Influence of the Drying Process on the Volatile Profile of Different Capsicum Species
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Drying Peppers
2.3. Volatiles Organic Compounds (VOCs) from Fresh and Dried Samples
2.4. Statistic Analysis
3. Results and Discussion
3.1. Evaluation of Aroma Compounds from Fresh to Dry Samples
3.2. Dried Chili: Volatile Markers for Species Discrimination
4. Conclusions and Future Perspectives
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Fowles, J.; Mitchell, J.; McGrath, H. Assessment of cancer risk from ethylene oxide residues in spices imported into New Zealand. Food Chem. Toxicol. 2001, 39, 1055–1062. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Zhang, M.; Mujumdar, A.S. Recent developments in smart drying technology. Dry. Technol. 2015, 33, 260–276. [Google Scholar] [CrossRef]
- Vilela, C.A.A.; Artur, P.O. Drying of Curcuma longa L. in diferent shapes. Food Sci. Technol. 2008, 28, 387–394. [Google Scholar] [CrossRef]
- Taiti, C.; Costa, C.; Migliori, C.A.; Comparini, D.; Figorilli, S.; Mancuso, S. Correlation between volatile compounds and spiciness in domesticated and wild fresh chili peppers. Food Bioprocess Technol. 2019, 12, 1366–1380. [Google Scholar] [CrossRef]
- Korkmaz, A.; Hayaloglu, A.A.; Atasoy, A.F. Evaluation of the volatile compounds of fresh ripened Capsicum annuum and its spice pepper (dried red pepper flakes and isot). LWT 2017, 84, 842–850. [Google Scholar] [CrossRef]
- Van Ruth, S.M.; Roozen, J.P.; Cozijnsen, J.L.; Posthumus, M.A. Volatile compounds of rehydrated French beans, bell peppers and leeks. Part II. Gas Chromathraphy/sniffing port analysis and sensory evaluation. Food Chem. 1995, 54, 1e7. [Google Scholar] [CrossRef]
- Ko, A.-Y.; Rahman, M.; El-Aty, A.A.; Jang, J.; Choi, J.-H.; Mamun, M.; Shim, J.-H. Identification of volatile organic compounds generated from healthy and infected powdered chili using solvent-free solid injection coupled with GC/MS: Application to adulteration. Food Chem. 2014, 156, 326–332. [Google Scholar] [CrossRef]
- Pino, J.; González, M.; Ceballos, L.; Centurión-Yah, A.R.; Trujillo-Aguirre, J.; Latournerie-Moreno, L.; Sauri-Duch, E. Characterization of total capsaicinoids, colour and volatile compounds of Habanero chilli pepper (Capsicum chinense Jack.) cultivars grown in Yucatan. Food Chem. 2007, 104, 1682–1686. [Google Scholar] [CrossRef]
- Ziino, M.; Condurso, C.; Romeo, V.; Tripodi, G.; Verzera, A. Volatile compounds and capsaicinoid content of fresh hot peppers (Capsicum annuum L.) of different Calabrian varieties. J. Sci. Food Agric. 2009, 89, 774–780. [Google Scholar] [CrossRef]
- Toontom, N.; Posri, W.; Lertsiri, S.; Meenune, M. Effect of drying methods on Thai dried chilli’s hotness and pungent odour characteristics and consumer liking. Int. Food Res. J. 2016, 23, 289–299. [Google Scholar]
- Luning, P.A.; Ebbenhorst-Seller, T.; de Rijk, T.; Roozen, J.P. Effect of hot-air drying on flavour compounds of bell peppers (Capsicum annuum). J. Sci. Food Agric. 1995, 68, 355–365. [Google Scholar] [CrossRef]
- Rodríguez-Burruezo, A.; Kollmannsberger, H.; González-Mas, M.C.; Nitz, S.; Fernando, N. HS-SPME comparative analysis of genotypic diversity in the volatile fraction and aroma-contributing compounds of Capsicum fruits from the annuum-chinense-frutescens complex. J. Agric. Food Chem. 2010, 58, 4388–4400. [Google Scholar] [CrossRef] [PubMed]
- Toontom, N.; Meenune, M.; Posri, W.; Lertsiri, S. Effect of drying method on physical and chemical quality, hotness and volatile flavour characteristics of dried chilli. Int. Food Res. J. 2012, 19, 1023. [Google Scholar]
- Bianchi, G.; Lo Scalzo, R. Characterization of hot pepper spice phytochemicals, taste compounds content and volatile profiles in relation to the drying temperature. J. Food Biochem. 2018, 42, e12675. [Google Scholar] [CrossRef]
- Guclu, G.; Keser, D.; Kelebek, H.; Keskin, M.; Sekerli, Y.E.; Soysal, Y.; Selli, S. Impact of production and drying methods on the volatile and phenolic characteristics of fresh and powdered sweet red peppers. Food Chem. 2021, 338, 128129. [Google Scholar] [CrossRef]
- Liu, M.; Hu, L.; Deng, N.; Cai, Y.; Li, H.; Zhang, B.; Wang, J. Effects of different hot-air drying methods on the dynamic changes in color, nutrient and aroma quality of three chili pepper (Capsicum annuum L.) varieties. Food Chem. X 2024, 22, 101262. [Google Scholar] [CrossRef] [PubMed]
- Kamal, M.M.; Ali, M.R.; Rahman, M.M.; Shishir, M.R.I.; Yasmin, S.; Sarker, M.S.H. Effects of processing techniques on drying characteristics, physicochemical properties and functional compounds of green and red chilli (Capsicum annum L.) powder. J. Food Sci. Technol. 2019, 56, 3185–3194. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Lee, K.W.; Park, J.; Lee, H.J.; Hwang, I.K. Effect of drying in antioxidant activity and changes of ascorbic acid and colour by different drying and storage in Korean red pepper (Capsicum annuum L.). Int. J. Food Sci. Technol. 2006, 41, 90–95. [Google Scholar] [CrossRef]
- Pitt, J.I.; Hocking, A.D. Fungi and Food Spoilage, 2nd ed.; Blackie Academic and Professional, Blackie Academic & Professional: London, UK, 1997. [Google Scholar]
- Blake, R.S.; Monks, P.S.; Ellis, A.M. Proton-transfer reaction mass spectrometry. Chem. Rev. 2009, 109, 861–896. [Google Scholar] [CrossRef]
- Jordan, A.; Haidacher, S.G.H.E.H.; Hanel, G.; Hartungen, E.; Märk, L.; Seehauser, H.; Märk, T.D. A high resolution and high sensitivity proton-transfer-reaction time-of-flight mass spectrometer (PTR-TOF-MS). Int. J. Mass Spectrom. 2009, 286, 122–128. [Google Scholar] [CrossRef]
- Sjöström, M.; Wold, S.; Söderström, B. PLS discriminant plots. In Pattern Recognition in Practice; Elsevier: Amsterdam, The Netherlands, 1986; pp. 461–470. [Google Scholar]
- Sabatier, R.; Vivien, M.; Amenta, P. Two approaches for discriminant partial least squares. In Between Data Science and Applied Data Analysis: Proceedings of the 26th Annual Conference of the Gesellschaft für Klassifikation eV, University of Mannheim, Mannheim, Germany, 22–24 July 2002; Springer: Berlin/Heidelberg, Germany, 2003. [Google Scholar]
- Infantino, A.; Taiti, C.; Grottoli, A.; Mancuso, S.; Costa, C.; Garzoli, S. Examination of Volatile Signatures of Fusarium Bulb Rot in Garlic Using Proton-Transfer-Reaction Time-of-Flight Mass Spectrometry and Solid-Phase Microextraction Gas Chromatography/Mass Spectrometry. Separations 2023, 10, 556. [Google Scholar] [CrossRef]
- Moscovini, L.; Ortenzi, L.; Pallottino, F.; Figorilli, S.; Violino, S.; Pane, C.; Capparella, V.; Vasta, S.; Costa, C. An open-source machine-learning application for predicting pixel-to-pixel NDVI regression from RGB calibrated images. Comput. Electron. Agric. 2024, 216, 108536. [Google Scholar] [CrossRef]
- Chollet, F. Keras: Deep learning library for python-convnets, recurrent neural networks, and more. 2015. Available online: https://keras.io/ (accessed on 10 March 2024).
- Kennard, R.W.; Stone, L.A. Computer aided design of experiments. Technometrics 1969, 11, 137–148. [Google Scholar] [CrossRef]
- Antonucci, F.; Costa, C.; Pallottino, F.; Paglia, G.; Rimatori, V.; De Giorgio, D.; Menesatti, P. Quantitative method for shape description of almond cultivars (Prunus amygdalus Batsch). Food Bioprocess Technol. 2012, 5, 768–785. [Google Scholar] [CrossRef]
- Swierenga, H.; De Groot, P.J.; De Weijer, A.P.; Derksen, M.W.J.; Buydens, L.M.C. Improvement of PLS model transferability by robust wavelength selection. Chemom. Intell. Lab. Syst. 1998, 41, 237–248. [Google Scholar] [CrossRef]
- Chong, I.G.; Jun, C.H. Performance of some variable selection methods when multicollinearity is present. Chemom. Intell. Lab. Syst. 2005, 78, 103–112. [Google Scholar] [CrossRef]
- Pane, C.; Manganiello, G.; Nicastro, N.; Ortenzi, L.; Pallottino, F.; Cardi, T.; Costa, C. Machine learning applied to canopy hyperspectral image data to support biological control of soil-borne fungal diseases in baby leaf vegetables. Biol. Control. 2021, 164, 104784. [Google Scholar] [CrossRef]
- Aprea, E.; Romano, A.; Betta, E.; Biasioli, F.; Cappellin, L.; Fanti, M.; Gasperi, F. Volatile compound changes during shelf life of dried Boletus edulis: Comparison between SPME-GC-MS and PTR–ToF–MS analysis. J. Mass. Spectrom. 2015, 50, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Comparini, D.; Taiti, C.; Lanza, M.; Vita, F.; Pandolfi, C.; Luti, S.; Spinelli, F.; Pazzagli, L.; Mancuso, S. Comparison of wild and domesticated hot peppers fruit: Volatile emissions, pungency and protein profiles. Adv. Hortic. Sci. 2021, 35, 305–327. [Google Scholar] [CrossRef]
- Taiti, C.; Costa, C.; Menesatti, P.; Comparini, D.; Bazihizina, N.; Azzarello, E.; Masi, E.; Mancuso, S. Class-modeling approach to PTR-TOFMS data: A peppers case study. J. Sci. Food Agric. 2015, 95, 1757–1763. [Google Scholar] [CrossRef]
- Junior, S.B.; Março, P.H.; Valderrama, P.; Damasceno, F.C.; Aranda, M.S.; Zini, C.A.; Caramao, E.A.; Melo, A.M.T.; Filho, J.T.; Godoy, H.T. Analysis of volatile compounds in Capsicum spp. by headspace solid-phase microextraction and GC × GC-TOFMS. Anal. Methods 2015, 7, 521–529. [Google Scholar] [CrossRef]
- Pesis, E. The role of the anaerobic metabolites, acetaldehyde and ethanol, in fruit ripening, enhancement of fruit quality and fruit deterioration. Postharvest Biol. Technol. 2005, 37, 1–19. [Google Scholar] [CrossRef]
- Landaud, S.; Helinck, S.; Bonnarme, P. Formation of volatile sulfur compounds and metabolism of methionine and other sulfur compounds in fermented food. Appl. Microbiol. Biotechnol. 2008, 77, 1191–1205. [Google Scholar] [CrossRef] [PubMed]
- Cremer, D.R.; Eichner, K. Formation of volatile compounds during heating of spice paprika (Capsicum annuum) powder. J. Agric. Food Chem. 2000, 48, 2454–2460. [Google Scholar] [CrossRef]
- Guadayol, J.M.; Baquero, T.; Caixach, J. Aplication of headspace techniques to the extraction of volatile organic compounds from paprika oleoresin. Grasas Y Aceites 1997, 48, 1–5. [Google Scholar] [CrossRef]
- Mateo, J.; Aguirrezábal, M.; Domínguez, C.; Zumalacárregui, J.M. Volatile compounds in Spanish paprika. J. Food Compos. Anal. 1997, 10, 225–232. [Google Scholar] [CrossRef]
- Fu, Y.; Zhang, Y.; Soladoye, O.P.; Aluko, R.E. Maillard reaction products derived from food protein-derived peptides: Insights into flavor and bioactivity. Crit. Rev. Food Sci. Nutr. 2020, 60, 3429–3442. [Google Scholar] [CrossRef] [PubMed]
- Starkenmann, C.; Niclass, Y. New cysteine-S-conjugate precursors of volatile sulfur compounds in bell peppers (Capsicum annuum L. cultivar). J. Agric. Food Chem. 2011, 59, 3358–3365. [Google Scholar] [CrossRef] [PubMed]
- Jun, H.R.; Cho, I.H.; Choi, H.K.; Kim, Y.S. Comparison of volatile components in fresh and dried red peppers (Capsicum annuum L.). Food Sci. Biotechnol. 2005, 14, 392–398. [Google Scholar]
- Liu, M.; Deng, N.; Hou, X.; Zhang, B.; Li, H.; Wang, J. Characterisation of flavour profiles and microbial communities of fermented peppers with different fermentation years by combining flavouromics and metagenomics. Food Chem. 2024, 443, 138550. [Google Scholar] [CrossRef]
- Maleknia, S.D.; Bell, T.L.; Adams, M.A. PTR-MS analysis of reference and plant-emitted volatile organic compounds. Int. J. Mass Spectrom. 2007, 262, 203–210. [Google Scholar] [CrossRef]
- Demarcke, M.; Amelynck, C.; Schoon, N.; Dhooghe, F.; Van Langenhove, H.; Dewulf, J. Laboratory studies in support of the detection of sesquiterpenes by proton-transfer-reaction-mass-spectrometry. Int. J. Mass Spectrom. 2009, 279, 156–162. [Google Scholar] [CrossRef]
- Naef, R.; Velluz, A.; Jaquier, A. New volatile sulfur-containing constituents in a simultaneous distillation− extraction extract of red bell peppers (Capsicum annuum). J. Agric. Food Chem. 2008, 56, 517–527. [Google Scholar] [CrossRef] [PubMed]
- Eggink, P.M.; Maliepaard, C.; Tikunov, Y.; Haanstra, J.P.W.; Pohu-Flament, L.M.M.; de Wit-Maljaars, S.C.; Willeboordse-Vos, F.; Bos, S.; Benning-de Waard, C.; de Grauw-van Leeuwen, P.J.; et al. Prediction of sweet pepper (Capsicum annuum) flavor over different harvests. Euphytica 2012, 187, 117–131. [Google Scholar] [CrossRef]
- Kollmannsberger, H.; Rodríguez-Burruezo, A.; Nitz, S.; Nuez, F. Volatile and capsaicinoid composition of ají (Capsicum baccatum) and rocoto (Capsicum pubescens), two Andean species of chile peppers. J. Sci. Food Agric. 2011, 91, 1598–1611. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, M.; Schieberle, P. Important odorants of sweet bell pepper powder (Capsicum annuum cv. annuum): Differences between samples of Hungarian and Morrocan origin. Eur. Food Res. Technol. 2000, 211, 175–180. [Google Scholar] [CrossRef]
- Azcarate, C.; Barringer, S.A. Effect of enzyme activity and frozen storage on jalapeño pepper volatiles by selected ion flow tube—Mass spectrometry. J. Food Sci. 2010, 75, C710–C721. [Google Scholar] [CrossRef]
- Patel, K.; Ruiz, C.; Calderon, R.; Marcelo, M.; Rojas, R. Characterisation of volatile profiles in 50 native Peruvian chili pepper using solid phase microextraction–gas chromatography mass spectrometry (SPME–GCMS). Food Res. Int. 2016, 89, 471–475. [Google Scholar] [CrossRef]

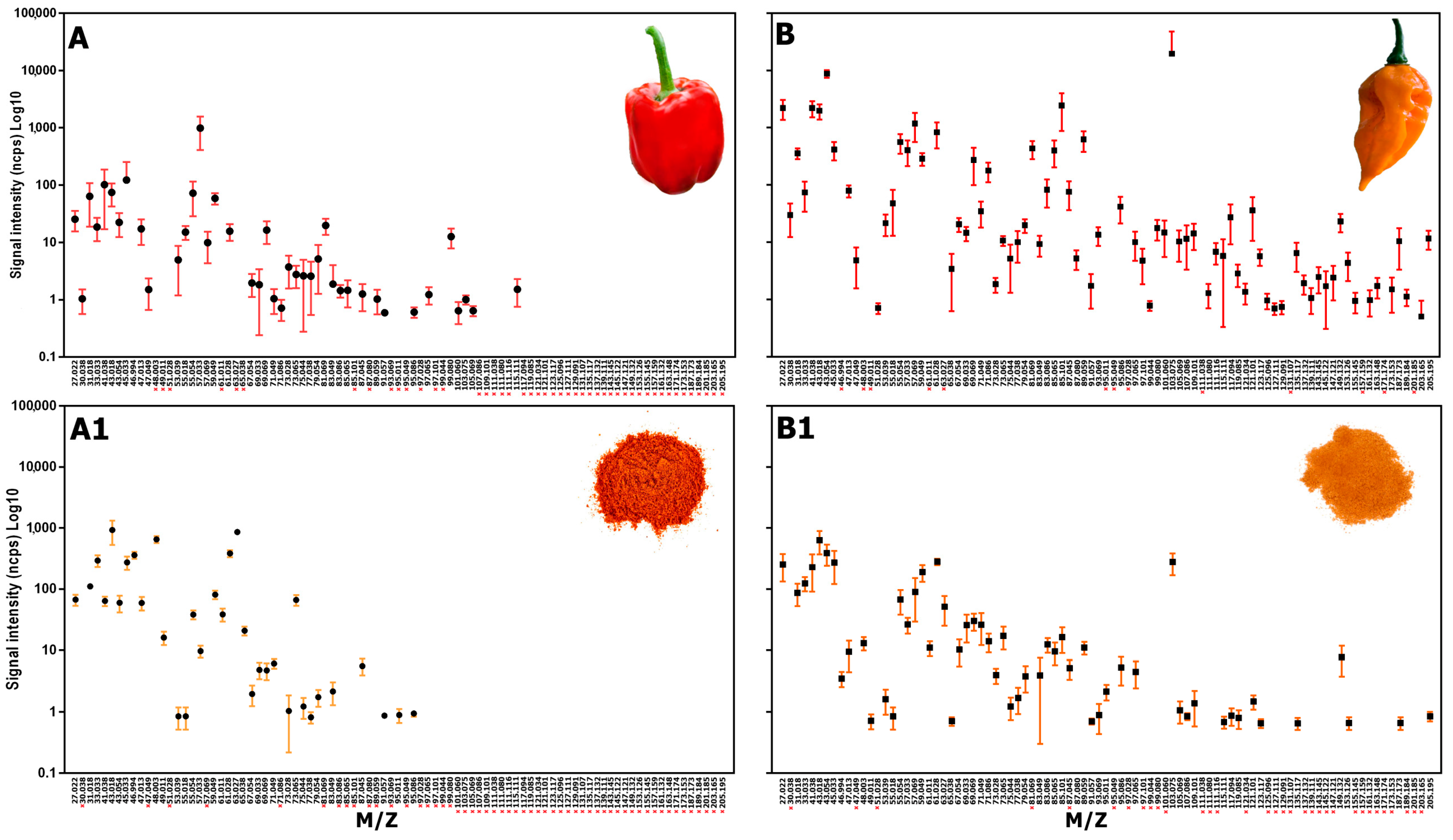
| Species | Varieties | Capsicum Fresh Sample | Capsicum Dry Sample | From Fresh to Dry | |||||
|---|---|---|---|---|---|---|---|---|---|
| Total VOC Emissions (Average and SD) | Total VOCs Emission of Each Species (Average) | Total Signals Detected (N°) | Total VOC Emissions (Average and SD) | Total VOC Emissions of Each Species (Average) | Total Signals Detected (N°) | Total VOCs Emissions Reduction/Increase (%) | Difference of Number of Signals Detected from Fresh to Dry | ||
| C. chacoense | Chacoense | 18,572.5 ± 2410.0 | 18,572.5 | 52 | 1333.3 ± 194.3 | 1333.3 | 32 | −92.8 | 20 |
| C. annuum | Bell Pepper | 1700.9 ± 746.7 | 5414.7 | 40 | 4424.4 ± 386.2 | 1949.1 | 34 | 160.1 | 6 |
| Little Nubian | 10,783.5 ± 3231.7 | 60 | 2247.3 ± 676.1 | 31 | −79.2 | 29 | |||
| Jalapeno | 11,905.1 ± 1674.7 | 50 | 954.2 ± 133.8 | 25 | −92.0 | 25 | |||
| Orange Hornamental | 4117.8 ± 748.8 | 45 | 1515.9 ± 358.8 | 36 | −63.2 | 9 | |||
| Red Hornamental | 2588.9 ± 616.1 | 46 | 629.9 ± 71.9 | 33 | −75.7 | 13 | |||
| Sweet Chocolate | 1931.9 ± 584.0 | 33 | 3979.5 ± 355.4 | 41 | 106.0 | −8 | |||
| Thai Hot | 4875.4 ± 827.0 | 46 | 1111.8 ± 141.4 | 29 | −77.2 | 17 | |||
| C. baccatum | Brasileiro | 6507.8 ± 1418.0 | 11,191.0 | 62 | 929.5 ± 165.9 | 844.9 | 43 | −85.7 | 19 |
| Campana | 10,137.5 ± 1090.6 | 55 | 1027.9 ± 284.5 | 26 | −89.9 | 29 | |||
| Hot lemon | 16,303.5 ± 2880.5 | 59 | 577.4 ± 84.0 | 30 | −96.5 | 29 | |||
| C. chinense | Monkey’s Nipple | 9012.6 ± 750.6 | 23,693.0 | 60 | 718.4 ± 112.9 | 2353.1 | 35 | −95.6 | 25 |
| Chupetinho | 36,121.7 ± 3833.3 | 67 | 1140.7 ± 113.0 | 31 | −96.8 | 36 | |||
| Fatalii | 34,114.1 ± 3131.6 | 78 | 3232.9 ± 440.9 | 56 | −90.5 | 22 | |||
| H. Chocolate | 36,472.8 ± 5335.1 | 78 | 1917.5 ± 331.4 | 46 | −94.7 | 32 | |||
| H. Orange | 29,084.2 ± 5042.9 | 72 | 5148.1 ± 584.4 | 43 | −82.3 | 29 | |||
| H. Red | 18,841.1 ± 2247.7 | 65 | 2801.5 ± 353.3 | 50 | −85.1 | 15 | |||
| H. Red Caribbean | 29,179.2 ± 1146.2 | 73 | 1383.7 ± 196.3 | 41 | −95.3 | 32 | |||
| H. Yellow | 23,646.0 ± 4056.2 | 62 | 2482.2 ± 250.2 | 49 | −89.5 | 13 | |||
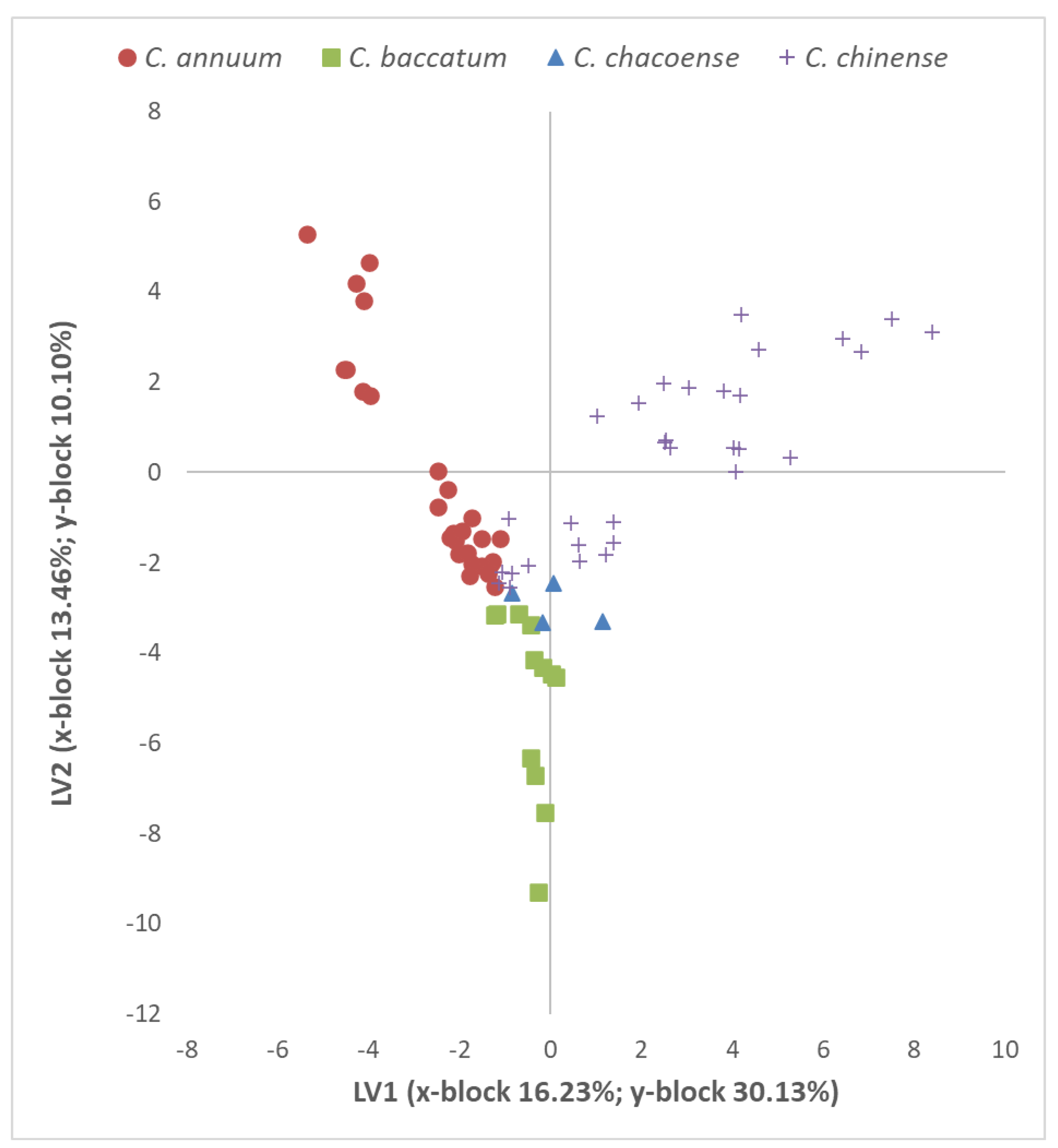
| C. annuum | C. baccatum | C. chacoense | C. chinense | Total | |
|---|---|---|---|---|---|
| N | 84 | 36 | 12 | 96 | 228 |
| N. units (X-block) | 70 | 72 | 56 | 89 | 89 |
| N. units (Y-block) | 2 | 2 | 2 | 2 | 2 |
| Preprocessing | Autoscale | Autoscale | Autoscale | Autoscale | Normalize |
| N. LV | 2 | 2 | 2 | 3 | 6 |
| % Cumulated variance X-block | 50.13 | 62.64 | 72.10 | 52.79 | 82.01 |
| % Cumulated variance Y-block | 37.92 | 42.61 | 44.40 | 41.56 | 87.74 |
| Mean specificity | 1 | 1 | 1 | 1 | 0.97 |
| Mean sensitivity | 1 | 1 | 1 | 1 | 0.99 |
| Random probability (%) | 50 | 50 | 50 | 50 | 50 |
| Mean class. err. | 0 | 0 | 0 | 0 | 0.02 |
| Mean RMSEC | 0.69 | 0.51 | 0.50 | 0.68 | 0.25 |
| %Corr. class. model | 100 | 100 | 100 | 100 | 98.2 |
| % Corr. class. independent test | 100 | 100 | 100 | 100 | 94.7 |
| C. annuum | C. baccatum | C. chacoense | C. chinense | Total | |
|---|---|---|---|---|---|
| N | 84 | 36 | 12 | 96 | 228 |
| N. units (X-block) | 70 | 72 | 56 | 89 | 89 |
| N. units (Y-block) | 2 | 2 | 2 | 2 | 2 |
| Random probability (%) | 50 | 50 | 50 | 50 | 50 |
| % Corr. class. model | 100 | 100 | 100 | 100 | 100 |
| % Corr. class. independent test | 90.5 | 100 | 100 | 100 | 98.2 |
| C. annuum | C. baccatum | C. chacoense | C. chinense | Total | |
|---|---|---|---|---|---|
| #1 | 61.011 (TI Thioacetaldehyde) | 30.038 (TI Ethylene isotope) | 63.027 (TI Dimethylsulfide) | 61.011 (TI Thioacetaldehyde) | 43.054 (TI Alkyl fragment (alcohols)) |
| #2 | 30.038 (TI Ethylene isotope) | 47.049 (TI Ethanol) | 46.994 (TI Thioformaldehyde) | 46.994 (TI Thioformaldehyde) | 103.075 (TI Ethyl 3-methylbutanoate/3-methylbutanoic acid) |
| #3 | 73.065 (TI Butanone/butanal) | 63.027 (TI Dimethylsulfide) | 48.003 (TI Methanethiol) | 30.038 (TI Ethylene isotope) | 57.033 (TI C3 Aldehyde and ketone fragments) |
| #4 | 115.111 (TI Heptanal) | 48.003 (TI Methanethiol) | 47.013 (TI Formic Acid/Formates) | 103.075 (TI Ethyl 3-methylbutanoate/3-methylbutanoic acid) | 41.038 (TI Alkyl fragment (alcohols and esters) |
| #5 | 47.049 (TI Ethanol) | 27.022 (TI Alkyl fragment) | 205.195 (TI Sesquiterpenes) | 97.064 (TI 2-Ethylfuran) | 45.033 (TI Acetaldehyde) |
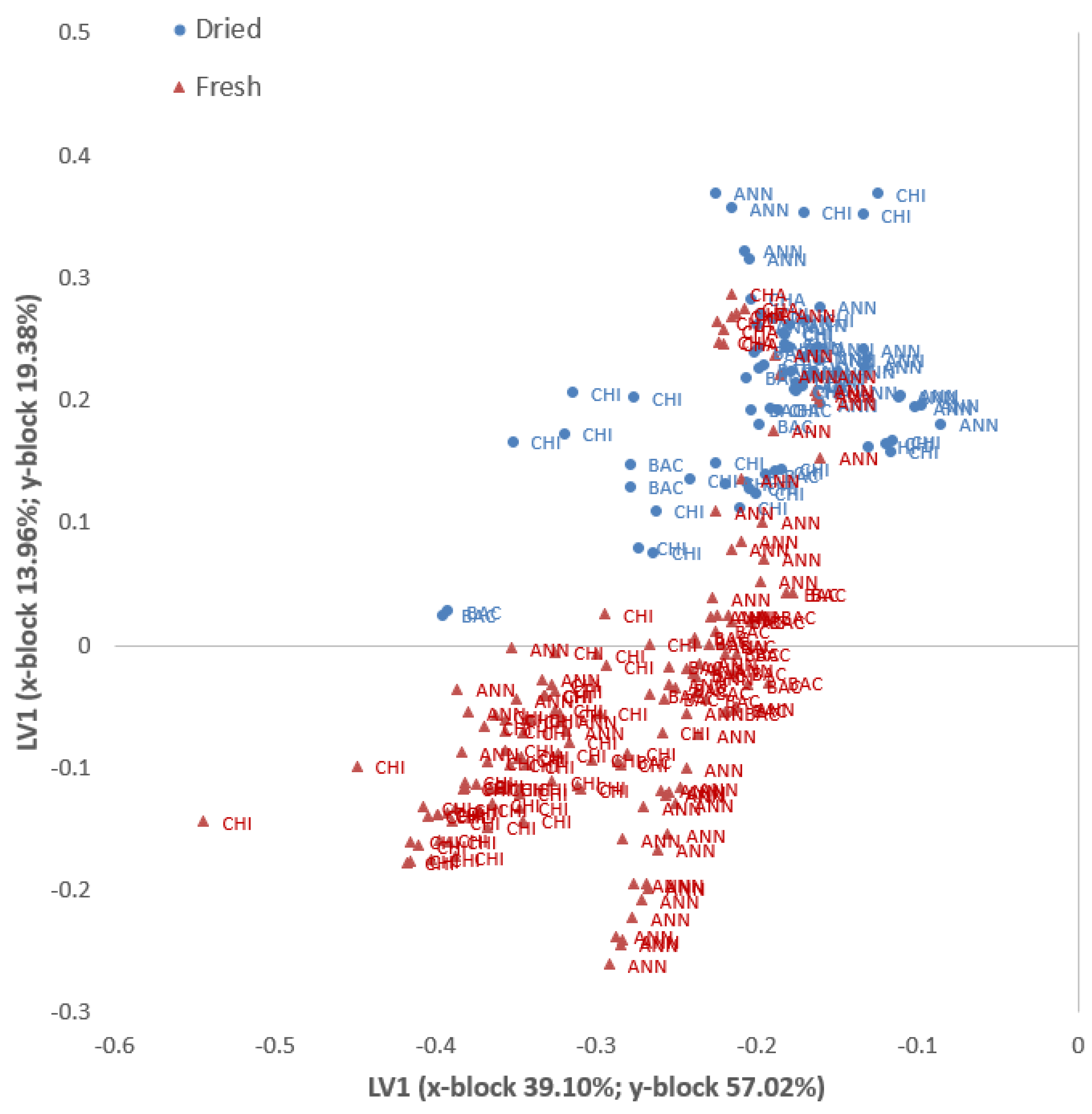
| Fresh | Dried | |
|---|---|---|
| N | 152 | 76 |
| N. units (X-block) | 84 | 64 |
| N. units (Y-block) | 4 | 4 |
| Preprocessing | Autoscale | Autoscale |
| N. LV | 10 | 9 |
| % Cumulated variance X-block | 76.94 | 76.58 |
| % Cumulated variance Y-block | 60.92 | 60.74 |
| Mean specificity | 0.99 | 0.99 |
| Mean sensitivity | 1 | 1 |
| Random probability (%) | 25 | 25 |
| Mean class. err. | 0.002 | 0.004 |
| Mean RMSEC | 0.39 | 0.38 |
| % Corr. class. model | 100 | 100 |
| % Corr. class. independent test | 98.6 | 89.5 |
| N° Compounds | m/z | Chemical Formulae | Tentatively Identification | VIP Scores | |||
|---|---|---|---|---|---|---|---|
| C. annuum | C. baccatum | C. chacoense | C. chinense | ||||
| 1 | 61.011 | C2H5S+ | S compound (Thioacetaldehyde) | 1.233 | * 1.579 | 1.049 | 0.957 |
| 2 | 87.045 | C4H7O+ | 2,3-Butanedione/Diacetyl | 0.534 | 0.951 | * 2.400 | 0.655 |
| 3 | 89.059 | C4H9O+ | Ethyl acetate | * 1.510 | 0.478 | 0.205 | 1.510 |
| 4 | 94.998 | C2H7OS2+ | S compound Dimethyl disulfide | 0.836 | * 1.559 | 0.749 | 0.758 |
| 5 | 109.101 | C8H13+ | Terpenes fragments | * 1.749 | 1.077 | 0.760 | * 1.862 |
| 6 | 117.094 | C6H13O2+ | Hexanoic acid/hexanoates | 0.827 | * 1.721 | 1.213 | 0.850 |
| 7 | 119.085 | C9H11+ | Terpenes fragments | 1.484 | 1.028 | 0.898 | * 1.581 |
| 8 | 121.014 | C4H9S2+ | 3-methyl-5-propyl-1,2-dithiolane | 0.923 | 1.218 | * 2.807 | 0.746 |
| 9 | 205.195 | C15H25+ | Sesquiterpenes | * 1.695 | 0.494 | * 1.319 | * 1.772 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Taiti, C.; Comparini, D.; Moscovini, L.; Violino, S.; Costa, C.; Mancuso, S. Influence of the Drying Process on the Volatile Profile of Different Capsicum Species. Plants 2024, 13, 1131. https://doi.org/10.3390/plants13081131
Taiti C, Comparini D, Moscovini L, Violino S, Costa C, Mancuso S. Influence of the Drying Process on the Volatile Profile of Different Capsicum Species. Plants. 2024; 13(8):1131. https://doi.org/10.3390/plants13081131
Chicago/Turabian StyleTaiti, Cosimo, Diego Comparini, Lavinia Moscovini, Simona Violino, Corrado Costa, and Stefano Mancuso. 2024. "Influence of the Drying Process on the Volatile Profile of Different Capsicum Species" Plants 13, no. 8: 1131. https://doi.org/10.3390/plants13081131






