essential oils (EOs), pressurized liquid extracts (PLE) and carbon dioxide supercritical fluid extracts (SFE-CO2) from Algerian Thymus munbyanus as valuable sources of antioxidants to be used on an industrial level. Food Chem. 2018, 260, 289–298. [Google Scholar] [CrossRef] [PubMed]
- Tohidi, B.; Rahimmalek, M.; Arzani, A. Essential oil composition, total phenolic, flavonoid contents, and antioxidant activity of Thymus species collected from different regions of Iran. Food Chem. 2017, 220, 153–161. [Google Scholar] [CrossRef]
- Pavlić, B.; Bera, O.; Teslić, N.; Vidović, S.; Parpinello, G.; Zeković, Z. Chemical profile and antioxidant activity of sage herbal dust extracts obtained by supercritical fluid extraction. Ind. Crops Prod. 2018, 120, 305–312. [Google Scholar] [CrossRef]
- Pavlić, B.; Teslić, N.; Zengin, G.; Đurović, S.; Rakić, D.; Cvetanović, A.; Gunes, A.K.; Zeković, Z. Antioxidant and enzyme-inhibitory activity of peppermint extracts and essential oils obtained by conventional and emerging extraction techniques. Food Chem. 2021, 338, 127724. [Google Scholar] [CrossRef]
- Kulisic, T.; Radonic, A.; Milos, M. Antioxidant properties of thyme (Thymus vulgaris L.) and wild thyme (Thymus serpyllum L.) essential oils. Ital. J. food Sci. 2005, 17, 315–324. [Google Scholar]
- Verma, R.S.; Rahman, L.U.; Chanotiya, C.S.; Verma, R.K.; Singh, A.; Yadav, A.; Chauhan, A.; Yadav, A.K.; Singh, A.K. Essential oil composition of Thymus serpyllum cultivated in the Kumaon region of western Himalaya, India. Nat. Prod. Commun. 2009, 4, 987–988. [Google Scholar] [CrossRef] [PubMed]
- Topal, U.; Sasaki, M.; Goto, M.; Otles, S. Chemical compositions and antioxidant properties of essential oils from nine species of Turkish plants obtained by supercritical carbon dioxide extraction and steam distillation. Int. J. Food Sci. Nutr. 2008, 59, 619–634. [Google Scholar] [CrossRef] [PubMed]
- Zeković, Z.; Pavlić, B.; Cvetanović, A.; Đurović, S. Supercritical fluid extraction of coriander seeds: Process optimization, chemical profile and antioxidant activity of lipid extracts. Ind. Crops Prod. 2016, 94, 353–362. [Google Scholar] [CrossRef]
- Shashiashvili, N.; Jokhadze, M.; Tushurashvili, P.; Bakuridze, A.; Berashvili, D. Analysis of Perilla nankinensis decne essential oil using gas chromatography coupled with time-of-flight mass spectrometry. Georgian Med. News 2014, 229, 92–96. [Google Scholar]
- Ozel, M.Z.; Kaymaz, H. Superheated water extraction, steam distillation and Soxhlet extraction of essential oils of Origanum onites. Anal. Bioanal. Chem. 2004, 379, 1127–1133. [Google Scholar] [CrossRef] [PubMed]
- Ili, P.; Kaska, Y.; Ozel, M.Z.; Genç, O.; Turgut, S.; Turgut, G. Chemichal composition of Origanum onites L. and Thymbra spicata L., and their cardiorespiratory effects in rabbits. Fresenius Environ. Bull. 2007, 16, 1401–1406. [Google Scholar]
- Kutlular, Ö.; Özel, M.Z. Analysis of essential oils of Origanum onites by superheated water extraction using GCxGC-TOF/MS. J. Essent. Oil Bear. Plants 2009, 12, 462–470. [Google Scholar] [CrossRef]
- Pereira, C.G.; Meireles, M.A.A. Supercritical fluid extraction of bioactive compounds: Fundamentals, applications and economic perspectives. Food Bioprocess Technol. 2010, 3, 340–372. [Google Scholar] [CrossRef]
- Babovic, N.; Djilas, S.; Jadranin, M.; Vajs, V.; Ivanovic, J.; Petrovic, S.; Zizovic, I. Supercritical carbon dioxide extraction of antioxidant fractions from selected Lamiaceae herbs and their antioxidant capacity. Innov. Food Sci. Emerg. Technol. 2010, 11, 98–107. [Google Scholar] [CrossRef]
- Petrović, N.V.; Petrović, S.S.; Džamić, A.M.; Ćirić, A.D.; Ristić, M.S.; Milovanović, S.L.; Petrović, S.D. Chemical composition, antioxidant and antimicrobial activity of Thymus praecox supercritical extracts. J. Supercrit. Fluids 2016, 110, 117–125. [Google Scholar] [CrossRef]
- Leon-Méndez, G.; Osorio-Fortich, M.; Ortega-Toro, R.; Pajaro-Castro, N.; Torrenegra-Alarcón, M.; Herrera-Barros, A. Design of an emulgel-type cosmetic with antioxidant activity using active essential oil microcapsules of thyme (Thymus vulgaris L.), Cinnamon (Cinnamomum verum J.), and clove (Eugenia caryophyllata T.). Int. J. Polym. Sci. 2018, 2018, 2874391. [Google Scholar] [CrossRef]
- Gladikostić, N.; Ikonić, B.; Teslić, N.; Zeković, Z.; Božović, D.; Putnik, P.; Bursać Kovačević, D.; Pavlić, B. Essential Oils from Apiaceae, Asteraceae, Cupressaceae and Lamiaceae Families Grown in Serbia: Comparative Chemical Profiling with In Vitro Antioxidant Activity. Plants 2023, 12, 745. [Google Scholar] [CrossRef]
- Guimarães, A.C.; Meireles, L.M.; Lemos, M.F.; Guimarães, M.C.C.; Endringer, D.C.; Fronza, M.; Scherer, R. Antibacterial activity of terpenes and terpenoids present in essential oils. Molecules 2019, 24, 2471. [Google Scholar] [CrossRef]
- Santajit, S.; Indrawattana, N. Mechanisms of antimicrobial resistance in ESKAPE pathogens. BioMed Res. Int. 2016, 2016, 2475067. [Google Scholar] [CrossRef]
- Jovanović, A.A.; Petrović, P.M.; Zdunić, G.M.; Šavikin, K.P.; Kitić, D.; Đorđević, V.B.; Bugarski, B.M.; Branković, S. Influence of lyophilized Thymus serpyllum L. extracts on the gastrointestinal system: Spasmolytic, antimicrobial and antioxidant properties. S. Afr. J. Bot. 2021, 142, 274–283. [Google Scholar] [CrossRef]
- Brezoiu, A.-M.; Prundeanu, M.; Berger, D.; Deaconu, M.; Matei, C.; Oprea, O.; Vasile, E.; Negreanu-Pîrjol, T.; Muntean, D.; Danciu, C. Properties of Salvia officinalis L. and Thymus serpyllum L. extracts free and embedded into mesopores of silica and titania nanomaterials. Nanomaterials 2020, 10, 820. [Google Scholar] [CrossRef]
- Baj, T.; Biernasiuk, A.; Wróbel, R.; Malm, A. Chemical composition and in vitro activity of Origanum vulgare L., Satureja hortensis L., Thymus serpyllum L. and Thymus vulgaris L. essential oils towards oral isolates of Candida albicans and Candida glabrata. Open Chem. 2020, 18, 108–118. [Google Scholar] [CrossRef]
- Mancini, E.; Senatore, F.; Del Monte, D.; De Martino, L.; Grulova, D.; Scognamiglio, M.; Snoussi, M.; De Feo, V. Studies on chemical composition, antimicrobial and antioxidant activities of five Thymus vulgaris L. essential oils. Molecules 2015, 20, 12016–12028. [Google Scholar] [CrossRef]
- Jovanović, A.A.; Balanč, B.; Petrović, P.; Pravilović, R.; Djordjević, V. Pharmacological potential of Thymus serpyllum L. (wild thyme) extracts and essential oil: A review. J. Eng. Process. Manag. 2021, 13, 32–41. [Google Scholar] [CrossRef]
- Council of Europe. European Pharmacopoeia 7.0. In Section 2.9. 18—Preparations for Inhalation: Aerodynamic Assessment of Fine Particles; Council of Europe: Strasbourg, France, 2010; pp. 274–285. [Google Scholar]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT-Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef] [PubMed]
- Duvnjak, D.; Pantić, M.; Pavlović, V.; Nedović, V.; Lević, S.; Matijašević, D.; Sknepnek, A.; Nikšić, M. Advances in batch culture fermented Coriolus versicolor medicinal mushroom for the production of antibacterial compounds. Innov. Food Sci. Emerg. Technol. 2016, 34, 1–8. [Google Scholar] [CrossRef]
| Compound | RT [min] | RIexp − RIlit | SFE-1 | SFE-2 | SFE-3 | SFE-4 | SFE-5 | SFE-6 | SFE-7 | SFE-8 | SFE-9 | SFE-10 | SFE-11 | SOX-Hex | SOX-MeCl | HD-EO |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| [µg/mL] | ||||||||||||||||
| (-)-(Z)-β-caryophyllene | 26.141 | 1408 | ND | ND | ND | 7.92 | 4.32 | 4.22 | 4.97 | ND | ND | ND | ND | ND | ND | 0.65 |
| (-)-isocaryophyllene | 26.819 | 1408 | ND | ND | ND | ND | ND | ND | ND | ND | ND | 1.28 | 0.95 | ND | ND | 2.95 |
| 1-octen-3-ol | 16.169 | 979 | ND | ND | ND | ND | ND | ND | ND | 10.96 | ND | ND | ND | ND | ND | ND |
| 2,2-dimethoxybutane | 9.566 | ND | ND | ND | ND | 1.91 | ND | ND | ND | ND | ND | ND | ND | ND | ND | |
| 2-methyl-5-(1-methylethyl)-2,5-cyclohexadiene-1,4-dione/thymoquinone | 22.785 | 16.45 | 15.37 | 15.06 | 18.05 | 7.48 | 7.43 | 12.10 | 31.75 | 16.13 | ND | 43.72 | ND | 6.87 | ND | |
| 3,7-dimethyl-, (E)-2,6-octadien-1-ol | 22.678 | 1254 | 8.06 | ND | 3.29 | 12.21 | 3.96 | 3.02 | 7.74 | ND | ND | ND | ND | ND | 7.23 | ND |
| (E)-3,7-dimethyl-2,6-octadien-1-ol, formate/geraniol (59%) | 22.736 | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | 13.26 | ND | ND | |
| 2-hexenal | 12.586 | 855 | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | 3.59 |
| 3-octanol | 16.672 | 988 | 4.51 | 4.25 | 3.85 | 2.18 | 1.57 | 1.43 | 2.00 | 12.73 | 3.25 | 11.14 | 13.77 | ND | ND | 34.67 |
| (R)-5-methyl-2-(1-methylethenyl)-4-hexen-1-ol | 22.661 | ND | ND | ND | ND | ND | ND | ND | ND | 5.37 | 43.15 | ND | ND | ND | ND | |
| Benzaldehyde | 15.883 | 969–960 | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | 1.32 |
| Borneol | 21.231 | 1165 | 6.41 | 5.97 | 6.03 | 7.74 | 2.72 | 2.76 | 5.89 | 19.68 | 6.26 | 18.81 | 16.71 | 1.95 | 2.65 | 30.98 |
| Camphene | 15.562 | 954 | 1.92 | 3.42 | 2.43 | ND | 1.03 | 1.19 | ND | 1.28 | 3.28 | 8.75 | 14.57 | ND | ND | 85.47 |
| Camphor | 20.636 | 1141 | ND | ND | ND | ND | ND | ND | ND | ND | ND | 1.53 | 2.02 | ND | ND | ND |
| Carvacrol | 23.76 | 1298 | 23.99 | 20.94 | 28.29 | 76.03 | 14.55 | 17.99 | 61.03 | 35.71 | 34.16 | 108.15 | 72.38 | 19.83 | 21.02 | 87.35 |
| Caryophyllene oxide | 29.464 | 1583 | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | 0.82 | 0.88 |
| cis-linalool oxide | 18.687 | 1067 | ND | ND | ND | ND | ND | ND | ND | ND | ND | 1.37 | 1.11 | ND | ND | ND |
| cis-sabinenehydrate | 18.71 | 1070 | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | 7.48 |
| Dihydrocarvone | 21.909 | 1191/1200 | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | 1.22 |
| Eucalyptol | 17.76 | 1035–1031 | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | 5.63 | ND | ND | 19.91 |
| Geraniol 90% | 23.066 | 1249 | ND | ND | ND | 0.80 | ND | ND | ND | ND | ND | ND | ND | ND | ND | 2.84 |
| Germacrene D | 14.795 | 930 | ND | ND | ND | ND | ND | ND | ND | ND | ND | 5.93 | 9.68 | ND | ND | 57.21 |
| Hexanal | 10.914 | 801 | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | 2.73 |
| Isothymol methyl ether 90% | 22.504 | 1244 | 16.32 | 21.54 | 17.97 | 9.60 | 6.65 | 6.45 | 8.14 | 37.58 | 17.28 | 38.54 | 46.74 | 3.40 | 5.94 | 151.73 |
| Limonene | 17.657 | 1035–1029 | ND | 6.44 | 8.11 | 6.95 | 5.99 | 5.51 | 7.59 | ND | 9.56 | ND | ND | ND | ND | ND |
| Linalool | 19.322 | 1096 | 7.28 | 5.74 | 4.95 | 4.22 | 2.46 | 3.11 | 4.83 | 16.59 | 5.80 | 21.74 | 23.36 | 1.36 | 1.27 | 38.87 |
| Linalool acetate 91% | 22.694 | 1257 | ND | 7.52 | ND | ND | ND | ND | ND | 20.83 | ND | ND | 26.32 | ND | ND | 43.36 |
| m-Cymene | 17.554 | 1023 | ND | 79.34 | 58.01 | ND | ND | ND | 15.96 | ND | ND | ND | ND | ND | 8.58 | 832.26 |
| Naphthalene-d8 (I.S.) | 21.521 | 2.00 | 2.00 | 2.00 | 2.00 | 2.00 | 2.00 | 2.00 | 2.00 | 2.00 | 2.00 | 2.00 | 2.00 | 2.00 | 2.00 | |
| Neryl acetate | 25.215 | 1359 | 4.09 | 3.43 | 3.20 | 5.23 | 1.65 | 2.22 | 3.40 | 6.73 | 4.89 | 9.82 | 10.28 | 1.91 | 1.35 | 15.70 |
| Nonane | 13.959 | 900–900 | 25.16 | 26.32 | 25.03 | 28.21 | 27.57 | 20.66 | 24.03 | ND | 25.27 | 28.58 | 22.74 | 27.15 | 18.90 | 14.76 |
| o-cymene | 17.543 | 1021 | 53.16 | ND | ND | 14.40 | 24.41 | 25.18 | ND | 76.62 | 74.17 | 167.72 | 284.66 | 10.68 | ND | ND |
| Terpinen-4-ol | 21.372 | 1174 | 4.37 | 3.83 | 4.01 | 4.90 | 1.34 | 1.41 | ND | ND | 4.05 | 11.16 | 10.66 | ND | ND | 14.45 |
| Thymol | 23.554 | 1290 | 13.79 | 9.60 | 11.95 | 28.97 | 6.73 | 8.97 | 26.17 | 18.29 | 15.50 | 49.36 | 35.07 | 8.31 | 8.30 | 43.11 |
| Thymol methyl ether 91% | 22.273 | 1232 | 5.23 | 6.08 | 4.85 | 2.92 | 2.10 | 2.14 | 2.80 | 10.19 | 5.04 | 13.05 | 16.04 | ND | 1.83 | 44.05 |
| α-copaene | 25.529 | 1376 | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | 1.26 |
| α-humulene | 27.158 | 1454 | ND | ND | ND | ND | ND | ND | ND | ND | ND | 1.26 | ND | ND | ND | 3.05 |
| α-phellandrene | 17.05 | 1010–1002 | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | 1.47 | ND | ND | 9.30 |
| α-pinene | 15.047 | 939 | 1.93 | 3.00 | 1.93 | ND | ND | 0.89 | ND | ND | 2.50 | 7.33 | 11.67 | ND | ND | 76.23 |
| α-terpinene | 17.314 | 1018–1017 | ND | 1.52 | ND | ND | ND | ND | ND | 0.96 | ND | ND | 4.52 | ND | ND | 48.36 |
| α-terpineol | 21.669 | 1188 | 7.88 | 5.80 | 6.08 | 9.94 | 3.86 | 4.67 | 12.27 | 17.85 | 6.75 | 31.81 | 22.38 | 4.75 | 5.42 | 28.56 |
| α-terpinolene | 19.082 | 1088 | 1.17 | 1.06 | 1.19 | 1.13 | 0.86 | 0.84 | 1.19 | 2.48 | 1.50 | 2.94 | 3.45 | ND | ND | 18.94 |
| α-terpinyl acetate | 24.785 | 1349 | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | 177.69 |
| β-bisabolene | 27.844 | 1505 | 12.35 | 15.15 | 15.82 | 20.31 | 9.22 | 10.91 | 20.61 | 16.35 | 29.41 | 50.32 | 32.04 | 5.91 | 7.69 | 58.50 |
| β-bourbonene | 25.736 | 1387 | ND | 0.79 | 0.76 | ND | ND | ND | ND | 1.02 | 0.83 | 2.42 | 1.58 | ND | ND | 4.29 |
| β-caryophyllene | 26.463 | 1419 | 7.11 | 10.90 | 9.61 | ND | ND | ND | ND | 12.19 | 10.61 | 20.35 | 16.16 | 2.09 | 1.43 | 59.60 |
| β-cubebene | 26.612 | 1387 | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | 1.33 |
| β-myrcene | 16.478 | 988 | ND | ND | ND | ND | ND | ND | ND | ND | 1.35 | ND | ND | ND | ND | 37.41 |
| β-pinene | 16.295 | 979 | 8.26 | 9.26 | 6.68 | ND | ND | 2.75 | ND | ND | ND | 22.20 | 33.57 | ND | ND | 91.06 |
| β-thujene | 15.322 | 966 | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | 3.62 |
| γ-cadinene | 27.389 | 1491 | ND | ND | ND | ND | ND | ND | ND | ND | ND | 1.77 | 1.06 | ND | ND | 3.16 |
| γ-terpinene | 18.367 | 1059 | 2.50 | 4.38 | 2.90 | ND | 1.34 | 1.23 | ND | 2.62 | 4.13 | 4.15 | 12.72 | ND | 1.24 | 192.52 |
| δ-cadinene | 28.141 | 1523 | 0.76 | 1.00 | 1.07 | 1.42 | ND | 0.73 | 1.30 | 1.13 | 0.96 | 3.60 | 1.87 | ND | ND | 5.38 |
| Compound | SFE-2 | SFE-7 | HD-EO |
|---|---|---|---|
| Relative Percentage (%) | |||
| (-)-β-bourbonene | 0.021 | 0.049 | 0.341 |
| (1-methylpropyl)-benzene | ND | 0.003 | ND |
| (1R)-2,6,6-trimethylbicyclo [3.1.1]hept-2-ene | ND | ND | 0.007 |
| (1R,2S,6S,7S,8S)-8-isopropyl-1-methyl-3-methylenetricyclo[4.4.0.02,7]decane-rel- | 0.004 | ND | ND |
| (1S,4S,4aS)-1-isopropyl-4,7-dimethyl-1,2,3,4,4a,5-hexahydronaphthalene | ND | ND | 0.064 |
| (1S-cis)-1,2,3,5,6,8a-hexahydro-4,7-dimethyl-1-(1-methylethyl)-naphthalene/δ-cadinene | ND | ND | 0.406 |
| (2α,4aα,8aα)-3,4,4a,5,6,8a-hexahydro-2,5,5,8a-tetramethyl-2H-1-benzopyran | ND | ND | 0.059 |
| (3S,3aS,6R,7R,9aS)-1,1,7-trimethyldecahydro-3a,7-methanocyclopenta[8]annulene-3,6-diol | 0.004 | ND | ND |
| (3β)-9,19-cyclolanost-24-en-3-ol | 0.033 | ND | ND |
| (3β)-olean-12-en-3-ol, acetate | ND | 0.018 | ND |
| (9Z,12Z)-(E)-3,7-dimethylocta-2,6-dien-1-yl octadeca-9,12-dienoate | 0.053 | 0.110 | 0.012 |
| (9Z,12Z,15Z)-(E)-3,7-dimethylocta-2,6-dien-1-yl octadeca-9,12,15-trienoate | 0.085 | 0.093 | 0.009 |
| (All-E)-(±)-2,6,10,15,19,23-hexamethyl-1,6,10,14,18,22-tetracosahexaen-3-ol | 0.022 | 0.095 | ND |
| (All-E)-2,2-dimethyl-3-(3,7,12,16,20-pentamethyl-3,7,11,15,19-heneicosapentaenyl)-oxirane | ND | 0.020 | ND |
| (E)-1-(2,6,6-trimethyl-1,3-cyclohexadien-1-yl)-2-buten-1-one | ND | ND | 0.068 |
| (E)-1-phenyl-1-butene | ND | 0.003 | ND |
| (E)-2,6-dimethylocta-3,7-diene-2,6-diol | 0.008 | ND | ND |
| (E)-3,7,11-trimethyl-1,6,10-dodecatrien-3-ol | 0.007 | ND | ND |
| (E)-3,7-dimethyl-2,6-octadienal | ND | ND | 0.814 |
| (E)-3,7-dimethylocta-2,6-dien-1-yl dodecanoate | ND | 0.007 | 0.005 |
| (E)-3,7-dimethylocta-2,6-dien-1-yl palmitate | 0.047 | 0.013 | 0.052 |
| (E)-3,7-dimethylocta-2,6-dien-1-yl stearate | ND | 0.013 | 0.011 |
| (E)-3,7-dimethylocta-2,6-dien-1-yl tetradecanoate | ND | 0.006 | ND |
| (E)-3-eicosene | ND | ND | 0.001 |
| (E)-cinnamaldehyde | ND | ND | 0.003 |
| (E,E)-2,4-decadienal | 0.007 | ND | ND |
| (E,E)-2,4-heptadienal | 0.016 | 0.021 | 0.198 |
| (E,E)-2,4-hexadienal | ND | ND | 0.016 |
| (E,E)-2,6-dimethyl-2,4,6-octatriene | ND | 0.005 | ND |
| (E,E)-3,5-octadien-2-one | ND | 0.002 | 0.097 |
| (E,E)-3,7,11,15-tetramethyl-1,6,10,14-hexadecatetraen-3-ol | 0.024 | 0.030 | 0.053 |
| (E,E)-6,10,14-trimethyl-5,9,13-pentadecatrien-2-one | ND | ND | 0.005 |
| (E,Z)-2,6-dimethyl-2,4,6-octatriene | ND | ND | 0.094 |
| (R)-2(4H)-5,6,7,7a-tetrahydro-4,4,7a-trimethyl-benzofuranone | 0.046 | 0.070 | 0.039 |
| (R)-2-methyl-5-(6-methylhepta-1,5-dien-2-yl)cyclohex-2-enone | 0.006 | ND | 0.110 |
| (R)-4-methyl-1-(1-methylethyl)-3-cyclohexen-1-ol | ND | 0.092 | ND |
| (R)-α,α,4-trimethyl-3-cyclohexene-1-methanol/α-terpinyl propionate | ND | ND | 0.921 |
| (S,1Z,6Z)-8-isopropyl-1-methyl-5-methylenecyclodeca-1,6-diene | ND | ND | 0.047 |
| (S,E)-4-hydroxy-3,5,5-trimethyl-4-(3-oxobut-1-en-1-yl)cyclohex-2-enone | 0.004 | ND | ND |
| (Z)-11-hexadecen-1-ol | ND | ND | 0.130 |
| (Z)-13-docosenamide | 0.007 | ND | 0.002 |
| (Z)-13-octadecenal | 0.025 | ND | 0.009 |
| (Z)-2-(hexa-2,4-diyn-1-ylidene)-1,6-dioxaspiro[4.4]non-3-ene | ND | ND | 0.001 |
| (Z)-3,7-dimethyl-1,3,6-octatriene | 0.027 | ND | ND |
| (Z)-3,7-dimethyl-2,6-octadien-1-ol | ND | ND | 0.185 |
| (Z)-3,7-dimethyl-2,6-octadien-1-ol formate | ND | 0.025 | ND |
| (Z)-3,7-dimethylocta-2,6-dien-1-yl palmitate | 0.004 | ND | ND |
| (Z)-9-octadecenal | ND | 0.007 | ND |
| (Z)-benzoate, 3-hexen-1-ol | ND | ND | 0.034 |
| (Z,Z)-12-octadecadienoic acid, methyl ester | ND | ND | 0.009 |
| (Z,Z)-3,6-nonadienal | 0.004 | 0.004 | ND |
| [1S-(1α,4aβ,8aα)]-1,2,4a,5,8,8a-hexahydro-4,7-dimethyl-1-(1-methylethyl)-, [1S-(1α,4aβ,8aα)]-naphthalene | 0.026 | 0.069 | ND |
| [R-[R*,R*-(E)]]-3,7,11,15-tetramethyl-2-hexadecen-1-ol, acetate | ND | 0.010 | ND |
| [R-[R*,R*-(E)]]-3,7,11,15-tetramethyl-2-hexadecene | ND | ND | 0.009 |
| 1-(1,5-dimethyl-4-hexenyl)-4-methyl-benzene | ND | 0.009 | 0.113 |
| 1-(3,4-dimethoxyphenyl)-ethanone | ND | 0.002 | ND |
| 1-(3-hydroxy-4-methoxyphenyl)-ethanone | 0.014 | ND | ND |
| 1-(4-methylphenyl)-ethanone | 0.011 | 0.015 | 0.169 |
| 1-(hexahydropyrrolizin-3-ylidene)-3,3-dimethyl-butan-2-one | ND | ND | 0.001 |
| 1-(phenylmethylene)-1H-indene | ND | ND | 0.001 |
| 1,1,5-trimethyl-1,2-dihydronaphthalene | ND | ND | 0.020 |
| 1,1’-oxybis-octane | ND | ND | 0.008 |
| 1,2,3,4-tetramethyl-benzene | 0.019 | ND | ND |
| 1,2,3-trimethyl-benzene | ND | 0.005 | ND |
| 1,2,4a,5,6,8a-hexahydro-4,7-dimethyl-1-(1-methylethyl)-naphthalene | ND | ND | 0.132 |
| 1,2,4-trimethyl-benzene | 0.008 | ND | ND |
| 1,2-dihydro-1,1,6-trimethyl-naphthalene | 0.002 | ND | 0.020 |
| 1,3,5-triazine | 0.004 | ND | ND |
| 1,3,5-trimethoxy-benzene | ND | ND | 0.005 |
| 1,3-bis(1,1-dimethylethyl)-benzene | ND | ND | 0.029 |
| 1,4-dimethyl-naphthalene | 0.002 | ND | ND |
| 1,6-dimethyl-4-(1-methylethyl)-naphthalene | ND | 0.012 | 0.099 |
| 1,7,7-trimethyl-, (1S)-bicyclo[2.2.1]heptan-2-one | ND | 0.044 | ND |
| 1,7,7-trimethyl-bicyclo[2.2.1]heptan-2-ol, propanoate | ND | ND | 0.012 |
| 11-(1-ethylpropyl)-heneicosane | ND | ND | 0.011 |
| 11-decyl-tetracosane | 0.104 | 0.255 | 0.019 |
| 13-methyltetradecanal | ND | ND | 0.020 |
| 1-chloro-2-propanol, phosphate (3:1) | ND | ND | 0.008 |
| 1-decyl-cyclohexene | ND | 0.003 | ND |
| 1-docosene | ND | 0.002 | ND |
| 1-dodecanol | ND | ND | 0.013 |
| 1-eicosanol | ND | 0.013 | ND |
| 1-ethenyl-1-methyl-2-(1-methylethenyl)-4-(1-methylethylidene)-cyclohexane | ND | ND | 0.092 |
| 1-ethenyl-1-methyl-2,4-bis(1-methylethenyl)-, [1S-(1α,2β,4β)]-cyclohexane | ND | ND | 0.027 |
| 1-ethyl-2-propyl-cyclohexane | 0.016 | 0.014 | ND |
| 1-hepten-3-one | ND | ND | 0.126 |
| 1-hexacosene | 0.050 | 0.065 | 0.002 |
| 1-hexadecanol | ND | ND | 0.017 |
| 1-iodo-docosane | 0.008 | ND | 0.002 |
| 1-iodo-dotriacontane | 0.067 | ND | ND |
| 1-methyl-2-pentyl-cyclohexane | ND | 0.009 | ND |
| 1-methyl-2-pyrrolidinone | ND | ND | 0.001 |
| 1-methyl-3-(1-methylethenyl)-benzene | ND | ND | 0.007 |
| 1-methyl-4-(1-methylethenyl)-1,2-cyclohexanediol | 0.022 | 0.029 | ND |
| 1-methyl-4-(1-methylethenyl)-benzene | ND | ND | 0.215 |
| 1-methyl-4-(1-methylethyl)-1,3-cyclohexadiene/α-terpinene | 0.009 | 0.022 | 0.695 |
| 1-methyl-4-(1-methylethyl)-cyclohexanol | 0.010 | 0.023 | ND |
| 1-methyl-4-(1-methylethylidene)-cyclohexene | 0.015 | 0.042 | 0.037 |
| 1-methyl-4-propyl-benzene | 0.004 | 0.010 | ND |
| 1-methyl-naphthalene | 0.014 | 0.005 | ND |
| 1-nonadecene | ND | 0.003 | 0.003 |
| 1-nonanol | ND | 0.004 | 0.089 |
| 1-nonen-3-ol | ND | 0.015 | 0.204 |
| 1-octadecanol | 0.009 | ND | ND |
| 1-pentadecene | ND | ND | 0.005 |
| 1-phenyl-1-propanone | 0.015 | 0.015 | 0.042 |
| 1-tetracosene | ND | ND | 0.002 |
| 1-tetradecene | ND | ND | 0.007 |
| 1-tricosene | ND | ND | 0.003 |
| 1-undecanol | ND | ND | 0.005 |
| 2-(2-methyl-2-propenyl)-phenol | ND | ND | 0.042 |
| 2,2′,5,5′-tetrahydro-2,2′-bifuran | ND | ND | 0.005 |
| 2,2-dihydroxy-1-phenyl-ethanone | ND | ND | 0.057 |
| 2,3,3,4,7-pentamethyl-2,3-dihydro-benzofuran | ND | ND | 0.107 |
| 2,3-dehydro-1,8-cineole | 0.003 | 0.027 | 0.339 |
| 2,3-dihydro-benzofuran | 0.002 | 0.001 | ND |
| 2,4,6-trimethyl-octane | ND | 0.079 | ND |
| 2,4-decadienal | ND | 0.012 | ND |
| 2,4-dihydroxy-3,6-dimethyl-benzoic acid, methyl ester | 0.002 | 0.004 | ND |
| 2,4-di-tert-butylphenol | 0.039 | 0.020 | 0.040 |
| 2,6,10,10-tetramethyl-1-oxaspiro[4.5]deca-3,6-diene | ND | ND | 0.012 |
| 2,6,10,14-tetramethyl-hexadecane | ND | ND | 0.009 |
| 2,6,10,15-tetramethyl-heptadecane | ND | ND | 0.005 |
| 2,6,10-trimethyltridecane | ND | ND | 0.056 |
| 2,6,6-trimethyl-2-cyclohexene-1,4-dione | ND | 0.002 | 0.008 |
| 2,6-dimethyl-3,7-octadiene-2,6-diol | ND | 0.015 | ND |
| 2,6-dimethyl-6-(4-methyl-3-pentenyl)-bicyclo[3.1.1]hept-2-ene | 0.007 | ND | ND |
| 2,6-dimethyl-octadecane | 0.019 | ND | ND |
| 2,7,7-trimethyl-bicyclo[3.1.1]hept-2-en-6-one | ND | ND | 0.030 |
| 2-amino-1,5-dihydro-4H-imidazol-4-one | 0.002 | ND | ND |
| 2-butenyl-benzene | 0.004 | ND | ND |
| 2-butyl-1-octanol | 0.006 | ND | 0.003 |
| 2-ethyl-1,4-dimethyl-benzene | 0.010 | 0.007 | ND |
| 2-ethyl-1-hexanol | ND | ND | 0.040 |
| 2-fluorobenzoic acid, 2-formyl-4,6-dichlorophenyl ester | ND | ND | 0.002 |
| 2-fluorobenzoic acid, 4-nitrophenyl ester | 0.005 | ND | ND |
| 2-hydroxy-3-methyl-1,4-naphthalenedione | ND | 0.001 | ND |
| 2-hydroxy-benzoic acid, phenylmethyl ester | ND | 0.004 | 0.029 |
| 2-methoxy-1-methyl-4-(1-methylethyl)-benzene | 0.380 | 0.656 | 0.726 |
| 2-methoxy-3-(2-propenyl)-phenol | ND | ND | 0.078 |
| 2-methoxy-4-methyl-1-(1-methylethyl)-benzene | ND | ND | 0.872 |
| 2-methoxy-4-vinylphenol | ND | ND | 0.034 |
| 2-methyl-5-(1-methylethyl)-phenol/carvacrol | 2.058 | 1.317 | 0.958 |
| 2-methyl-5-(1-methylethyl)-bicyclo[3.1.0]hex-2-ene | ND | ND | 0.006 |
| 2-methyl-5-(1-methylethyl)-phenol, acetate/carvacrol acetate | ND | ND | 1.231 |
| 2-methylbutyl-3-methylbutanoate | ND | ND | 0.012 |
| 2-methyl-eicosane | ND | ND | 0.003 |
| 2-methyl-hexadecanal | 0.003 | ND | ND |
| 2-methyl-octacosane | ND | ND | 0.017 |
| 2-methyl-octadecane | 0.009 | ND | ND |
| 2-methyl-pentadecane | ND | ND | 0.005 |
| 2-methyltetracosane | ND | 0.012 | 0.004 |
| 2-phenylethyl-benzoic acid, ester | ND | ND | 0.002 |
| 2-phenyl-naphthalene | ND | ND | 0.002 |
| 2-propen-1-ol | ND | ND | 0.002 |
| 2-propenal | ND | 0.003 | 0.004 |
| 2-propenoic acid, anhydride | 0.052 | ND | ND |
| 2-propenyl-benzene | ND | 0.002 | ND |
| 2-propyl-furan | 0.011 | ND | ND |
| 3-(1,1-dimethylethyl)-4-methoxy-phenol | ND | ND | 0.032 |
| 3,3-diethoxy-1-propyne | ND | ND | 0.071 |
| 3,4-dihydro-1(2H)-naphthalenone | 0.006 | 0.004 | ND |
| 3,4-dimethyl-2,5-furandione | ND | 0.005 | ND |
| 3,5-diamino-1,2,4-triazole | ND | 0.002 | ND |
| 3,7,11,15-tetramethyl-2-hexadecen-1-ol | 0.040 | 0.037 | 0.008 |
| 3,7,11,15-tetramethylhexadec-2-en-1-yl acetate | 0.015 | ND | ND |
| 3,7,11-trimethyl-1-dodecanol | 0.005 | 0.004 | 0.006 |
| 3,7-dimethyl-2,6-octadien-1-ol | ND | 0.086 | 0.224 |
| 3,7-dimethyl-undecane | ND | ND | 0.003 |
| 3,8-dimethyl-undecane | ND | 0.003 | 0.010 |
| 3-allyl-6-methoxyphenol | 0.002 | ND | ND |
| 3-ethyl-4-methyl-1H-pyrrole-2,5-dione | 0.008 | 0.013 | ND |
| 3-fluoro-2-propynenitrile | 0.011 | 0.004 | 0.040 |
| 3-hydroxy-benzaldehyde | ND | ND | 0.010 |
| 3-hydroxypropyl ester oleic acid | ND | 0.010 | ND |
| 3-methyl-2-cyclohexen-1-one | ND | 0.003 | ND |
| 3-methyl-6-(1-methylethyl)-2-cyclohexen-1-one | ND | ND | 0.195 |
| 3-methyl-benzaldehyde | ND | ND | 0.066 |
| 3-methylhexacosane | ND | 0.006 | ND |
| 3-methylpentacosane | 0.021 | 0.044 | 0.007 |
| 3-methyl-phenol | ND | ND | 0.004 |
| 3-octanol | 0.035 | 0.130 | 0.483 |
| 3-pentanol | ND | 0.006 | ND |
| 4-(2,2,6-trimethyl-7-oxabicyclo[4.1.0]hept-1-yl)-3-buten-2-one | ND | 0.011 | 0.094 |
| 4-(2,6,6-trimethyl-1-cyclohexen-1-yl)-3-buten-2-one | ND | 0.011 | ND |
| 4,11,11-trimethyl-8-methylene-bicyclo[7.2.0]undec-4-ene | ND | ND | 0.067 |
| 4,5-dimethyl-nonane | ND | ND | 0.005 |
| 4,6-dimethyl-dodecane | ND | ND | 0.006 |
| 4,7,7-trimethylbicyclo[4.1.0]hept-3-en-2-one | ND | ND | 0.048 |
| 4,7-dimethyl-undecane | ND | ND | 0.004 |
| 4,8,12,16-tetramethylheptadecan-4-olide | 0.024 | 0.036 | 0.015 |
| 4-[(1E)-1,5-dimethyl-1,4-hexadien-1-yl]-1-methyl-cyclohexene | ND | ND | 0.086 |
| 4-ethenyl-1,2-dimethyl-benzene | ND | 0.002 | ND |
| 4-hydroxy-3,5,6-trimethyl-4-(3-oxo-1-butenyl)-2-cyclohexen-1-one | ND | 0.005 | ND |
| 4-hydroxy-3,5-dimethoxy-benzaldehyde | ND | 0.001 | ND |
| 4-isopropyl-6-methyl-1-methylene-1,2,3,4-tetrahydronaphthalene | 0.001 | 0.003 | 0.037 |
| 4-methoxy-6-(2-propenyl)-1,3-benzodioxole | ND | 0.002 | 0.045 |
| 4-methoxybenzoic acid, 2-methoxyethyl ester | ND | ND | 0.001 |
| 4-methyl-1-(1-methylethyl)-bicyclo[3.1.0]hex-3-en-2-one | ND | ND | 0.032 |
| 4-methyl-2,4-bis(p-hydroxyphenyl)pent-1-ene, 2TMS derivative | 0.012 | 0.035 | 0.030 |
| 4-methyl-4-vinylbutyrolactone | ND | 0.004 | ND |
| 4-methyl-6-hepten-4-olide | 0.002 | ND | ND |
| 4-methylene-1-(1-methylethyl)-cyclohexene | ND | ND | 0.004 |
| 4-tert-octylphenol, TMS derivative | ND | 0.011 | 0.014 |
| 5-(1-methylethyl)-bicyclo[3.1.0]hex-3-en-2-one | ND | ND | 0.028 |
| 5-(4-hexyloxybenzoyloxy)-2-(4-nitrophenyl)pyrimidine | ND | ND | 0.001 |
| 5-methyl-1,2,3,4-tetrathiane | ND | ND | 0.003 |
| 5-methyl-2-(1-methylethyl)-cyclohexanol | ND | ND | 0.184 |
| 5-methyl-2-(1-methylethyl)-phenol, acetate | ND | ND | 0.157 |
| 5-methyl-2-thiophenecarboxaldehyde | ND | ND | 0.004 |
| 5β-iodomethyl-1β-isopropenyl-4α,5α-dimethyl-6βbicyclo[4.3.0]nonane | ND | ND | 0.003 |
| 6,10,14-trimethyl-pentadecan-2-ol | ND | 0.003 | 0.023 |
| 6,10-dimethyl-5,9-undecadien-2-ol | ND | 0.003 | 0.038 |
| 6,9-heptadecadiene | ND | ND | 0.011 |
| 6-isopropenyl-4,8a-dimethyl-1,2,3,5,6,7,8,8a-octahydro-naphthalen-2-ol | ND | 0.021 | ND |
| 6-methyl-3,5-heptadiene-2-one | ND | 0.007 | 0.131 |
| 7-hexyl-docosane | 0.006 | 0.006 | ND |
| 8-heptadecene | ND | 0.008 | 0.012 |
| 9,12,15-octadecatrienal | 0.007 | ND | ND |
| 9H-fluoren-9-one | ND | ND | 0.003 |
| 9-methylene-9H-fluorene | ND | 0.004 | ND |
| 9-methyl-nonadecane | ND | 0.006 | ND |
| 9-octadecenal | 0.156 | 0.030 | 0.029 |
| 9-octyl-hexacosane | 0.056 | 0.125 | ND |
| Acetic acid, 1,7,7-trimethyl-bicyclo[2.2.1]hept-2-yl ester | 0.028 | 0.093 | ND |
| Aciphyllene | ND | ND | 0.017 |
| Ambrosin | ND | 0.002 | ND |
| Apocynin | ND | 0.017 | ND |
| Aromadendrene oxide-(1) | ND | ND | 0.027 |
| Azulene | 0.024 | ND | ND |
| Benzaldehyde | 0.024 | 0.024 | 0.143 |
| Benzeneacetaldehyde | ND | 0.004 | 0.147 |
| Benzothiazole | ND | 0.002 | ND |
| Benzyl alcohol | 0.013 | 0.031 | 0.018 |
| Benzyl benzoate | ND | 0.002 | 0.041 |
| Benzyl nitrile | ND | ND | 0.003 |
| Bis(2-ethylhexyl) phthalate | 0.017 | 0.012 | 0.003 |
| Bornyl acetate | ND | 0.019 | 0.526 |
| Bornyl isovalerate | 0.009 | ND | ND |
| Camphor | 0.015 | ND | 0.296 |
| Caprolactam | ND | 0.003 | ND |
| Carbonic acid, (1R)-(-)-menthyl tridecyl ester | ND | 0.005 | ND |
| Carbonic acid, decyl phenyl ester | ND | 0.048 | ND |
| Carbonic acid, nonyl phenyl ester | ND | 0.004 | ND |
| Carbonic acid, octadecyl phenyl ester | ND | 0.116 | ND |
| Caryophyllene | ND | 0.004 | 0.214 |
| Caryophyllenyl alcohol | 0.009 | 0.019 | 0.113 |
| cis,cis,cis-7,10,13-hexadecatrienal | ND | 0.003 | ND |
| cis-3-hexenyl-α-methylbutyrate | ND | ND | 0.145 |
| cis-dihydrocarvone | 0.007 | ND | ND |
| cis-linaloloxide | ND | 0.005 | ND |
| cis-vaccenic acid | 10.445 | 4.269 | 0.377 |
| Copaene | 0.003 | 0.009 | 0.162 |
| Coumarin | 0.026 | 0.038 | 0.012 |
| Dibutyl phthalate | 0.052 | 0.005 | 0.045 |
| dihydro-3-methylene-5-methyl-2-furanone | ND | 0.005 | ND |
| dihydro-5-methyl-2(3H)-furanone | ND | 0.011 | ND |
| dihydro-5-pentyl-2(3H)-furanone | ND | ND | 0.065 |
| Di-isononyl phthalate | ND | 0.069 | ND |
| Dimethyl sulfone | ND | 0.005 | ND |
| Diphenyl sulfone | 0.001 | ND | ND |
| D-limonene | ND | ND | 0.532 |
| Docosane | ND | 0.025 | 0.020 |
| Dodecanal | ND | ND | 0.008 |
| Dodecanoic acid | ND | 0.014 | 0.076 |
| Dodecyl acrylate | ND | ND | 0.062 |
| Dotriacontanal | ND | 0.055 | ND |
| Dotriacontane | ND | 0.109 | ND |
| Eicosanal | 0.011 | 0.089 | 0.007 |
| Eicosane | 0.013 | 0.004 | 0.008 |
| Endo-borneol | 0.298 | 0.561 | 0.978 |
| endo-pentanoic acid, 1,7,7-trimethylbicyclo[2.2.1]hept-2-yl ester | ND | ND | 0.008 |
| Estragole | ND | ND | 0.050 |
| Ethanedioic acid, dimethyl ester | ND | ND | 0.001 |
| Ethyl 4-(ethyloxy)-2-oxobut-3-enoate | ND | 0.005 | ND |
| Ethylpentamethyl-benzene | ND | ND | 0.008 |
| Eugenol | ND | 0.004 | ND |
| Fluoranthene | ND | ND | 0.007 |
| Fluorene | ND | ND | 0.008 |
| Fumaronitrile | ND | 0.012 | 0.015 |
| Geranic acid | ND | 0.039 | ND |
| Geraniol | 0.468 | 0.572 | 1.687 |
| Geranyl acetate | 0.092 | 0.202 | 0.338 |
| Geranyl formate | 0.015 | ND | ND |
| Geranyl isobutyrate | ND | ND | 0.119 |
| Geranyl oleate | 0.043 | ND | ND |
| Heneicosane | 0.018 | 0.017 | 0.017 |
| Heptadecane | 0.011 | 0.008 | 0.024 |
| Hexacosane | ND | ND | 0.013 |
| Hexadecanal | ND | 0.002 | 0.015 |
| Hexadecanoic acid, dodecyl ester | ND | ND | 0.002 |
| Hexadecanoic acid, methyl ester | ND | 0.008 | 0.030 |
| Hexanoic acid | ND | 0.059 | ND |
| Hexatriacontane | 0.073 | 0.710 | 0.056 |
| Humulene | 0.009 | 0.032 | 0.331 |
| Hydroxymethyl 2-hydroxy-2-methylpropionate | 0.003 | ND | ND |
| Isoaromadendrene epoxide | 0.009 | 0.018 | ND |
| Isophytol | ND | ND | 0.036 |
| Isothiazole | ND | 0.004 | ND |
| l-Alanine, N-(2-furoyl)-, heptyl ester | ND | ND | 0.003 |
| Limonene | 0.158 | 0.318 | 0.638 |
| Linalool | 0.043 | 0.143 | 0.346 |
| Linalyl acetate | 0.020 | 0.090 | ND |
| Lup-20(29)-en-3-one | 0.053 | ND | ND |
| Lupeol | 0.061 | 0.045 | ND |
| Methyl formate | 0.002 | ND | ND |
| Methyl salicylate | ND | ND | 0.117 |
| N,N-dimethyl-octanamide | ND | 0.002 | ND |
| n-decanoic acid | ND | ND | 0.045 |
| Neophytadiene | 0.091 | 0.079 | 0.014 |
| Neral | ND | ND | 0.803 |
| n-hexadecanoic acid | 0.413 | 0.786 | 0.257 |
| n-nonylcyclohexane | ND | 0.002 | ND |
| Nonacos-1-ene | 0.008 | 0.010 | ND |
| Nonanoic acid | ND | 0.012 | 0.068 |
| n-tridecan-1-ol | ND | ND | 0.011 |
| O-(2-furoyl)-O’-(pentafluoropropionyl)-1,2-benzenediol | ND | ND | 0.020 |
| O,O’-di(4-butylbenzoyl)-1,2-benzenediol | ND | ND | 0.135 |
| Octacosane | 0.704 | 0.924 | 0.012 |
| Octacosanol | ND | 0.013 | ND |
| Octadecane | ND | ND | 0.022 |
| Octan-2-yl palmitate | ND | ND | 0.002 |
| O-dichloroacetyl-O’-(3-methylbut-2-enoyl)-1,2-benzenediol | ND | 0.002 | ND |
| Oleic acid | ND | ND | 4.312 |
| Oxacycloheptadecan-2-one | ND | ND | 0.009 |
| p-(1-propenyl)-toluene | 0.020 | 0.031 | ND |
| p-(2-methylallyl)-phenol | 0.008 | ND | ND |
| p-cresol | 0.003 | 0.004 | ND |
| p-cumenol | ND | 0.004 | ND |
| p-cymene | 0.130 | 0.736 | 0.534 |
| p-cymene-2,5-diol | 0.104 | 0.068 | 0.076 |
| Pentacosanal | ND | 0.006 | ND |
| Pentacosane | 0.728 | 0.696 | 0.044 |
| Pentadecanal | ND | ND | 0.033 |
| Pentadecanoic acid | ND | 0.011 | 0.039 |
| Pentamethyl-ethanol | ND | ND | 0.004 |
| Pentanoic acid | 0.028 | ND | ND |
| Pentyl-benzene | 0.005 | ND | ND |
| Phenanthrene | 0.002 | ND | ND |
| Phenylethyl alcohol | 0.014 | 0.026 | ND |
| Phloroglucinaldehyde, tris(tert-butyldimethylsilyl) ether | 0.004 | ND | ND |
| Phosphorus pentafluoride | ND | ND | 0.002 |
| Phthalic acid, cyclobutyl tridecyl ester | ND | ND | 0.789 |
| Phthalic acid, hept-4-yl nonyl ester | ND | 0.016 | ND |
| Phthalic anhydride | 0.003 | 0.002 | ND |
| Phytol | 0.047 | ND | 0.111 |
| Phytyl decanoate | 0.032 | 0.050 | ND |
| p-mentha-1,5-dien-8-ol | 0.013 | 0.030 | 0.134 |
| Propanoic acid, 2-methyl-, 3-hydroxy-2,2,4-trimethylpentyl ester | ND | 0.006 | 0.016 |
| Propanoic acid, anhydride | ND | 0.002 | 0.047 |
| Propoxy-benzene | ND | 0.016 | ND |
| Squalene | 0.474 | 0.813 | 0.068 |
| Stigmasterol | 0.065 | 0.065 | ND |
| Succinic anhydride | ND | ND | 0.004 |
| Sulfur tetrafluoride | 0.002 | ND | ND |
| Tetracosanal | 0.013 | ND | ND |
| Tetracosane | 0.012 | 0.017 | 0.007 |
| Tetradecanoic acid | 0.010 | 0.028 | 0.096 |
| Thymol | ND | ND | 0.138 |
| Thymoquinone | 0.091 | 0.117 | 0.091 |
| trans-13-octadecenoic acid | ND | 8.888 | ND |
| trans-2-(2-pentenyl)furan | ND | ND | 0.026 |
| trans-2-methyl-5-(1-methylethenyl)-cyclohexanone | ND | 0.022 | 0.195 |
| trans-5-methyl-2-(1-methylethyl)-cyclohexanone | ND | ND | 0.143 |
| trans-geranic acid methyl ester | ND | ND | 0.096 |
| trans-geranic acid methyl ester | ND | ND | 0.096 |
| trans-geranylgeraniol | 0.046 | 0.014 | ND |
| trans-β-ionone | ND | ND | 0.100 |
| Tridecane | ND | ND | 0.010 |
| Trifluoroamine oxide | 0.002 | ND | ND |
| Undecane | 0.018 | 0.023 | 0.033 |
| Vanillin | 0.011 | 0.013 | ND |
| Xanthoxylin | 0.018 | 0.029 | 0.092 |
| α-calacorene | 0.005 | 0.010 | 0.136 |
| α-corocalene | ND | ND | 0.021 |
| α-cubebene | ND | 0.003 | ND |
| α-muurolene | 0.010 | 0.023 | 0.286 |
| α-terpineol | 0.241 | 0.368 | 0.447 |
| β-amyrin | 0.057 | 0.044 | ND |
| β-amyrone | ND | 0.029 | ND |
| β-bisabolene | 0.221 | 0.320 | 0.563 |
| β-ocimene | 0.027 | 0.062 | ND |
| β-phellandrene | ND | ND | 0.538 |
| β-sitosterol | 0.133 | ND | ND |
| γ-muurolene | 0.012 | 0.025 | 0.292 |
| γ-sitostenone | 0.067 | 0.084 | ND |
| γ-terpinene | 0.015 | 0.022 | 0.394 |
| Tested Bacteria | SFE-2 (mg/mL) | SFE-7 (mg/mL) | HD-EO (mg/mL) | CHL (µg/mL) 1 | |
|---|---|---|---|---|---|
| B. spizizeni ATCC 6633 | MIC | 0.31 ± 0.00 a2 | 0.83 ± 0.36 b | 0.31 ± 0.00 a | 1.95 ± 0.00 c |
| MBC | 0.31 ± 0.00 a | 0.83 ± 0.36 b | 0.31 ± 0.00 a | 62.5 ± 0.00 c | |
| E. faecalis ATCC 29212 | MIC | 13.33 ± 5.77 a | ND 3 | 1.25 ± 0.00 b | 2.60 ± 1.13 c |
| MBC | 20.00 ± 0.00 a | ND | 1.25 ± 0.00 b | ND | |
| E. faecalis clinical strain | MIC | 5.00 ± 0.00 a | 5.00 ± 0.00 a | 0.62 ± 0.00 b | 1.95 ± 0.00 c |
| MBC | 10.00 ± 0.00 a | ND | 1.25 ± 0.00 a | ND | |
| S. aureus ATCC 25923 | MIC | <0.02 | <0.02 | <0.02 | <0.98 |
| MBC | 0.31 ± 0.00 a | 0.62 ± 0.00 b | 0.62 ± 0.00 b | ND | |
| S. aureus MRSA clinical stain | MIC | <0.02 | <0.02 | <0.02 | <0.98 |
| MBC | 2.50 ± 0.00 a | 2.50 ± 0.00 a | 0.62 ± 0.00 b | 62.50 ± 00 c | |
| L. monocytogenes ATCC 19111 | MIC | 1.25 ± 0.00 a | 1.25 ± 0.00 a | 0.16 ± 0.00 c | <0.98 |
| MBC | 5.00 ± 0.00 a | 20.00 ± 0.00 b | 1.25 ± 0.00 c | ND | |
| P. mirabilis ATCC 12453 | MIC | 6.67 ± 2.69 a | 10.0 ± 00.0 a | 0.83 ± 0.36 c | 1.95 ± 0.00 d |
| MBC | 10.00 ± 0.00 a | 20.00 ± 0.00 b | 1.25 ± 0.00 c | ND | |
| P. hauseri ATCC 13315 | MIC | 0.83 ± 0.36 a | 2.50 ± 0.00 b | 0.16 ± 0.00 c | <0.98 |
| MBC | 2.50 ± 0.00 a | 10.00 ± 00.0 b | 0.62 ± 0.00 c | 500.00 ± 0.00 d | |
| Ps. aeruginosa clinical strain | MIC | 2.50 ± 0.00 a | 5.00 ± 0.00 b | 0.62 ± 0.00 c | 15.62 ± 0.00 d |
| MBC | 2.50 ± 0.00 a | 5.00 ± 0.00 b | 1.25 ± 0.00 c | 500.00 ± 0.00 d | |
| E. coli ATCC 25922 | MIC | ND | ND | 1.25 ± 0.00 a | 1.95 ± 0.00 b |
| MBC | ND | ND | 1.25 ± 0.00 a | 500.0 ± 0.0 b | |
| E. coli H7:O157 ATCC 35150 | MIC | ND | ND | 1.25 ± 0.00 a | 3.91 ± 0.00 b |
| MBC | ND | ND | 1.25 ± 0.00 a | 1000.00 ± 0.00 a | |
| S. Enteritidis ATCC 13076 | MIC | 20.00 ± 0.00 a | ND | 2.50 ± 0.00 b | 1.95 ± 0.00 c |
| MBC | 20.00 ± 0.00 a | ND | 2.50 ± 0.00 b | ND | |
| S. Typhimurium ATCC 14028 | MIC | 20.00 ± 0.00 a | ND | 1.25 ± 0.00 b | 1.95 ± 0.00 c |
| MBC | 20.00 ± 0.00 a | ND | 2.50 ± 0.00 b | ND | |
| S. sonnei ATCC 29930 | MIC | 5.00 ± 0.00 a | ND | 1.25 ± 0.00 b | 0.98 ± 0.00 c |
| MBC | ND | ND | 2.50 ± 0.00 b | 125.00 ± 0.00 d | |
| Y. enterocolitica ATCC 27729 | MIC | 0.83 ± 0.36 a | 2.50 ± 0.00 b | 0.10 ± 0.04 c | <0.98 |
| MBC | 10.00 ± 0.00 a | ND | 0.62 ± 0.00 c | ND | |
| Tested yeast | SFE-2 (mg/mL) | SFE-7 (mg/mL) | HD-EO (mg/mL) | NYS (µg/mL) | |
| C. albicans ATCC 1231 | MIC | 2.5 ± 0.00 a | ND | 1.25 ± 0.00 b | 250.00 ± 0.00 c |
| MFC | 10.00 ± 0.00 a | ND | 1.25 ± 0.00 b | 250.00 ± 0.00 c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mrkonjić, Ž.; Kaplan, M.; Milošević, S.; Božović, D.; Sknepnek, A.; Miletić, D.; Lazarević Mrkonjić, I.; Rakić, D.; Zeković, Z.; Pavlić, B. Green Extraction Approach for Isolation of Bioactive Compounds in Wild Thyme (Thymus serpyllum L.) Herbal Dust—Chemical Profile, Antioxidant and Antimicrobial Activity and Comparison with Conventional Techniques. Plants 2024, 13, 897. https://doi.org/10.3390/plants13060897
Mrkonjić Ž, Kaplan M, Milošević S, Božović D, Sknepnek A, Miletić D, Lazarević Mrkonjić I, Rakić D, Zeković Z, Pavlić B. Green Extraction Approach for Isolation of Bioactive Compounds in Wild Thyme (Thymus serpyllum L.) Herbal Dust—Chemical Profile, Antioxidant and Antimicrobial Activity and Comparison with Conventional Techniques. Plants. 2024; 13(6):897. https://doi.org/10.3390/plants13060897
Chicago/Turabian StyleMrkonjić, Živan, Muammer Kaplan, Sanja Milošević, Danica Božović, Aleksandra Sknepnek, Dunja Miletić, Ivana Lazarević Mrkonjić, Dušan Rakić, Zoran Zeković, and Branimir Pavlić. 2024. "Green Extraction Approach for Isolation of Bioactive Compounds in Wild Thyme (Thymus serpyllum L.) Herbal Dust—Chemical Profile, Antioxidant and Antimicrobial Activity and Comparison with Conventional Techniques" Plants 13, no. 6: 897. https://doi.org/10.3390/plants13060897






