Physiological and Enzymatic Antioxidant Responses of Solanum tuberosum Leaves to Arbuscular Mycorrhizal Fungal Inoculation under Water Stress
Abstract
:1. Introduction
2. Results
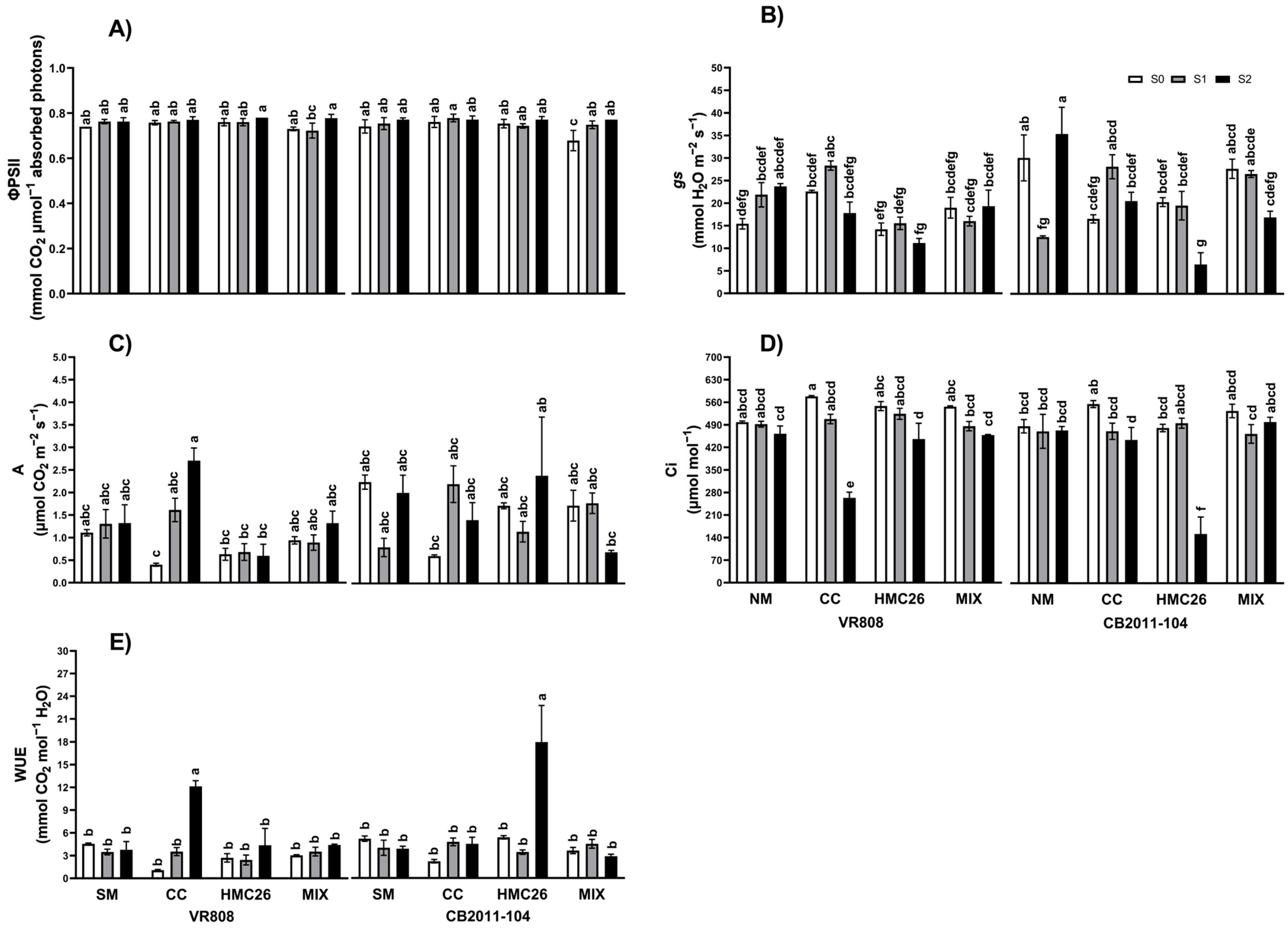
2.1. Photosynthesis Traits
2.2. Chlorophylls and Carotenoids
2.3. Enzymatic Antioxidant Activity
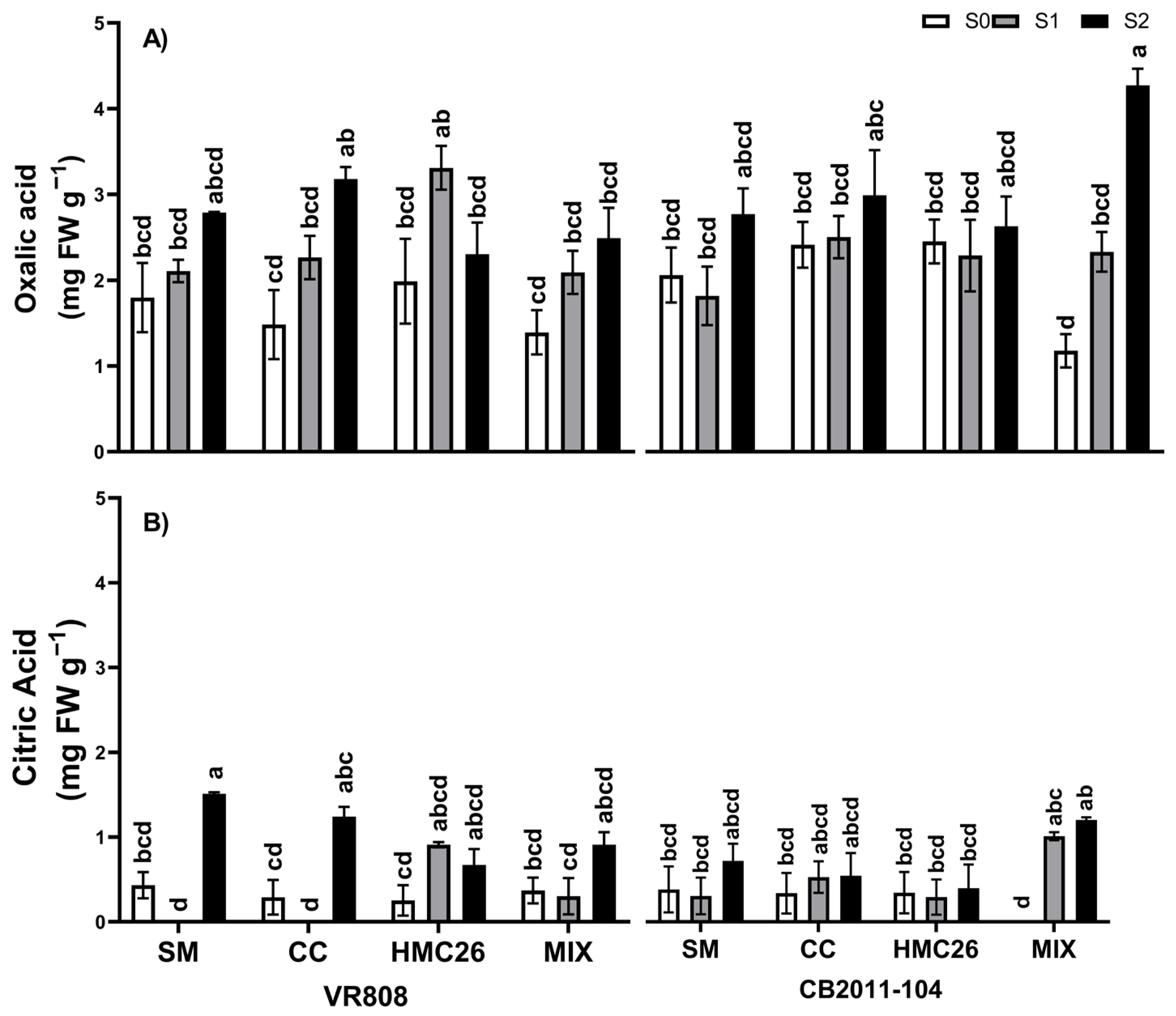
2.4. Concentrations of Low-Molecular-Weight Organic Acids
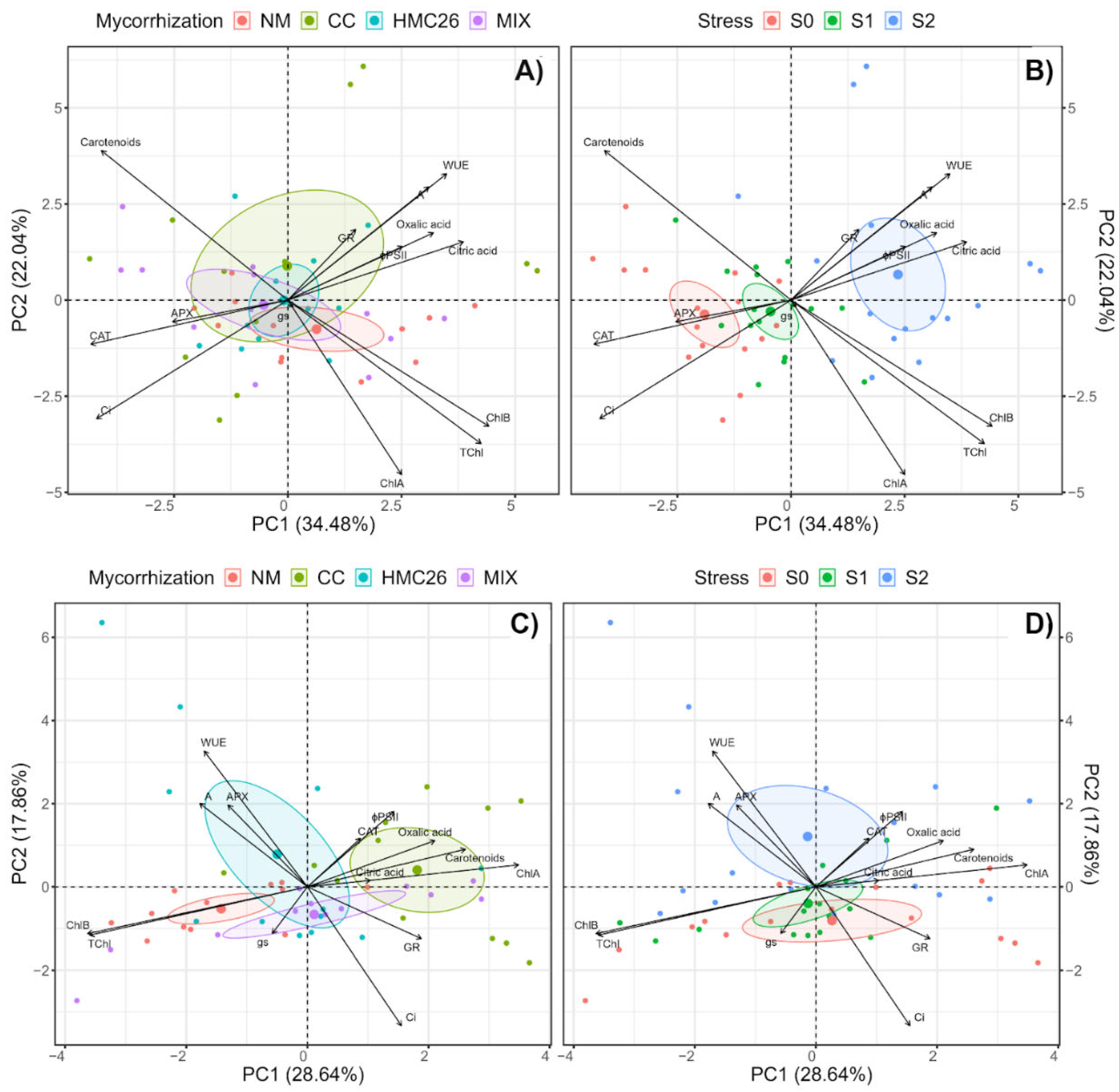
2.5. Multivariate Analysis
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. Potato Samples and Biological Materials
4.3. Photosynthetic Parameter Determination
4.4. Chlorophyll and Carotenoid Contents in Leaves
4.5. Evaluation of Enzyme Antioxidant Activities
4.6. Concentrations of Low-Molecular-Weight Organic Acids
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Hamooh, B.T.; Sattar, F.A.; Wellman, G.; Mousa, M.A.A. Metabolomic and Biochemical Analysis of Two Potato (Solanum tuberosum L.) Cultivars Exposed to In Vitro Osmotic and Salt Stresses. Plants 2021, 10, 98. [Google Scholar] [CrossRef] [PubMed]
- Aliche, E.; Theeuwen, T.; Oortwijn, M.; Visser, R.; van der Linden, C.G. Carbon partitioning mechanisms in potato under drought stress. Plant Physiol. Biochem. 2020, 146, 211–219. [Google Scholar] [CrossRef] [PubMed]
- Food and Agriculture Organization of the United Nations. FAOSTAT—Potato Production. 2023. Available online: https://www.fao.org/faostat/en/#data/QCL/visualize (accessed on 20 September 2023).
- Dahal, K.; Li, X.Q.; Tai, H.; Creelman, A.; Bizimungu, B. Improving Potato Stress Tolerance and Tuber Yield Under a Climate Change Scenario—A Current Overview. Front. Plant Sci. 2019, 10, 563. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.B.; So, J.M.; Bae, D.H. Global Warming Impacts on Severe Drought Characteristics in Asia Monsoon Region. Water 2020, 12, 1360. [Google Scholar] [CrossRef]
- Sam, T.T.; Nhi, P.T.T.; Mai, N.T.H.; Linh, D.Q.; Loi, P.T. The climate change effects on agricultural drought in the Be River Basin. IOP Conf. Ser. Earth Environ. Sci. 2023, 1170, 012006. [Google Scholar] [CrossRef]
- Chacón-Cerdas, R.; Barboza-Barquero, L.; Albertazzi, F.J.; Rivera-Méndez, W. Transcription factors controlling biotic stress response in potato plants. Physiol. Mol. Plant Pathol. 2020, 112, 101527. [Google Scholar] [CrossRef]
- Fahad, S.; Bajwa, A.A.; Nazir, U.; Anjum, S.A.; Farooq, A.; Zohaib, A.; Sadia, S.; Nasim, W.; Adkins, S.; Saud, S.; et al. Crop Production under Drought and Heat Stress: Plant Responses and Management Options. Front. Plant Sci. 2017, 8, 1147. [Google Scholar] [CrossRef] [PubMed]
- Hirut, B.; Shimelis, H.; Fentahun, M.; Bonierbale, M.; Gastelo, M.; Asfaw, A. Combining Ability of Highland Tropic Adapted Potato for Tuber Yield and Yield Components under Drought. PLoS ONE 2017, 12, e0181541. [Google Scholar] [CrossRef]
- Batool, T.; Ali, S.; Seleiman, M.F.; Naveed, N.H.; Ali, A.; Ahmed, K.; Abid, M.; Rizwan, M.; Shahid, M.R.; Alotaibi, M.; et al. Plant growth promoting rhizobacteria alleviates drought stress in potato in response to suppressive oxidative stress and antioxidant enzymes activities. Sci. Rep. 2020, 10, 16975. [Google Scholar] [CrossRef]
- Zhao, W.; Liu, L.; Shen, Q.; Yang, J.; Han, X.; Tian, F.; Wu, J. Effects of Water Stress on Photosynthesis, Yield, and Water Use Efficiency in Winter Wheat. Water 2020, 12, 2127. [Google Scholar] [CrossRef]
- Jafari, S.; Hashemi Garmdareh, S.E.; Azadegan, B. Effects of drought stress on morphological, physiological, and biochemical characteristics of stock plant (Matthiola incana L.). Sci. Hortic. 2019, 253, 128–133. [Google Scholar] [CrossRef]
- Li, J.; Cang, Z.; Jiao, F.; Bai, X.; Zhang, D.; Zhai, R. Influence of drought stress on photosynthetic characteristics and protective enzymes of potato at seedling stage. J. Saudi Soc. Agric. Sci. 2015, 16, 82–88. [Google Scholar] [CrossRef]
- Chen, Y.; Yao, Z.; Sun, Y.; Wang, E.; Tian, C.; Sun, Y.; Liu, J.; Sun, C.; Tian, L. Current Studies of the Effects of Drought Stress on Root Exudates and Rhizosphere Microbiomes of Crop Plant Species. Int. J. Mol. Sci. 2022, 23, 2374. [Google Scholar] [CrossRef]
- Santander, C.; Aroca, R.; Ruiz-Lozano, J.M.; Olave, J.; Cartes, P.; Borie, F.; Cornejo, P. Arbuscular mycorrhiza effects on plant performance under osmotic stress. Mycorrhiza 2017, 27, 639–657. [Google Scholar] [CrossRef] [PubMed]
- Püschel, D.; Bitterlich, M.; Rydlová, J.; Jansa, J. Facilitation of plant water uptake by an arbuscular mycorrhizal fungus: A Gordian knot of roots and hyphae. Mycorrhiza 2020, 30, 299–313. [Google Scholar] [CrossRef] [PubMed]
- Fritz, V.; Tereucán, G.; Santander, C.; Contreras, B.; Cornejo, P.; Ferreira, P.A.A.; Ruiz, A. Effect of Inoculation with Arbuscular Mycorrhizal Fungi and Fungicide Application on the Secondary Metabolism of Solanum tuberosum Leaves. Plants 2022, 11, 278. [Google Scholar] [CrossRef] [PubMed]
- Cayún, Y.; Alarcón, S.; Tereucán, G.; Cornejo, P.; Santander, C.; Gómez, F.; Contreras, B.; Ruiz, A. Effect of Arbuscular Mycorrhizal Fungi Inoculation on the Metabolic Activity of Solanum tuberosum Plants Under Fungicide Application. J. Soil Sci. Plant Nutr. 2023, 23, 3623–3639. [Google Scholar] [CrossRef]
- Wang, H.; Liang, L.; Liu, B.; Huang, D.; Liu, S.; Liu, R.; Siddique, K.H.M.; Chen, Y. Arbuscular Mycorrhizas Regulate Photosynthetic Capacity and Antioxidant Defense Systems to Mediate Salt Tolerance in Maize. Plants 2020, 9, 1430. [Google Scholar] [CrossRef] [PubMed]
- Chang, W.; Sui, X.; Fan, X.X.; Jia, T.T.; Song, F.Q. Arbuscular Mycorrhizal Symbiosis Modulates Antioxidant Response and Ion Distribution in Salt-Stressed Elaeagnus angustifolia Seedlings. Front. Microbiol. 2018, 9, 652. [Google Scholar] [CrossRef]
- Adavi, Z.; Tadayon, M.R.; Razmjoo, J.; Ghaffari, H. Antioxidant Enzyme Responses in Potato (Solanum tuberosum) Cultivars Colonized with Arbuscular Mycorrhizas. Potato Res. 2020, 63, 291–301. [Google Scholar] [CrossRef]
- Chen, J.; Guo, J.; Li, Z.; Liang, X.; You, Y.; He, Y.; Li, M.; Zhan, F. Effects of AMF on the growth and cadmium uptake of maize grown on polluted soils of three land-use types with different cadmium contents. Res. Sq. 2022, 10, 359. [Google Scholar] [CrossRef]
- Huang, D.; Ma, M.; Wang, Q.; Zhang, M.; Jing, G.; Li, C.; Ma, F. Arbuscular mycorrhizal fungi enhanced drought resistance in apple by regulating genes in the MAPK pathway. Plant Physiol. Biochem. 2020, 149, 245–255. [Google Scholar] [CrossRef] [PubMed]
- Hashem, A.; Kumar, A.; Al-Dbass, A.M.; Algarawi, A.A.; Al-Arjani, A.F.; Singh, G.; Farooq, M.; Abd-Allah, E.F. Arbuscular mycorrhizal fungi and biochar improves drought tolerance in chickpea. Saudi J. Biol. Sci. 2019, 26, 614–624. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Li, T.; Wu, Z.; Feng, H.; Yu, M.; Zhang, X.; Chen, B. Arbuscular mycorrhiza enhances drought tolerance of tomato plants by regulating the 14-3-3 genes in the ABA signaling pathway. App. Soil Ecol. 2018, 125, 213–221. [Google Scholar] [CrossRef]
- Valdebenito, A.; Nahuelcura, J.; Santander, C.; Cornejo, P.; Contreras, B.; Gómez-Alonso, S.; Ruiz, A. Physiological and Metabolic Effects of the Inoculation of Arbuscular Mycorrhizal Fungi in Solanum tuberosum Crops under Water Stress. Plants 2022, 11, 2539. [Google Scholar] [CrossRef] [PubMed]
- Varghese, R.; Cherukuri, A.K.; Doddrell, N.H.; Doss, C.G.; Simkin, A.J.; Ramamoorthy, S. Machine learning in photosynthesis: Prospects on sustainable crop development. Plant Sci. 2023, 335, 111795. [Google Scholar] [CrossRef] [PubMed]
- Muhammad, I.; Shalmani, A.; Ali, M.; Yang, Q.-H.; Ahmad, H.; Li, F.B. Mechanisms regulating the dynamics of photosynthesis under abiotic stresses. Front. Plant Sci. 2021, 11, 615942. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Morezuelas, A.; Barandalla, L.; Ritter, E.; Lacuesta, M.; Ruiz de Galarreta, J.I. Physiological response and yield components under greenhouse drought stress conditions in potato. J. Plant Physiol. 2022, 278, 153790. [Google Scholar] [CrossRef] [PubMed]
- Begum, N.; Qin, C.; Ahanger, M.A.; Raza, S.; Khan, M.I.; Ashraf, M.; Ahmed, N.; Zhang, L. Role of arbuscular mycorrhizal fungi in plant growth regulation: Implications in abiotic stress tolerance. Front. Plant Sci. 2019, 10, 1068. [Google Scholar] [CrossRef]
- Gimenez, C.; Gallardo, M.; Thompson, R.B. Plant–water relations. In Encyclopaedia of Soils Environment; Hillel, D., Ed.; Elsevier: Oxford, UK, 2005; pp. 231–238. [Google Scholar]
- Bhardwaj, A.K.; Chandra, K.K.; Kumar, R. Mycorrhizal inoculation under water stress conditions and its influence on the benefit of host microbe symbiosis of Terminalia arjuna species. Bull. Nat. Res. Cent. 2023, 47, 89. [Google Scholar] [CrossRef]
- Ye, Q.; Wang, H.; Li, H. Arbuscular mycorrhizal fungi improve growth, photosynthetic activity, and chlorophyll fluorescence of Vitis vinifera cv. ecolly under drought stress. Agronomy 2022, 12, 1563. [Google Scholar] [CrossRef]
- Hao, S.; Cao, H.; Wang, H.; Pan, X. The physiological responses of tomato to water stress and re-water in different growth periods. Sci. Hortic. 2019, 249, 143–154. [Google Scholar] [CrossRef]
- Rashidi, S.; Yousefi, A.R.; Pouryousef, M.; Goicoechea, N. Total phenol, anthocyanin, and terpenoid content, photosynthetic rate, and nutrient uptake of Solanum nigrum L. and Digitaria sanguinalis L. as affected by arbuscular mycorrhizal fungi inoculation. Weed Biol. Manag. 2020, 20, 95–108. [Google Scholar] [CrossRef]
- Tereucán, G.; Ruiz, A.; Nahuelcura, J.; Oyarzún, P.; Santander, C.; Winterhalter, P.; Ademar Avelar Ferreira, P.; Cornejo, P. Shifts in biochemical and physiological responses by the inoculation of arbuscular mycorrhizal fungi in Triticum aestivum growing under drought conditions. J. Sci. Food Agric. 2021, 102, 1927–1938. [Google Scholar] [CrossRef]
- Xiao, X.; Liao, X.; Yan, Q.; Xie, Y.; Chen, J.; Liang, G.; Chen, M.; Xiao, S.; Chen, Y.; Liu, J. Arbuscular mycorrhizal fungi improve the growth, water status, and nutrient uptake of Cinnamomum migao and the soil nutrient stoichiometry under drought stress and recovery. J. Fungi 2023, 9, 321. [Google Scholar] [CrossRef] [PubMed]
- Deja-Sikora, E.; Werner, K.; Hrynkiewicz, K. AMF species do matter: Rhizophagus irregularis and Funneliformis mosseae affect healthy and PVY-infected Solanum tuberosum L. in a different way. Front. Microbiol. 2023, 14, 1127278. [Google Scholar] [CrossRef]
- Roca, M.; Chen, K.; Pérez-Gálvez, A. Chlorophylls. In Handbook on Natural Pigments in Food and Beverages; Woodhead Publishing: Sawston, UK, 2016; pp. 125–158. [Google Scholar]
- Maoka, T. Carotenoids as natural functional pigments. J. Nat. Med. 2019, 74, 1–16. [Google Scholar] [CrossRef]
- Luo, Q.; Xie, H.; Chen, Z.; Ma, Y.; Yang, H.; Yang, B.; Ma, Y. Morphology, photosynthetic physiology and biochemistry of nine herbaceous plants under water stress. Front. Plant Sci. 2023, 14, 1147208. [Google Scholar] [CrossRef] [PubMed]
- Rasouli, F.; Amini, T.; Skrovankova, S.; Asadi, M.; Hassanpouraghdam, M.B.; Ercisli, S.; Buckova, M.; Mrazkova, M.; Mlcek, J. Influence of drought stress and mycorrhizal (Funneliformis mosseae) symbiosis on growth parameters, chlorophyll fluorescence, antioxidant activity, and essential oil composition of Summer savory (Satureja hortensis L.) plants. Front. Plant Sci. 2023, 14, 1151467. [Google Scholar] [CrossRef]
- Heidari, M.; Golpayegani, A. Effects of water stress and inoculation with plant growth promoting rhizobacteria (PGPR) on antioxidant status and photosynthetic pigments in Basil (Ocimum basilicum L.). J. Saudi Soc. Agric. Sci. 2012, 11, 57–61. [Google Scholar] [CrossRef]
- Zou, Y.N.; Zhang, F.; Srivastava, A.K.; Wu, Q.-S.; Kuča, K. Arbuscular mycorrhizal fungi regulate polyamine homeostasis in roots of Trifoliate orange for improved adaptation to soil moisture deficit stress. Front. Plant Sci. 2021, 11, 600792. [Google Scholar] [CrossRef]
- Chandrasekaran, M. Arbuscular mycorrhizal fungi mediated alleviation of drought stress via non-enzymatic antioxidants: A meta-analysis. Plants 2022, 11, 2448. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, Y.; Liu, C.; Gao, Y.; Han, L.; Chu, H. Arbuscular mycorrhizal fungi contribute to reactive oxygen species homeostasis of Bombax ceiba L. under drought stress. Front. Microbiol. 2022, 13, 991781. [Google Scholar] [CrossRef]
- Ma, W.Y.; Qin, Q.Y.; Zou, Y.N.; Kuča, K.; Giri, B.; Wu, Q.S.; Hashem, A.; Al-Arjani, A.-B.F.; Almutairi, K.F.; Abd_Allah, E.F.; et al. Arbuscular mycorrhiza induces low oxidative burst in drought-stressed walnut through activating antioxidant defense systems and heat shock transcription factor expression. Front. Plant Sci. 2022, 13, 1089420. [Google Scholar] [CrossRef] [PubMed]
- Haddidi, I.; Duc, N.H.; Tonk, S.; Rápó, E.; Posta, K. Defense enzymes in mycorrhizal tomato plants exposed to combined drought and heat stresses. Agronomy 2020, 10, 1657. [Google Scholar] [CrossRef]
- Bahraminia, M.; Zarei, M.; Ronaghi, A.; Sepehri, M.; Etesami, H. Ionomic and biochemical responses of maize plant (Zea mays L.) inoculated with Funneliformis mosseae to water-deficit stress. Rhizosphere 2020, 16, 100269. [Google Scholar] [CrossRef]
- Nahuelcura, J.; Ortega, T.; Peña, F.; Berríos, D.; Valdebenito, A.; Contreras, B.; Santander, C.; Cornejo, P.; Ruiz, A. Antioxidant response, phenolic compounds and yield of Solanum tuberosum tubers inoculated with arbuscular mycorrhizal fungi and growing under water stress. Plants 2023, 12, 4171. [Google Scholar] [CrossRef]
- Meier, S.; Alvear, M.; Aguilera, P.; Ginocchio, R.; Borie, F.; Cornejo, P. Influence of copper on root exudate patterns in some metallophytes and agricultural plants. Ecotoxicol. Environ. Saf. 2012, 75, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Menezes-Blackburn, D.; Paredes, C.; Zhang, H.; Giles, C.D.; Darch, T.; Stutter, M.; George, T.S.; Shand, C.; Lumsdon, D.; Cooper, P.; et al. Organic acids regulation of chemical–microbial phosphorus transformations in soils. Environ. Sci. Technol. 2016, 50, 11521–11531. [Google Scholar] [CrossRef]
- Andrino, A.; Guggenberger, G.; Kernchen, S.; Mikutta, R.; Sauheitl, L.; Boy, J. Production of organic acids by arbuscular mycorrhizal fungi and their contribution in the mobilization of phosphorus bound to iron oxides. Front. Plant Sci. 2021, 12, 661842. [Google Scholar] [CrossRef]
- Chen, J.; Guo, J.; Li, Z.; Liang, X.; You, Y.; Li, M.; He, Y.; Zhan, F. Effects of an arbuscular mycorrhizal fungus on the growth of and cadmium uptake in maize grown on polluted wasteland, farmland and slopeland soils in a lead-zinc mining area. Toxics 2022, 10, 359. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Wang, W.; Yang, J.; Qin, S.; Yang, Y.; Sun, C.; Pei, G.; Zeeshan, M.; Liao, H.; Liu, L.; et al. Mycorrhizal symbiosis promotes the nutrient content accumulation and affects the root exudates in maize. BMC Plant Biol. 2022, 22, 64. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Fan, X.; Feng, Y.; Wang, X.; Gao, H.; Song, F. Arbuscular mycorrhizal fungi influence the uptake of cadmium in industrial hemp (Cannabis sativa L.). Chemosphere 2023, 330, 138728. [Google Scholar] [CrossRef] [PubMed]
- Wahab, A.; Muhammad, M.; Munir, A.; Abdi, G.; Zaman, W.; Ayaz, A.; Khizar, C.; Reddy, S.P. Role of arbuscular mycorrhizal fungi in regulating growth, enhancing productivity, and potentially influencing ecosystems under abiotic and biotic stresses. Plants 2023, 12, 3102. [Google Scholar] [CrossRef] [PubMed]
- Nasir, M.W.; Toth, Z. Response of different potato genotypes to drought stress. Agriculture 2021, 11, 763. [Google Scholar] [CrossRef]
- Gervais, T.; Creelman, A.; Li, X.Q.; Bizimungu, B.; De Koeyer, D.; Dahal, K. Potato response to drought stress: Physiological and growth basis. Front. Plant Sci. 2021, 12, 698060. [Google Scholar] [CrossRef] [PubMed]
- Alarcón, S.; Tereucán, G.; Cornejo, P.; Contreras, B.; Ruiz, A. Metabolic and antioxidant effects of inoculation with arbuscular mycorrhizal fungi in crops of flesh-coloured Solanum tuberosum treated with fungicides. J. Sci. Food Agric. 2021, 102, 2270–2280. [Google Scholar] [CrossRef] [PubMed]
- Rashidi, S.; Yousefi, A.R.; Pouryousef, M.; Goicoechea, N. Effect of arbuscular mycorrhizal fungi on the accumulation of secondary metabolites in roots and reproductive organs of Solanum nigrum, Digitaria sanguinalis and Ipomoea purpurea. Chem. Biol. Technol. Agric. 2022, 9, 23. [Google Scholar] [CrossRef]
- Bahadur, A.; Batool, A.; Nasir, F.; Jiang, S.; Mingsen, Q.; Zhang, Q.; Pan, J.; Liu, Y.; Feng, H. Mechanistic insights into arbuscular mycorrhizal fungi-mediated drought stress tolerance in plants. Int. J. Mol. Sci. 2019, 20, 4199. [Google Scholar] [CrossRef]
- Das, S.; Sarkar, S. Arbuscular mycorrhizal fungal contribution towards plant resilience to drought conditions. Front. Fungal Biol. 2024, 5, 1355999. [Google Scholar] [CrossRef]
- Yilmaz, A.; Yildirim, E.; Yilmaz, H.; Soydemir, H.E.; Güler, E.; Ciftci, V.; Yaman, M. Use of arbuscular mycorrhizal fungi for boosting antioxidant enzyme metabolism and mitigating saline stress in Sweet Basil (Ocimum basilicum L.). Sustainability 2023, 15, 5982. [Google Scholar] [CrossRef]
- Jakovljević, D.Z.; Topuzović, M.D.; Stanković, M.S.; Bojović, B.M. Changes in antioxidant enzyme activity in response to salinity-induced oxidative stress during early growth of Sweet Basil. Hortic. Environ. Biote. 2017, 58, 240–246. [Google Scholar] [CrossRef]
- Kramm, V. (Ed.) Manual del Cultivo de la Papa en Chile [en Línea]; no. 375; Boletín INIA—Instituto de Investigaciones Agropecuarias: Chillán, Chile, 2017; Available online: https://hdl.handle.net/20.500.14001/6706 (accessed on 6 November 2023).
- Lichtenthaler, H.K. Chlorophylls and carotenoids: Pigments of photosynthetic biomembranes. Methods Enzymol. 1987, 148, 350–382. [Google Scholar]
- Wellburn, A.R. The spectral determination of chlorophylls a and b, as well as total carotenoids, using various solvents with spectrophotometers of different resolution. J. Plant Physiol. 1994, 144, 307–313. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nahuelcura, J.; Bravo, C.; Valdebenito, A.; Rivas, S.; Santander, C.; González, F.; Cornejo, P.; Contreras, B.; Ruiz, A. Physiological and Enzymatic Antioxidant Responses of Solanum tuberosum Leaves to Arbuscular Mycorrhizal Fungal Inoculation under Water Stress. Plants 2024, 13, 1153. https://doi.org/10.3390/plants13081153
Nahuelcura J, Bravo C, Valdebenito A, Rivas S, Santander C, González F, Cornejo P, Contreras B, Ruiz A. Physiological and Enzymatic Antioxidant Responses of Solanum tuberosum Leaves to Arbuscular Mycorrhizal Fungal Inoculation under Water Stress. Plants. 2024; 13(8):1153. https://doi.org/10.3390/plants13081153
Chicago/Turabian StyleNahuelcura, Javiera, Catalina Bravo, Analía Valdebenito, Sheina Rivas, Christian Santander, Felipe González, Pablo Cornejo, Boris Contreras, and Antonieta Ruiz. 2024. "Physiological and Enzymatic Antioxidant Responses of Solanum tuberosum Leaves to Arbuscular Mycorrhizal Fungal Inoculation under Water Stress" Plants 13, no. 8: 1153. https://doi.org/10.3390/plants13081153




