Potential Anti-Infectious Activity of Essential Oil Chemotypes of Lippia origanoides Kunth on Antibiotic-Resistant Staphylococcus aureus Strains
Abstract
:1. Introduction
2. Results
2.1. Chemical Composition of the Three Chemotypes of the L. origanoides EO
2.2. Activity of the L. origanoides EO on Staphylococcal Growth
2.3. Activity of the L. origanoides EO on the Formation of the Biofilm of S. aureus
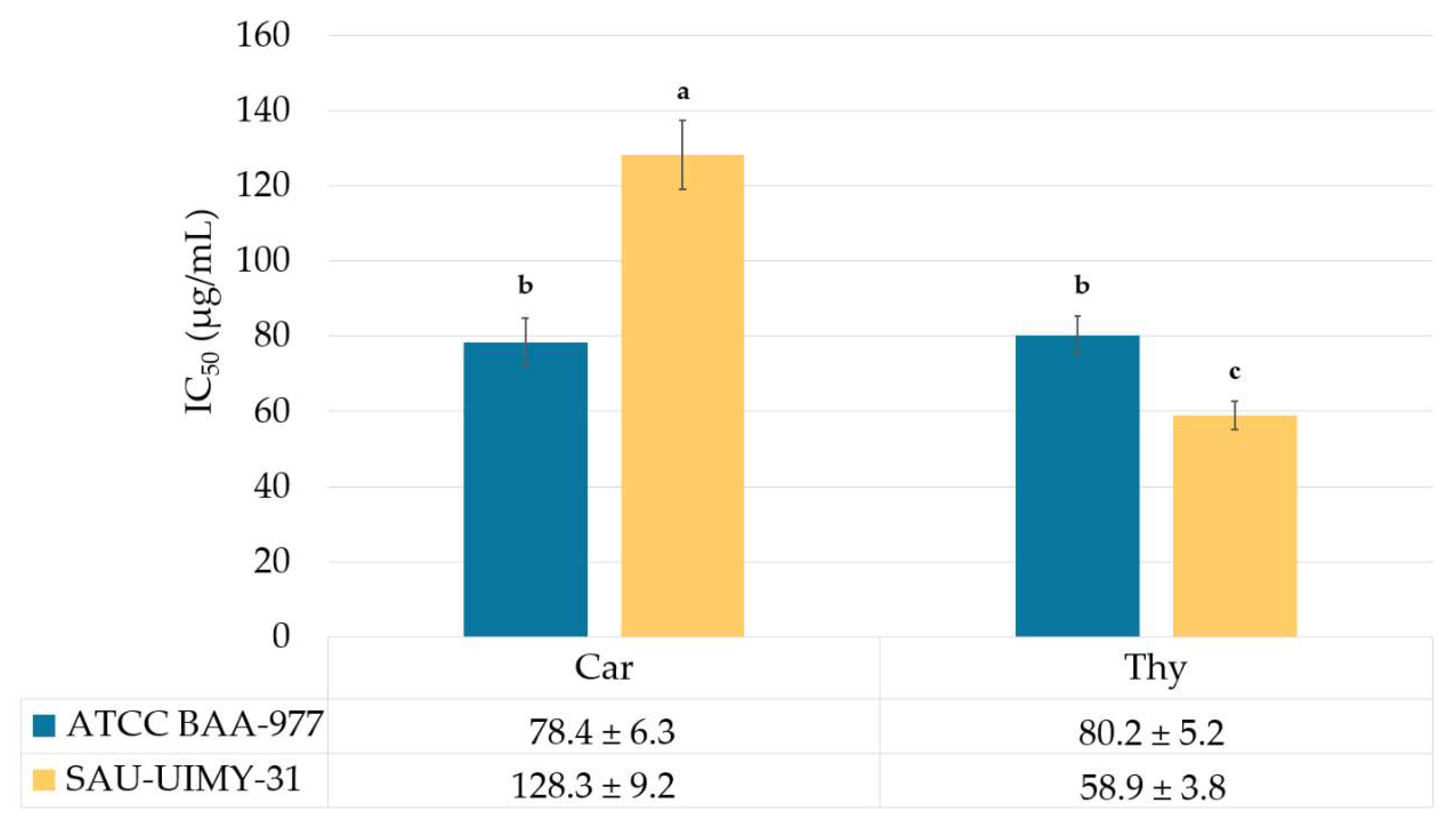
2.4. Correlation of the Chemical Composition of the L. origanoides EO and Its Antibiofilm Activity
2.5. Antihemolysis Activity of the L. origanoides EO on S. aureus
3. Discussion
3.1. Chemical Composition of the Three Chemotypes of the L. origanoides EO
3.2. Activity of the L. origanoides EO on Staphylococcal Growth
3.3. Activity of the L. origanoides EO on the Formation of the Biofilm of S. aureus
3.4. Correlation of the Chemical Composition of the L. origanoides EO and Its Antibiofilm Activity
3.5. Antihemolysis Activity of the L. origanoides EO on S. aureus
4. Materials and Methods
4.1. EO Extraction and Chemical Characterization
4.2. Anti-Infectious Assays
4.2.1. Staphylococcus aureus Strains
4.2.2. Activity of EO on Staphylococcal Growth
4.2.3. Activity of the EO on the Formation of Biofilm
4.2.4. Antihemolytic Activity of EO
4.3. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wohlleben, W.; Mast, Y.; Stegmann, E.; Ziemert, N. Antibiotic drug discovery. Microb. Biotechnol. 2016, 9, 541–548. [Google Scholar] [CrossRef]
- Lee Ventola, C. The antibiotic resistance crisis part 1: Causes and threats. Pharm. Ther. 2015, 40, 277–283. [Google Scholar]
- Prestinaci, F.; Pezzotti, P.; Pantosti, A. Antimicrobial resistance: A global multifaceted phenomenon. Pathog. Glob. Health 2015, 109, 309–318. [Google Scholar] [CrossRef]
- Chinemerem Nwobodo, D.; Ugwu, M.C.; Oliseloke Anie, C.; Al-Ouqaili, M.T.S.; Chinedu Ikem, J.; Victor Chigozie, U.; Saki, M. Antibiotic resistance: The challenges and some emerging strategies for tackling a global menace. J. Clin. Lab. Anal. 2022, 36, e24655. [Google Scholar] [CrossRef]
- Mancuso, G.; Midiri, A.; Gerace, E.; Biondo, C. Bacterial antibiotic resistance: The most critical pathogens. Pathogens 2021, 10, 1310. [Google Scholar] [CrossRef]
- Turner, N.A.; Sharma-Kuinkel, B.K.; Maskarinec, S.A.; Eichenberger, E.M.; Shah, P.P.; Carugati, M.; Holland, T.L.; Fowler, V.G. Methicillin-Resistant Staphylococcus aureus: An overview of basic and clinical research. Nat. Rev. Microbiol. 2019, 17, 203–218. [Google Scholar] [CrossRef]
- Yu, Z.; Tang, J.; Khare, T.; Kumar, V. The alarming antimicrobial resistance in ESKAPEE pathogens: Can essential oils come to the rescue? Fitoterapia 2020, 140, 104433. [Google Scholar] [CrossRef]
- Kumar, V.; Yasmeen, N.; Pandey, A.; Ahmad Chaudhary, A.; Alawam, A.S.; Ahmad Rudayni, H.; Islam, A.; Lakhawat, S.S.; Sharma, P.K.; Shahid, M. Antibiotic adjuvants: Synergistic tool to combat multi-drug resistant pathogens. Front. Cell. Infect. Microbiol. 2023, 13, 1293633. [Google Scholar] [CrossRef]
- Reichling, J. Anti-biofilm and virulence factor-reducing activities of essential oils and oil components as a possible option for bacterial infection control. Planta Med. 2020, 86, 520–537. [Google Scholar] [CrossRef]
- Álvarez-Martínez, F.J.; Barrajón-Catalán, E.; Herranz-López, M.; Micol, V. Antibacterial plant compounds, extracts and essential oils: An updated review on their effects and putative mechanisms of action. Phytomedicine 2021, 90, 153626. [Google Scholar] [CrossRef]
- Brożyna, M.; Paleczny, J.; Kozłowska, W.; Chodaczek, G.; Dudek-Wicher, R.; Felińczak, A.; Gołębiewska, J.; Górniak, A.; Junka, A. The antimicrobial and antibiofilm in vitro activity of liquid and vapour phases of selected essential oils against Staphylococcus aureus. Pathogens 2021, 10, 1207. [Google Scholar] [CrossRef]
- Romanescu, M.; Oprean, C.; Lombrea, A.; Badescu, B.; Teodor, A.; Constantin, G.D.; Andor, M.; Folescu, R.; Muntean, D.; Danciu, C.; et al. Current state of knowledge regarding who high priority pathogens—Resistance mechanisms and proposed solutions through candidates such as essential oils: A systematic review. Int. J. Mol. Sci. 2023, 24, 9727. [Google Scholar] [CrossRef]
- Kintzios, S.E. Profile of the multifaceted prince of the herbs. In Oregano the Genera Origanum and Lippia, 1st ed.; Kintzios, S.E., Ed.; Taylor & Francis Group: Oxfordshire, UK, 2002; pp. 1–6. [Google Scholar]
- O’Leary, N.; Denham, S.S.; Salimena, F.; Múlgura, M.E. Species delimitation in Lippia section Goniostachyum (verbenaceae) using the phylogenetic species concept. Bot. J. Linn. Soc. 2012, 170, 197–219. [Google Scholar] [CrossRef]
- Atlas de Las Plantas de La Medicina Tradicional Mexicana. Available online: http://www.medicinatradicionalmexicana.unam.mx/apmtm/index.html (accessed on 25 March 2024).
- Calvo-Irabien, L.M. Native mexican aromatic flora and essential oils: Current research status, gaps in knowledge and agro-industrial potential. Ind. Crops. Prod. 2018, 111, 807–822. [Google Scholar] [CrossRef]
- Siqueira-Lima, P.S.; Passos, F.R.S.; Lucchese, A.M.; Menezes, I.R.A.; Coutinho, H.D.M.; Lima, A.A.N.; Zengin, G.; Quintans, J.S.S.; Quintans-Júnior, L.J. Central nervous system and analgesic profiles of Lippia genus. Rev. Bras. Farm. 2019, 29, 125–135. [Google Scholar] [CrossRef]
- Candelaria-Dueñas, S.; Serrano-Parrales, R.; Ávila-Romero, M.; Meraz-Martínez, S.; Orozco-Martínez, J.; Ávila-Acevedo, J.G.; García-Bores, A.M.; Cespedes-Acuña, C.L.; Peñalosa-Castro, I.; Hernandez-Delgado, T. Evaluation of the antimicrobial activity of some components of the essential oils of plants used in the traditional medicine of the Tehuacán-Cuicatlán valley, Puebla, México. Antibiotics 2021, 10, 295. [Google Scholar] [CrossRef]
- Zapién-Chavarría, K.A.; Plascencia-Terrazas, A.; Venegas-Ortega, M.G.; Varillas-Torres, M.; Rivera-Chavira, B.E.; Adame-Gallegos, J.R.; González-Rangel, M.O.; Nevárez-Moorillón, G.V. Susceptibility of multidrug-resistant and biofilm-forming uropathogens to mexican oregano essential oil. Antibiotics 2019, 8, 186. [Google Scholar] [CrossRef]
- Gómez-Sequeda, N.; Cáceres, M.; Stashenko, E.E.; Hidalgo, W.; Ortiz, C. Antimicrobial and antibiofilm activities of essential oils against Escherichia coli o157: H7 and methicillin-resistant Staphylococcus aureus (MRSA). Antibiotics 2020, 9, 730. [Google Scholar] [CrossRef]
- Dhifi, W.; Bellili, S.; Jazi, S.; Bahloul, N.; Mnif, W. Essential oils’ chemical characterization and investigation of some biological activities: A critical review. Medicines 2016, 3, 25. [Google Scholar] [CrossRef]
- Feyaerts, A.F.; Mathé, L.; Luyten, W.; De Graeve, S.; Van Dyck, K.; Broekx, L.; Van Dijck, P. Essential oils and their components are a class of antifungals with potent vapour-phase-mediated anti-candida activity. Sci. Rep. 2018, 8, 3958. [Google Scholar] [CrossRef]
- El-Tarabily, K.A.; El-Saadony, M.T.; Alagawany, M.; Arif, M.; Batiha, G.E.; Khafaga, A.F.; Elwan, H.A.M.; Elnesr, S.S.; El-Hack, M.E.A. Using essential oils to overcome bacterial biofilm formation and their antimicrobial resistance. Saudi J. Biol. Sci. 2021, 28, 5145–5156. [Google Scholar] [CrossRef] [PubMed]
- Bassolé, I.H.N.; Juliani, H.R. Essential oils in combination and their antimicrobial properties. Molecules 2012, 17, 3989–4006. [Google Scholar] [CrossRef] [PubMed]
- Chouhan, S.; Sharma, K.; Guleria, S. Antimicrobial activity of some essential oils—Present status and future perspectives. Medicines 2017, 4, 58. [Google Scholar] [CrossRef] [PubMed]
- Chraibi, M.; Farah, A.; Elamin, O.; Iraqui, H.; Fikri-Benbrahim, K. Characterization, antioxidant, antimycobacterial, antimicrobial effects of Moroccan rosemary essential oil, and its synergistic antimicrobial potential with carvacrol. J. Adv. Pharm. Technol. Res. 2020, 11, 25–29. [Google Scholar] [PubMed]
- Trindade, H.; Pedro, L.G.; Figueiredo, A.C.; Barroso, J.G. Chemotypes and terpene synthase genes in Thymus genus: State of the art. Ind. Crops Prod. 2018, 124, 530–547. [Google Scholar] [CrossRef]
- Stashenko, E.; Martínez, J.; Ruiz, C.; Arias, G.; Duran, C.; Salgar, W.; Cala, M. Lippia origanoides chemotype differentiation based on essential oil GC-MS and principal components analysis. J. Sep. Sci. 2010, 33, 93–103. [Google Scholar] [CrossRef] [PubMed]
- Calvo-Irabién, L.M.; Parra-Tabla, V.; Acosta-Arriola, V.; Escalante-Erosa, F.; Díaz-Vera, L.; Dzib, G.R.; Peña-Rodríguez, L.M. Phytochemical diversity of the essential oils of mexican oregano (Lippia graveolens Kunth) populations along an edapho-climatic gradient. Chem. Biodivers. 2014, 11, 1010–1021. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, A.F.; Andrade, E.H.A.; Salimena, F.R.G.; Maia, J.G.S. Circadian and seasonal study of the cinnamate chemotype from Lippia origanoides Kunth. Biochem. Syst. Ecol. 2014, 55, 249–259. [Google Scholar] [CrossRef]
- Méndez González, M.E.; Durán García, R.; Borges Argáez, R.; Peraza Sánchez, S.; Dorantes Euan, A.; Tapia Muñoz, J.L. Flora Medicinal: De los Mayas Peninsulares, 1st ed.; Centro de Investigación Científica de Yucatán Pronatura Península de Yucatán: Mérida, Mexico, 2012; pp. 154–196. [Google Scholar]
- Fimbres-García, J.O.; Flores-Sauceda, M.; Othon-Díaz, E.D.; García-Galaz, A.; Tapia-Rodríguez, M.R.; Silva-Espinoza, B.A.; Ayala-Zavala, J.F. Facing resistant bacteria with plant essential oils: Reviewing the oregano case. Antibiotics 2022, 11, 1777. [Google Scholar] [CrossRef] [PubMed]
- Hao, Y.; Kang, J.; Yang, R.; Li, H.; Cui, H.; Bai, H.; Tsitsilin, A.; Li, J.; Shi, L. Multidimensional exploration of essential oils generated via eight oregano cultivars: Compositions, chemodiversities, and antibacterial capacities. Food Chem. 2022, 374, 131629. [Google Scholar] [CrossRef] [PubMed]
- Martínez, A.; Manrique-Moreno, M.; Klaiss-Luna, M.C.; Stashenko, E.; Zafra, G.; Ortiz, C. Effect of essential oils on growth inhibition, biofilm formation and membrane integrity of Escherichia coli and Staphylococcus aureus. Antibiotics 2021, 10, 1474. [Google Scholar] [CrossRef]
- Martínez, A.; Stashenko, E.E.; Sáez, R.T.; Zafra, G.; Ortiz, C. Effect of essential oil from Lippia origanoides on the transcriptional expression of genes related to quorum sensing, biofilm formation, and virulence of Escherichia coli and Staphylococcus aureus. Antibiotics 2023, 12, 845. [Google Scholar] [CrossRef] [PubMed]
- Ben Abdallah, F.; Lagha, R.; Gaber, A. Biofilm inhibition and eradication properties of medicinal plant essential oils against methicillin-resistant Staphylococcus aureus clinical isolates. Pharmaceuticals 2020, 13, 369. [Google Scholar] [CrossRef] [PubMed]
- Kwiecinski, J.M.; Jacobsson, G.; Horswill, A.R.; Josefsson, E.; Jin, T. Biofilm formation by Staphylococcus aureus clinical isolates correlates with the infection type. Infect. Dis. 2019, 51, 446–451. [Google Scholar] [CrossRef] [PubMed]
- Tan, L.; Huang, Y.; Shang, W.; Yang, Y.; Peng, H.; Hu, Z.; Wang, Y.; Rao, Y.; Hu, Q.; Rao, X.; et al. Accessory gene regulator (agr) allelic variants in cognate Staphylococcus aureus strain display similar phenotypes. Front. Microbiol. 2022, 13, 700894. [Google Scholar] [CrossRef] [PubMed]
- dos Santos Rodrigues, J.B.; de Carvalho, R.J.; de Souza, N.T.; de Sousa Oliveira, K.; Franco, O.L.; Schaffner, D.; de Souza, E.L.; Magnani, M. Effects of oregano essential oil and carvacrol on biofilms of Staphylococcus aureus from food-contact surfaces. Food Control. 2017, 73, 1237–1246. [Google Scholar] [CrossRef]
- Wu, X.; Wang, H.; Xiong, J.; Yang, G.-X.; Hu, J.-F.; Zhu, Q.; Chen, Z. Staphylococcus aureus biofilm: Formulation, regulatory, and emerging natural products-derived therapeutics. Biofilm 2024, 7, 100175. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Shin, M.; Kang, J.; Kang, D. Effect of sub-lethal treatment of carvacrol and thymol on virulence potential and resistance to several bactericidal treatments of Staphylococcus aureus. J. Food Saf. 2022, 42, e13004. [Google Scholar] [CrossRef]
- Knowles, J.R.; Roller, S.; Murray, D.B.; Naidu, A.S. Antimicrobial action of carvacrol at different stages of dual-species biofilm development by Staphylococcus aureus and Salmonella enterica serovar Typhimurium. Appl. Environ. Microbiol. 2005, 71, 797–803. [Google Scholar] [CrossRef] [PubMed]
- Nostro, A.; Roccaro, A.S.; Bisignano, G.; Marino, A.; Cannatelli, M.A.; Pizzimenti, F.C.; Cioni, P.L.; Procopio, F.; Blanco, A.R. Effects of oregano, carvacrol and thymol on Staphylococcus aureus and Staphylococcus epidermidis biofilms. J. Med. Microbiol. 2007, 56, 519–523. [Google Scholar] [CrossRef]
- Miladi, H.; Zmantar, T.; Chaabouni, Y.; Fedhila, K.; Bakhrouf, A.; Mahdouani, K.; Chaieb, K. Antibacterial and efflux pump inhibitors of thymol and carvacrol against food-borne pathogens. Microb. Pathog. 2016, 99, 95–100. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, A.; Van Vuuren, S.; Viljoen, A. Unravelling the complex antimicrobial interactions of essential oils—The case of Thymus vulgaris (Thyme). Molecules 2014, 19, 2896–2910. [Google Scholar] [CrossRef] [PubMed]
- Cáceres, M.; Hidalgo, W.; Stashenko, E.; Torres, R.; Ortiz, C. Essential oils of aromatic plants with antibacterial, anti-biofilm and anti-quorum sensing activities against pathogenic bacteria. Antibiotics 2020, 9, 147. [Google Scholar] [CrossRef] [PubMed]
- Oliveira Ribeiro, S.; Fontaine, V.; Mathieu, V.; Abdesselam, Z.; Dominique, B.; Caroline, S.; Florence, S. Antibacterial activities of homemade matrices miming essential oils compared to commercial ones. Antibiotics 2021, 10, 584. [Google Scholar] [CrossRef] [PubMed]
- Dahham, S.S.; Tabana, Y.M.; Iqbal, M.A.; Ahamed, M.B.K.; Ezzat, M.O.; Majid, A.S.A.; Majid, A.M.S.A. The anticancer, antioxidant and antimicrobial properties of the sesquiterpene β-caryophyllene from the essential oil of Aquilaria crassna. Molecules 2015, 20, 11808–11829. [Google Scholar] [CrossRef] [PubMed]
- Thi Huong, L.; Chung, N.; Duc, D.; Dai, D.; Pham The, H.; Ninh The, S. Essential oil of Syzygium boisianum (Gagnep.) Merr. & L.M.Perry: Chemical compositions, antimicrobial activity, and molecular docking. Vietnam J. Chem. 2023, 61, 333–338. [Google Scholar]
- Huong, L.T.; Sam, L.N.; Dai, D.N.; Pham, T.V.; Son, N.T. Essential oils of two ginger plants Newmania orthostachys N.S. Lý & Škorničk. and N. Serpens N.S. Lý & Škorničk.: Chemical compositions and antimicrobial activity. J. Essent. Oil Bear. Plants 2022, 25, 1221–1228. [Google Scholar]
- Martínez-Natarén, D.A.; Parra-Tabla, V.; Ferrer-Ortega, M.M.; Calvo-Irabién, L.M. Genetic diversity and genetic structure in wild populations of mexican oregano (Lippia graveolens H.B.K.) and its relationship with the chemical composition of the essential oil. Plant Syst. Evol. 2014, 300, 535–547. [Google Scholar] [CrossRef]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry, 4th ed.; Allured: Carol Stream, IL, USA, 2007; pp. 10–51. [Google Scholar]
- Babushok, V.I.; Linstrom, P.J.; Zenkevich, I.G. Retention indices for frequently reported compounds of plant essential oils. J. Phys. Chem. Ref. Data 2011, 40, 043101. [Google Scholar] [CrossRef]
- Zellner, B. d’Acampora; Bicchi, C.; Dugo, P.; Rubiolo, P.; Dugo, G.; Mondello, L. Linear retention indices in gas chromatographic analysis: A review. Flavour Fragr. J. 2008, 23, 297–314. [Google Scholar] [CrossRef]
- Uc-Cachón, A.H.; de Dzul-Beh, A.J.; Palma-Pech, G.A.; Jiménez-Delgadillo, B.; Flores-Guido, J.S.; Gracida-Osorno, C.; Molina-Salinas, G.M. Antibacterial and antibiofilm activities of mayan medicinal plants against methicillin-susceptible and -resistant strains of Staphylococcus aureus. J. Ethnopharmacol. 2021, 279, 114369. [Google Scholar] [CrossRef] [PubMed]
- de Jesús Dzul-Beh, A.; Uc-Cachón, A.H.; González-Sánchez, A.A.; Dzib-Baak, H.E.; Ortiz-Andrade, R.; Barrios-García, H.B.; Jiménez-Delgadillo, B.; Molina-Salinas, G.M. Antimicrobial potential of the mayan medicine plant Matayba oppositifolia (A. Rich.) britton against antibiotic-resistant priority pathogens. J. Ethnopharmacol. 2023, 300, 115738. [Google Scholar] [CrossRef] [PubMed]
- Molina-Salinas, G.M.; Ramos-Guerra, M.C.; Vargas-Villarreal, J.; Mata-Cárdenas, B.D.; Becerril-Montes, P.; Said-Fernández, S. Bactericidal activity of organic extracts from Flourensia cernua DC against strains of Mycobacterium tuberculosis. Arch. Med. Res. 2006, 37, 45–49. [Google Scholar] [CrossRef] [PubMed]
- Loges, L.A.; Silva, D.B.; Paulino, G.V.B.; Landell, M.F.; Macedo, A.J. Polyketides from marine-derived Aspergillus welwitschiae inhibit Staphylococcus aureus virulence factors and potentiate vancomycin antibacterial activity in vivo. Microb. Pathog. 2020, 143, 104066. [Google Scholar] [CrossRef] [PubMed]

| Compound Name | Class | AI a | AI ADAMS b | Chemotype | Compound Identification d | ||
|---|---|---|---|---|---|---|---|
| Car | Thy | Ses | |||||
| % area c | % area c | % area c | |||||
| myrcene | M | 992 | 988 | 1.15 | 2.15 | 0.34 | RI, MS, Coi |
| p-cymene | M | 1029 | 1020 | 18.12 | 15.26 | 2.85 | RI, MS, Coi |
| limonene | M | 1033 | 1024 | 0.25 | 0.41 | 1.06 | RI, MS, Coi |
| eucalyptol | OM | 1036 | 1026 | 0.74 | 2.63 | 2.77 | RI, MS, Coi |
| γ-terpinene | M | 1061 | 1054 | 1.30 | 0.24 | 0.39 | RI, MS, Coi |
| thymol methyl ether | OM | 1238 | 1232 | 1.31 | 3.33 | 0.07 | RI, MS |
| thymol | OM | 1295 | 1289 | 2.74 | 57.30 | 0.13 | RI, MS, Coi |
| carvacrol | OM | 1309 | 1298 | 68.51 | 0.20 | 0.19 | RI, MS, Coi |
| α-copaene | S | 1388 | 1374 | nd | nd | 3.29 | RI, MS, Coi |
| β-caryophyllene | S | 1438 | 1417 | 1.46 | 7.75 | 33.00 | RI, MS, Coi |
| β-copaene | S | 1445 | 1430 | 0.04 | 0.08 | 1.00 | RI, MS |
| α-humulene | S | 1471 | 1452 | 0.80 | 3.84 | 13.42 | RI, MS, Coi |
| aromadendrene-allo | S | 1479 | 1458 | nd | nd | 1.73 | RI, MS |
| γ-muurolene | S | 1491 | 1478 | nd | nd | 4.23 | RI, MS |
| β-selinene | S | 1502 | 1489 | nd | 0.13 | 5.05 | RI, MS |
| α-selinene | S | 1510 | 1498 | nd | 0.21 | 7.91 | RI, MS |
| γ-cadinene | S | 1529 | 1513 | nd | nd | 1.36 | RI, MS |
| δ-cadinene | S | 1539 | 1522 | nd | 0.08 | 7.21 | RI, MS |
| caryophyllene oxide | OS | 1600 | 1582 | 0.79 | 1.33 | 1.32 | RI, MS, Coi |
| Total of compound class (%) | |||||||
| M | 20.82 | 18.06 | 4.64 | ||||
| OM | 73.3 | 63.46 | 3.16 | ||||
| S | 2.3 | 12.09 | 78.20 | ||||
| OS | 0.79 | 1.33 | 1.32 | ||||
| Total identified (%) | 97.21 | 94.94 | 87.32 | ||||
| S. aaureus Strain | Drug-Resistant Profile | L. origanoides EO | Positive Control | |||||
|---|---|---|---|---|---|---|---|---|
| Car | Thy | Ses | ||||||
| MIC | MBC | MIC | MBC | MIC | MBC | MIC | ||
| ATCC 43300 | MRSA | 500 | 500 | 500 | 500 | >500 | >500 | RIF = 0.04 |
| ATCC 25923 | MSSA | 500 | 500 | 500 | 500 | 500 | >500 | RIF = 0.08 |
| ATCC BAA-977 | MSSA | 500 | 500 | 500 | 500 | >500 | >500 | RIF = 0.08 |
| SAU-UIMY-44 | MDR, MRSA, VRSA | 500 | >500 | 500 | >500 | >500 | >500 | TET = 0.5 |
| SAU-UIMY-31 | MSSA | 500 | 500 | 250 | 500 | 250 | 250 | RIF = 0.08 |
| SAU-UIMY-24 | MDR, MRSA, VSSA | 250 | 500 | 250 | 500 | 500 | 500 | TET = 0.5 |
| SAU-UIMY-5 | MDR, MRSA | 250 | 250 | 250 | 250 | 250 | 250 | RIF = 0.04 |
| SAU-UIMY- 1 | XDR, MRSA, VISA | 125 | 250 | 125 | 250 | >500 | >500 | AMK = 16 |
| SAU-UIMY-26 | MDR, MSSA, VISA | 125 | 125 | 125 | 125 | >500 | >500 | TET = 0.5 |
| SAU-UIMY-10 | MDR, MRSA, VSSA | 125 | 125 | 125 | 125 | 250 | 500 | TET = 0.5 |
| SAU-UIMY-39 | MDR, MSSA, VSSA | 125 | 125 | 125 | 250 | 250 | 250 | TET = 0.5 |
| SAU-UIMY-16 | MDR, MRSA, VSSA | 125 | 125 | 62.5 | 125 | 125 | 250 | TET = 0.5 |
 | ||||||||
| S. aureus Strain | Origin | IC50 (µg/mL) | Biofilm Biomass (OD Values) | |||
|---|---|---|---|---|---|---|
| Lippia origanoides Chemotypes | Positive Control | |||||
| Car | Thy | Sesq | EDTA | |||
| ATCC 43300 | - | 90.0 ± 1.9 a,b | 149.3 ± 1.9 a,b | 156.9 ± 2.1 a,b | 79.5 ± 2.9 | 0.728 ± 0.08 |
| SAU-UIMY-26 | Surgical wound | 379.0 ± 7.0 e | 568.3 ± 20.9 f | 706.6 ± 173.5 g | 406.9 ± 1.2 | 0.198 ± 0.01 |
| SAU-UIMY-44 | Blood | 228.3 ± 7.5 b,c,d | 291.7 ± 14.9 b,c,d,e | 684.86 ± 90.4 f,g | 458.1 ± 3.3 | 0.324 ± 0.03 |
| SAU-UIMY-16 | Bronchial secretion | 213.8 ± 5.4 b,c,d | 192.8 ± 7.7 b,c,d | 659.3 ± 62.8 f,g | 123.9 ± 5.0 | 0.581 ± 0.06 |
| SAU-UIMY-24 | Surgical wound | 196.9 ± 6.8 b,c,d | 198.6 ± 3.3 b,c,d | 307.8 ± 12.8 d,e | 55.8 ± 2.6 | 0.407 ± 0.07 |
| SAU-UIMY-10 | Urine | 169.6 ± 4.5 b,c | 162.4 ± 7.4 b,c | 350.0 ± 2.0 d,e | 257.8 ± 4.6 | 0.420 ± 0.07 |
| SAU-UIMY-31 | Breast abscess | 99.3 ± 3.9 a,b | 82.4 ± 5.1 a,b | 79.1 ± 6.1 a,b | 199 ± 1.8 | 0.902 ± 0.2 |
| SAU-UIMY-1 | Bronchial secretion | 77.3 ± 4.8 a,b | 60.6 ± 1.7 a,b | 30.8 ± 1.5 a | 84.8 ± 1.8 | 1.29 ± 0.27 |
| SAU-UIMY-39 | Blood | 35.5 ± 1.8 a,b | 63.3 ± 4.1 a,b | 103.2 ± 5.2 a,b | 104.8 ± 3.1 | 0.482 ± 0.08 |
 | ||||||
| S. aureus Strain | p-cymene | eucalyptol | thymol | carvacrol | β-caryophyllene | α-humulene | α+β-selinene | γ+δ-cadinene |
|---|---|---|---|---|---|---|---|---|
| SAU-UIMY-1 | 0.95 | −0.95 | 0.47 | 0.95 | −0.95 | −0.95 | −0.95 | −0.95 |
| SAU-UIMY-31 | 0.74 | −0.74 | 0.21 | 0.74 | −0.74 | −0.74 | −0.74 | −0.74 |
| SAU-UIMY-16 | −0.53 | 0.53 | −0.90 | −0.53 | 0.53 | 0.53 | 0.53 | 0.53 |
| SAU-UIMY-10 | −0.63 | 0.63 | −0.79 | −0.63 | 0.63 | 0.63 | 0.63 | 0.63 |
| SAU-UIMY-24 | −0.74 | 0.74 | −0.69 | −0.74 | 0.74 | 0.74 | 0.74 | 0.74 |
| SAU-UIMY-26 | −0.84 | 0.84 | −0.26 | −0.84 | 0.84 | 0.84 | 0.84 | 0.84 |
| SAU-UIMY-39 | −0.95 | 0.95 | −0.47 | −0.95 | 0.95 | 0.95 | 0.95 | 0.95 |
| SAU-UIMY-44 | −0.95 | 0.95 | −0.47 | −0.95 | 0.95 | 0.95 | 0.95 | 0.95 |
| ATCC 43300 | −0.84 | 0.84 | −0.26 | −0.84 | 0.84 | 0.84 | 0.84 | 0.84 |
| S. aureus Strain | Drug-Resistant Profile | Origin of Clinical Isolate | Susceptible To | Resistant To |
|---|---|---|---|---|
| ATCC 43300 | MDR, MRSA | — | DAP, LVX, LZD, MXF, RIF, SNC, SXT, TET, VAN | AMC, AMP, CLI, CRO, ERY, GEN, OXA, PEN, MET, SAM |
| ATCC 25923 | MSSA | — | AMC, AMP, CLI, CRO, DAP, ERY, GEN, LVX, LZD, MET, MXF, PEN, OXA, RIF, SAM, SNC, SXT, TET, VAN | — |
| ATCC BAA-977 | MSSA | — | CHL, CIP, CLI, CPT, DAP, GEN, LZD, OXA, RIF, SXT, TGC, VAN | ERY, PEN |
| SAU-UIMY-39 | MDR, MSSA, VSSA | blood | AMC, CRO, DAP, GEN, LVX, LZD, MET, MXF, OXA, RIF, SAM, SNC, SXT, TET, VAN | AMP, CLI, ERY, PEN |
| SAU-UIMY-44 | MDR, MRSA, VRSA | blood | SXT, TET | AMC, AMP, CIP, CLI, CRO, ERY, LVX, MET, MFX, OXA, PEN, RIF, SAM, SNC, VAN |
| SAU-UIMY-31 | MSSA | breast abscess | AMC, CLI, CRO, DAP, ERY, GEN, LVX, LZD, MET, MXF, OXA, RIF, SAM, SNC, SXT, TET, VAN | AMP, PEN |
| SAU-UIMY-16 | MDR, MRSA, VSSA | bronchial secretion | DAP, GEN, LZD, SNC, SXT, TET, VAN | AMC, AMP, CLI, CRO, ERY, LVX, MET, MXF, OXA, PEN, RIF, SAM, SNC |
| SAU-UIMY-1 | XDR, MRSA, VISA | bronchial secretion | DAP, LZD | AMC, AMP, CIP, CLI, CRO, ERY, LVX, MET, MXF, OXA, PEN, RIF, SAM, SXT, TET |
| SAU-UIMY-5 | MDR, MRSA | surgical wound | DAP, GEN, LZD, RIF, SNC, SXT, TET, VAN | AMC, AMP, CIP, CLI, CRO, ERY, LVX, MET, MXF, OXA, PEN, SAM, |
| SAU-UIMY-24 | MDR, MRSA, VSSA | surgical wound | CIP, DAP, ERY, GEN, LVX, LZD, MXF, RIF, SNC, SXT, TET, VAN | AMC, AMP, CLI, CRO, MET, OXA, PEN, SAM, |
| SAU-UIMY-26 | MDR, MSSA, VISA | surgical wound | AMC, CLI, CRO, DAP, ERY, LVX, LZD, MET, MXF, OXA, RIF, SAM, SXT, TET | AMP, GEN, PEN, SNC, |
| SAU-UIMY-10 | MDR, MRSA, VSSA | urine | DAP, SXT, TET, VAN | AMC, AMP, CIP, CLI, CRO, ERY, GEN, LVX, LZD, MET, MXF, OXA, PEN, RIF, SAM, SNC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Uc-Cachón, A.H.; Calvo-Irabien, L.M.; Dzul-Beh, A.d.J.; Dzib-Baak, H.E.; Grijalva-Arango, R.; Molina-Salinas, G.M. Potential Anti-Infectious Activity of Essential Oil Chemotypes of Lippia origanoides Kunth on Antibiotic-Resistant Staphylococcus aureus Strains. Plants 2024, 13, 1172. https://doi.org/10.3390/plants13091172
Uc-Cachón AH, Calvo-Irabien LM, Dzul-Beh AdJ, Dzib-Baak HE, Grijalva-Arango R, Molina-Salinas GM. Potential Anti-Infectious Activity of Essential Oil Chemotypes of Lippia origanoides Kunth on Antibiotic-Resistant Staphylococcus aureus Strains. Plants. 2024; 13(9):1172. https://doi.org/10.3390/plants13091172
Chicago/Turabian StyleUc-Cachón, Andrés Humberto, Luz María Calvo-Irabien, Angel de Jesús Dzul-Beh, Haziel Eleazar Dzib-Baak, Rosa Grijalva-Arango, and Gloria María Molina-Salinas. 2024. "Potential Anti-Infectious Activity of Essential Oil Chemotypes of Lippia origanoides Kunth on Antibiotic-Resistant Staphylococcus aureus Strains" Plants 13, no. 9: 1172. https://doi.org/10.3390/plants13091172







