Heterotrophy Compared to Photoautotrophy for Growth Characteristics and Pigment Compositions in Batch Cultures of Four Green Microalgae
Abstract
:1. Introduction
2. Results
2.1. Comparison of Growth Characteristics in Light-Limited Photoautotrophy and in Heterotrophy
2.1.1. Dependence of Growth on Light Intensity in Light-Limited Photoautotrophy
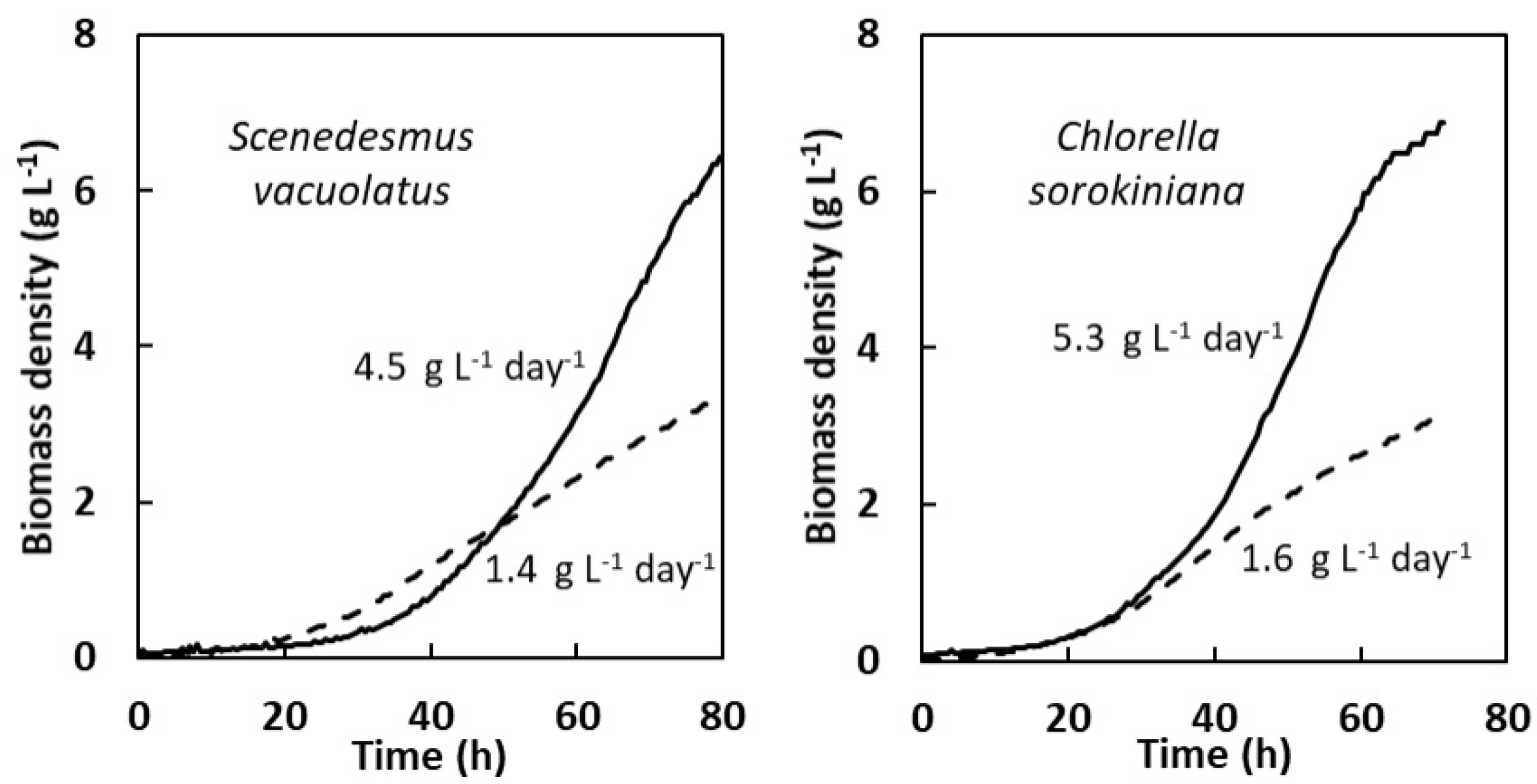
2.1.2. Heterotrophic Growth: Effect of Organic Carbon Source (Acetate, Glucose) and of Weak Light
2.2. Comparison of Pigment Content of the Biomass in Light-Limited Photoautotrophy and in Heterotrophy
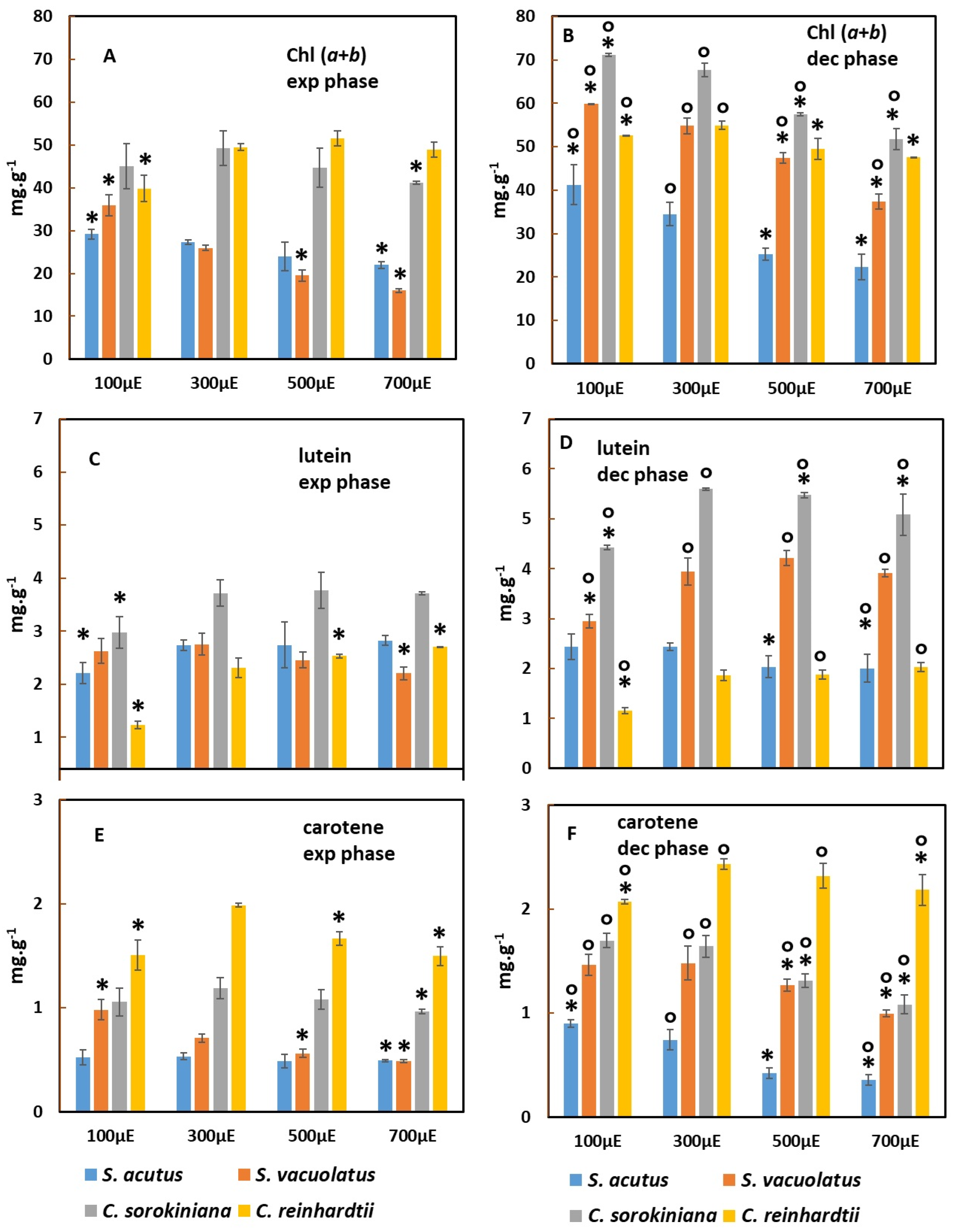
2.2.1. Effect of Light-Intensity on Pigment Content during Exponential and Deceleration Phases of Light-Limited Photoautotrophic Growth
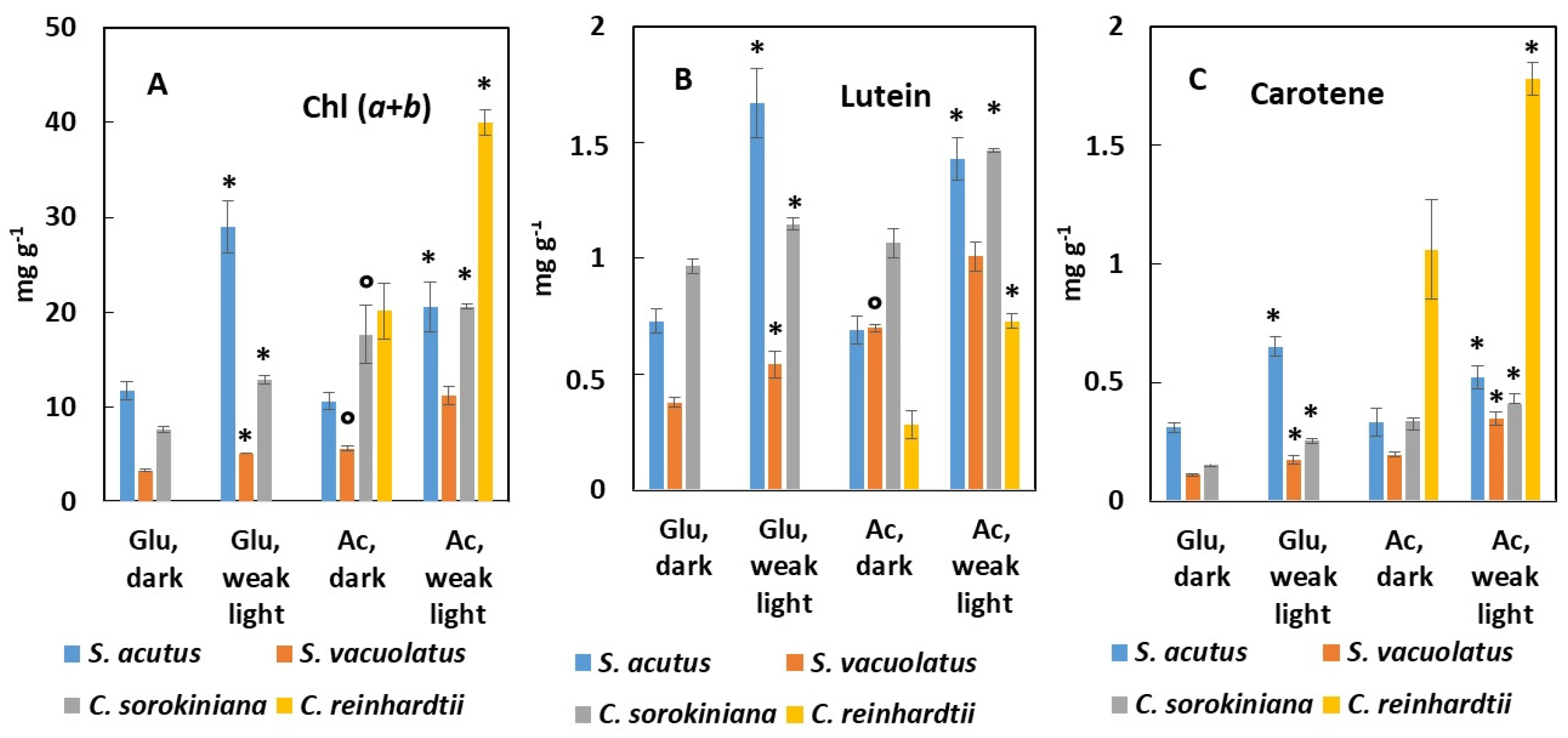
2.2.2. Pigment Content of Heterotrophically Grown Cells: Effect of Carbon Source (Glucose or Acetate) and of Weak Light
3. Discussion
3.1. Comparing Photoautotrophic and Heterotrophic Growth Performances on the Basis of Batch Experiments
3.2. Photoacclimation Processes Revealed by Changes in Pigment Content during Light-Limited Photoautotrophic Batch Growth
3.3. Species- and Substrate-Dependent Pigmentation during Heterotrophy in Darkness or under Weak Light
4. Materials and Methods
4.1. Microalgal Strains and Growth Media
4.2. Cultivation Conditions
4.2.1. Pre-Cultivation
4.2.2. Cultivation Conditions
4.3. Sampling and Analysis
4.3.1. Biomass Estimation
4.3.2. Specific Growth Rate and Productivity Determination
4.3.3. Pigment Analysis
4.3.4. Quantification of Acetate by HPLC
4.3.5. Statistics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- González, L.E.; Díaz, G.C.; Aranda, D.A.G.; Cruz, Y.R.; Fortes, M.M. Biodiesel production based in microalgae: A biorefinery approach. Nat. Sci. 2015, 07, 358–369. [Google Scholar] [CrossRef]
- Chew, K.W.; Yap, J.Y.; Show, P.L.; Suan, N.H.; Juan, J.C.; Ling, T.C.; Lee, D.J.; Chang, J.S. Microalgae biorefinery: High value products perspectives. Bioresour. Technol. 2017, 229, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Margalith, P.Z. Production of ketocarotenoids by microalgae. Appl. Microbiol. Biotechnol. 1999, 51, 431–438. [Google Scholar] [CrossRef] [PubMed]
- Del Campo, J.A.; García-González, M.; Guerrero, M.G. Outdoor cultivation of microalgae for carotenoid production: Current state and perspectives. Appl. Microbiol. Biotechnol. 2007, 74, 1163–1174. [Google Scholar] [CrossRef] [PubMed]
- Mulders, K.J.M.; Lamers, P.P.; Martens, D.E.; Wijffels, R.H. Phototrophic pigment production with microalgae: Biological constraints and opportunities. J. Phycol. 2014, 50, 229–242. [Google Scholar] [CrossRef] [PubMed]
- Aflalo, C.; Meshulam, Y.; Zarka, A.; Boussiba, S. On the relative efficiency of two- vs. one-stage production of astaxanthin by the green alga Haematococcus pluvialis. Biotechnol. Bioeng. 2007, 98, 300–305. [Google Scholar] [CrossRef] [PubMed]
- Bumbak, F.; Cook, S.; Zachleder, V.; Hauser, S.; Kovar, K. Best practices in heterotrophic high-cell-density microalgal processes: Achievements, potential and possible limitations. Appl. Microbiol. Biotechnol. 2011, 91, 31–46. [Google Scholar] [CrossRef] [PubMed]
- Perez-Garcia, O.; Escalante, F.M.; De-Bashan, L.E.; Bashan, Y. Heterotrophic cultures of microalgae: Metabolism and potential products. Water Res. 2011, 45, 11–36. [Google Scholar] [CrossRef]
- Carone, M.; Corato, A.; Dauvrin, T.; Le Thanh, T.; Durante, L.; Joris, B.; Franck, F.; Remacle, C. Heterotrophic growth of microalgae. In Grand Challenges in Algae Biotechnology; Hallmann, A., Rampelotto, P.H., Eds.; Springer Nature: Cham, Switzerland, 2019; pp. 71–107. [Google Scholar]
- Sutherland, D.L.; Ralph, P.J. Differing growth responses in four related microalgal genera grown under autotrophic, mixotrophic and heterotrophic conditions. J. Appl. Phycol. 2021, 33, 3539–3553. [Google Scholar] [CrossRef]
- Cheniae, G.M.; Martin, I.F. Absence of oxygen evolving capacity in dark grown Chlorella: The photoactivation of oxygen evolving centers. Photochem. Photobiol. 1973, 17, 441–459. [Google Scholar] [CrossRef]
- Im, C.S.; Eberhard, S.; Huang, K.; Beck, C.F.; Grossman, A.R. Phototropin involvement in the expression of genes encoding chlorophyll and carotenoid biosynthesis enzymes and LHC apoproteins in Chlamydomonas reinhardtii. Plant J. 2006, 48, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Killam, A.; Myers, J. A special effect of light on the growth of Chlorella vulgaris. Am. J. Bot. 1956, 43, 569–572. [Google Scholar] [CrossRef]
- Shi, X.M.; Jiang, Y.; Chen, F. High-yield production of lutein by the green microalga Chlorella protothecoides in heterotrophic fed-batch culture. Biotechnol. Prog. 2002, 18, 723–727. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Nagarajan, D.; Zhang, Q.; Chang, J.S.; Lee, D.J. Heterotrophic cultivation of microalgae for pigment production: A review. Biotechnol. Adv. 2018, 36, 54–67. [Google Scholar] [CrossRef] [PubMed]
- Richardson, K.; Beardall, J.; Raven, J.A. Adaptation of unicellular algae to irradiance: An analysis of strategies. New Phytol. 1983, 93, 157–191. [Google Scholar] [CrossRef]
- Falkowski, P.G.; LaRoche, J. Acclimation to spectral irradiance in algae. J. Phycol. 1991, 27, 8–14. [Google Scholar] [CrossRef]
- Doucha, J.; Lívanský, K. Production of high-density Chlorella culture grown in fermenters. J. Appl. Phycol. 2012, 24, 35–43. [Google Scholar] [CrossRef]
- Myers, J. Physiology of the algae. Annu. Rev. Microbiol. 1951, 5, 157–180. [Google Scholar] [CrossRef] [PubMed]
- De Marchin, T.; Erpicum, M.; Franck, F. Photosynthesis of Scenedesmus obliquus in outdoor open thin-layer cascade system in high and low CO2 in Belgium. J. Biotechnol. 2015, 215, 2–12. [Google Scholar] [CrossRef] [PubMed]
- Masojídek, J.; Ranglová, K.; Lakatos, G.E.; Benavides, A.M.S.; Torzillo, G. Variables governing photosynthesis and growth in microalgae mass cultures. Processes 2021, 9, 820. [Google Scholar] [CrossRef]
- Cui, B.; Liu, C.; Rong, H.; Luo, S.; Guo, D.; Ji, B. CO2 favors the lipid and biodiesel production of microalgal-bacterial granular sludge. Results Eng. 2023, 17, 100980. [Google Scholar] [CrossRef]
- Ogbona, J.C.; Yada, H.; Tanaka, H. Kinetic study on light-limited batch cultivation of photosynthetic cells. J. Ferment. Bioeng. 1995, 80, 259–264. [Google Scholar] [CrossRef]
- Gérin, S.; Delhez, T.; Corato, A.; Remacle, C.; Franck, F. A novel culture medium for freshwater diatoms promotes efficient photoautotrophic batch production of biomass, fucoxanthin, and eicosapentaenoic acid. J. Appl. Phycol. 2020, 32, 1581–1596. [Google Scholar] [CrossRef]
- Harris, E.H. The Chlamydomonas Sourcebook: A Comprehensive Guide to Biology and Laboratory Use; Academic Press, Inc.: San Diego, CA, USA, 1989. [Google Scholar]
- Torzillo, G.; Vonshak, A. Environmental stress physiology with reference to mass cultures. In Handbook of Microalgal Culture: Applied Phycolology and Biotechnology; Richmond, A., Hu, Q., Eds.; John Wiley & Sons: Oxford, UK, 2013; pp. 90–113. [Google Scholar]
- Falkowski, P.G. Kinetics of adaptation to irradiance in Dunaliella tertiolecta. Photosynthetica 1984, 18, 62–68. [Google Scholar]
- Ma, R.; Zhang, Z.; Tang, Z.; Ho, S.; Shi, X.; Liu, L.; Xie, Y.; Chen, J. Enhancement of co-production of lutein and protein in Chlorella sorokiniana FZU60 using different bioprocess operation strategies. Bioresour. Bioprocess 2021, 8, 82. [Google Scholar] [CrossRef] [PubMed]
- Myers, J.; Phillips, J.N.; Graham, J.R. On the mass culture of algae. Plant Physiol. 1951, 26, 539–548. [Google Scholar] [CrossRef]
- Becker, E.W. Microalgae Biotechnology and Microbiology; Cambridge University Press: Cambridge, UK, 1994. [Google Scholar]
- Huesemann, M.H.; Van Wagenen, J.; Miller, T.; Chavis, A.; Hobbs, S.; Crowe, B. A screening model to predict microalgae biomass growth in photobioreactors and raceway ponds. Biotechnol. Bioeng. 2013, 110, 1583–1594. [Google Scholar] [CrossRef] [PubMed]
- Melis, A. Solar energy conversion efficiencies in photosynthesis: Minimizing the chlorophyll antennae to maximize efficiency. Plant Sci. 2009, 177, 272–280. [Google Scholar] [CrossRef]
- Formighieri, C.; Franck, F.; Bassi, R. Regulation of the pigment optical density of an algal cell: Filling the gap between photosynthetic productivity in the laboratory and in mass culture. J. Biotechnol. 2012, 162, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Tamiya, H.; Hase, E.; Shibata, K.; Mituya, A.; Iwamura, T.; Nihei, T.; Sasa, T. Kinetics of growth of Chlorella, with special reference to its dependence on quantity of available light and temperature. In Algal Culture from Laboratory to Pilot Plant; Burlew, J.S., Ed.; Carnegie Insitution of Washigton Publication 600: Washington, DC, USA, 1953; pp. 204–232. [Google Scholar]
- Qiang, H.; Zarmi, Y.; Richmond, A. Combined effects of light intensity, light-path and culture density on output rate of Spirulina platensis (Cyanobacteria). Eur. J. Phycol. 1998, 33, 165–171. [Google Scholar] [CrossRef]
- Chen, F.; Johns, M.R. Heterotrophic growth of Chlamydomonas reinhardtii on acetate in chemostat culture. Process Biochemistry 1996, 31, 601–604. [Google Scholar] [CrossRef]
- Wu, Z.; Shi, X. Optimization for high-density cultivation of heterotrophic Chlorella based on a hybrid neural network model. Lett. Appl. Microbiol. 2007, 44, 13–18. [Google Scholar] [CrossRef] [PubMed]
- Je, S.; Yamaoka, Y. Biotechnological approaches for biomass and lipid production using microalgae Chlorella and its future perspectives. J. Microbiol. Biotechnol. 2022, 32, 1357–1372. [Google Scholar] [CrossRef] [PubMed]
- Endo, H.; Nakajima, K.; Chino, R.; Shirota, M. Growth characteristics and cellular components of Chlorella regularis, heterotrophically fast growing strain. Agric. Biol. Chem. 1974, 38, 9–18. [Google Scholar] [CrossRef]
- Samejima, H.; Myers, J. On the heterotrophic growth of Chlorella pyrenoidosa. J. Gen. Microbiol. 1958, 18, 107–117. [Google Scholar] [CrossRef] [PubMed]
- Patil, S.; Prakash, G.; Lali, A. Reduced chlorophyll antenna mutants of Chlorella saccharophila for higher photosynthetic efficiency and biomass productivity under high light intensities. J. Appl. Phycol. 2020, 32, 1559–1567. [Google Scholar] [CrossRef]
- Dall’Osto, L.; Lico, C.; Alric, J.; Giuliano, G.; Havaux, M.; Bassi, R. Lutein is needed for efficient chlorophyll triplet quenching in the major LHCII antenna complex of higher plants and effective photoprotection in vivo under strong light. BMC Plant Biol. 2006, 6, 32. [Google Scholar] [CrossRef] [PubMed]
- Neale, P.J.; Melis, A. Algal photosynthetic membrane complexes and the photosynthesis-irradiance curve: A comparison of light-adaptation responses in Chlamydomonas reinhardtii (Chlorophyta). J. Phycol. 1986, 22, 531–538. [Google Scholar] [CrossRef]
- Ochiai, S.; Hase, E. Studies on chlorophyll formation in Chlorella protothecoides I. Enhancing effects of light and added δ-aminolevulinic acid, and suppressive effect of glucose on chlorophyll formation. Plant Cell Physiol. 1970, 11, 663–673. [Google Scholar]
- Bohne, F.; Linden, H. Regulation of carotenoid biosynthesis genes in response to light in Chlamydomonas reinhardtii. Biochim. Biophys. Acta 2002, 1579, 26–34. [Google Scholar] [CrossRef] [PubMed]
- Schoefs, B.; Franck, F. Protochlorophyllide reduction: Mechanisms and evolution. Photochem. Photobiol. 2003, 78, 543–557. [Google Scholar] [CrossRef] [PubMed]
- Yamasaki, S.; Nomata, J.; Fujita, Y. Differential operation of dual protochlorophyllide reductases for chlorophyll biosynthesis in response to environmental oxygen levels in the cyanobacterium Leptolyngbya boryana. Plant Physiol. 2006, 142, 911–922. [Google Scholar] [CrossRef] [PubMed]
- Karlander, E.P.; Krauss, R.W. Responses of heterotrophic cultures of Chlorella vulgaris Beyerinck to darkness and light. I. Pigment and pH changes. Plant Physiol. 1966, 41, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Van Baalen, C.; Hoare, D.S.; Brandt, E. Heterotrophic growth of blue-green algae in dim light. J. Bacteriol. 1971, 105, 685–689. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Xu, H.; Wu, Q. Large-scale biodiesel production from microalga Chlorella protothecoides through heterotrophic cultivation in bioreactors. Biotechnol. Bioeng. 2007, 98, 764–771. [Google Scholar] [CrossRef] [PubMed]
- Kessler, E.; Schäfer, M.; Hümmer, C.; Kloboucek, A.; Huss, V.A.R. Physiological, biochemical, and molecular characters for the taxonomy of the subgenera of Scenedesmus (Chlorococcales, Chlorophyta). Bot. Acta. 1997, 110, 244–250. [Google Scholar] [CrossRef]
- Wynne, M.J.; Hallan, J.K. Reinstatement of Tetradesmus G. M. Smith (Sphaeropleales, Chlorophyta). Feddes Repert. 2015, 126, 83–86. [Google Scholar] [CrossRef]
- Newman, S.M.; Boynton, J.E.; Gillham, N.W.; Randolph-Anderson, B.L.; Johnson, A.M.; Harris, E.H. Transformation of chloroplast ribosomal RNA genes in Chlamydomonas: Molecular and genetic characterization of integration events. Genetics 1990, 126, 875–888. [Google Scholar] [CrossRef] [PubMed]
- Nichols, W.; Bold, H.C. Trichosarcina polymorpha Gen et Sp. Nov. J. Phycol. 1965, 1, 34–38. [Google Scholar] [CrossRef]
- Gorman, D.S.; Levine, R.P. Cytochrome f and plastocyanin: Their sequence in the photosynthetic electron chain of Chlamydomonas reinhardtii. Proc. Natl. Acad. Sci. USA 1965, 54, 1665–1669. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Hseu, R.; Lin, L. Identification of Chlorella spp. isolates using ribosomal DNA sequences. Bot. Bull. Acad. Sin. 2001, 42, 115–121. [Google Scholar]
- Shi, K.; Wu, Z.; Chen, F. Kinetic modeling of lutein production by heterotrophic Chlorella at various pH and temperatures. Mol. Nutr. Food Res. 2006, 50, 763–768. [Google Scholar] [CrossRef] [PubMed]
- Vonshak, A.; Novoplansky, N.; Silva Benavides, A.M.; Torzillo, G.; Beardall, J.; Palacios, Y.M. Photosynthetic characterization of two Nannochloropsis species and its relevance to outdoor cultivation. J. Appl. Phycol. 2020, 32, 909–922. [Google Scholar] [CrossRef]
- Cardol, P.; Gloire, G.; Havaux, M.; Remacle, C.; Franck, F. Photosynthesis and state transitions in mitochondrial mutants of Chlamydomonas reinhardtii affected in respiration. Plant Physiol. 2003, 133, 2010–2020. [Google Scholar] [CrossRef] [PubMed]
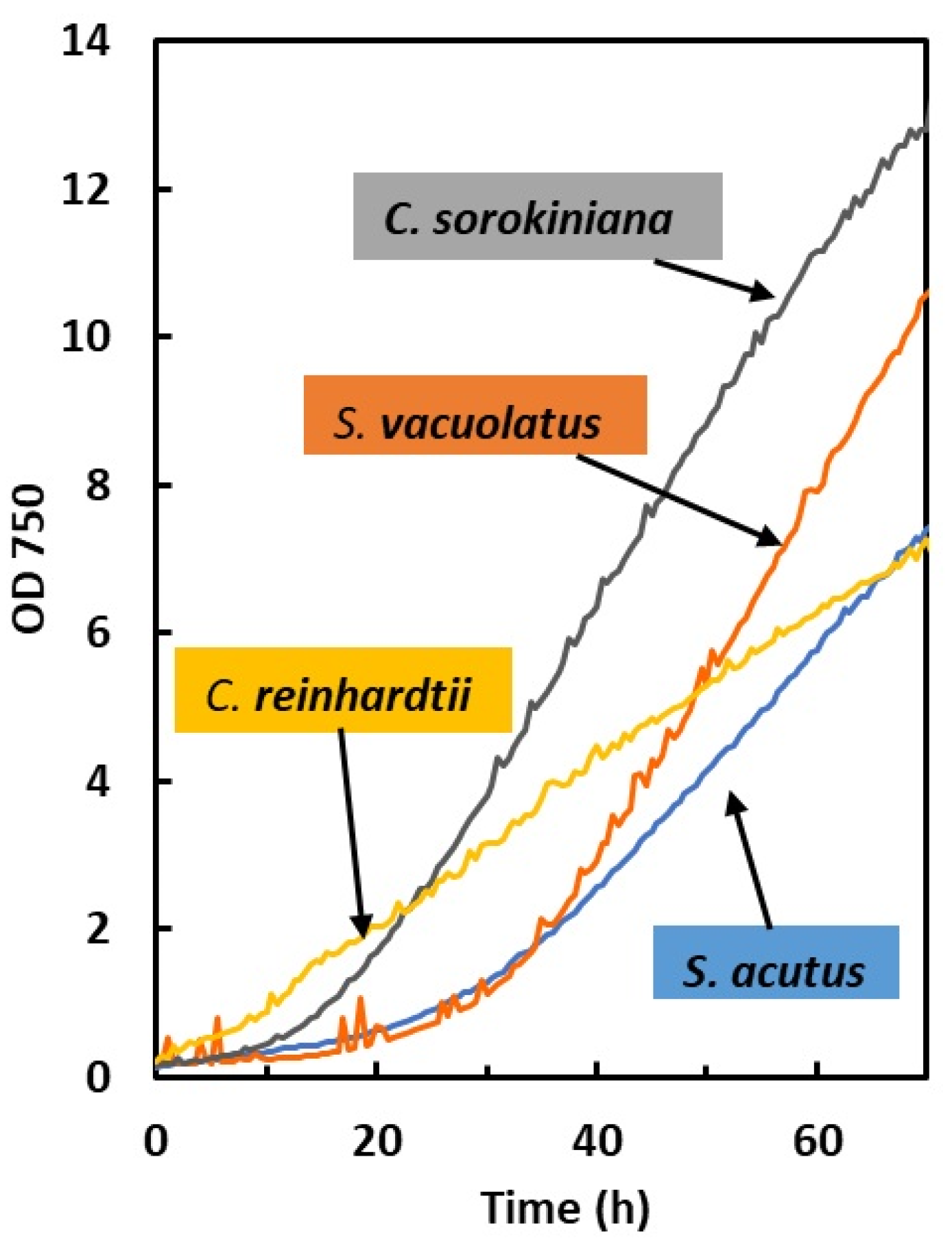
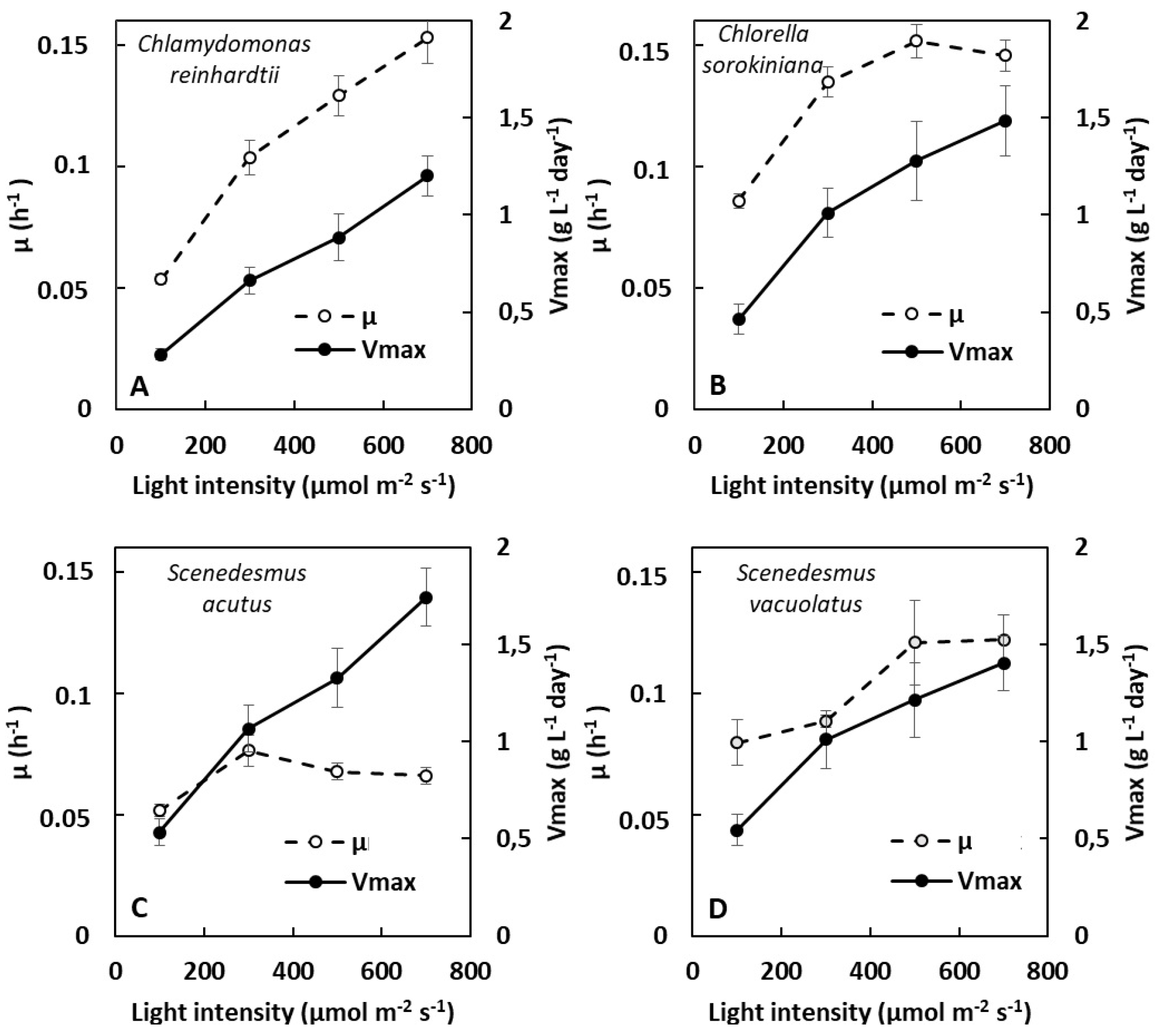
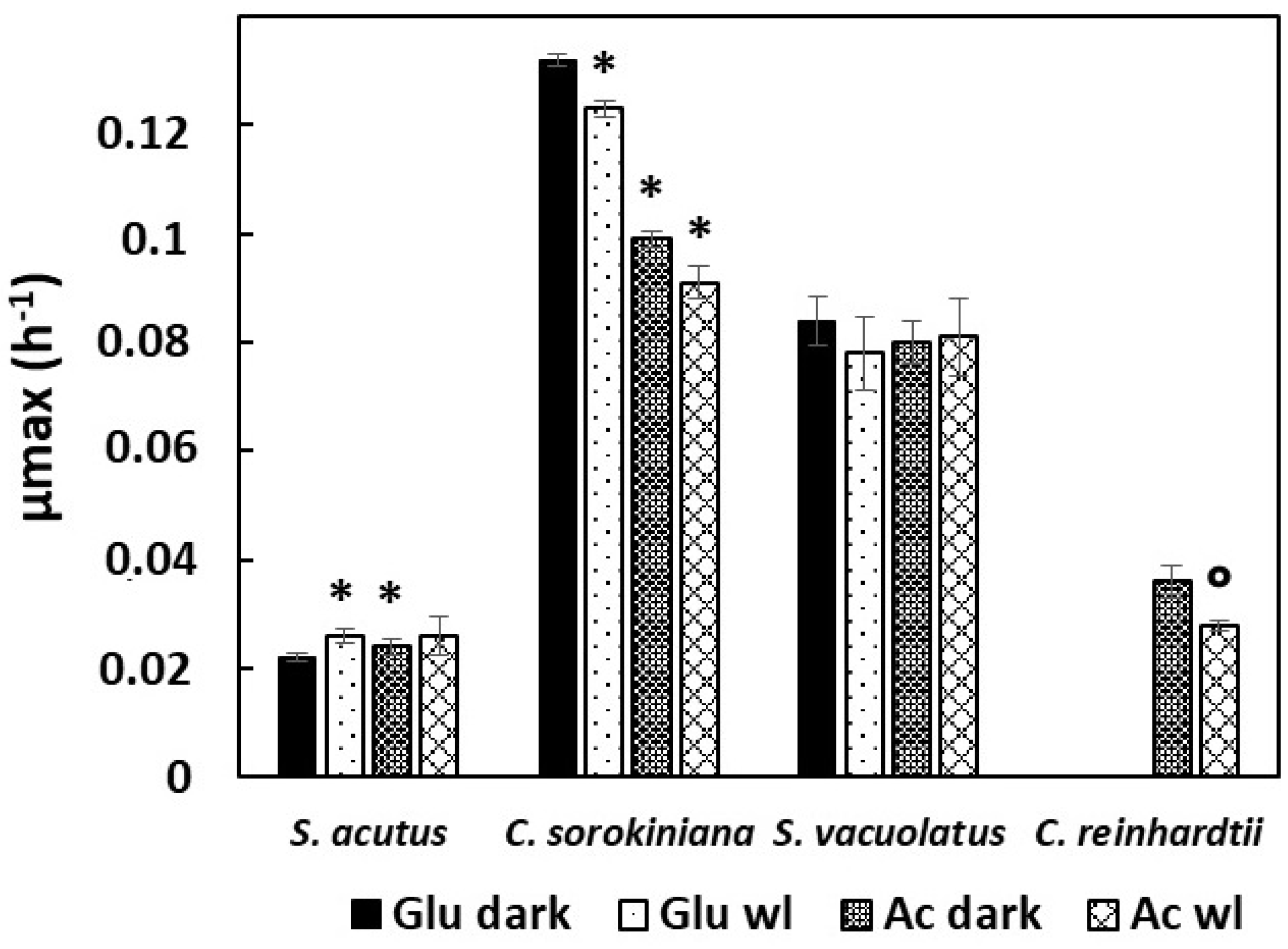
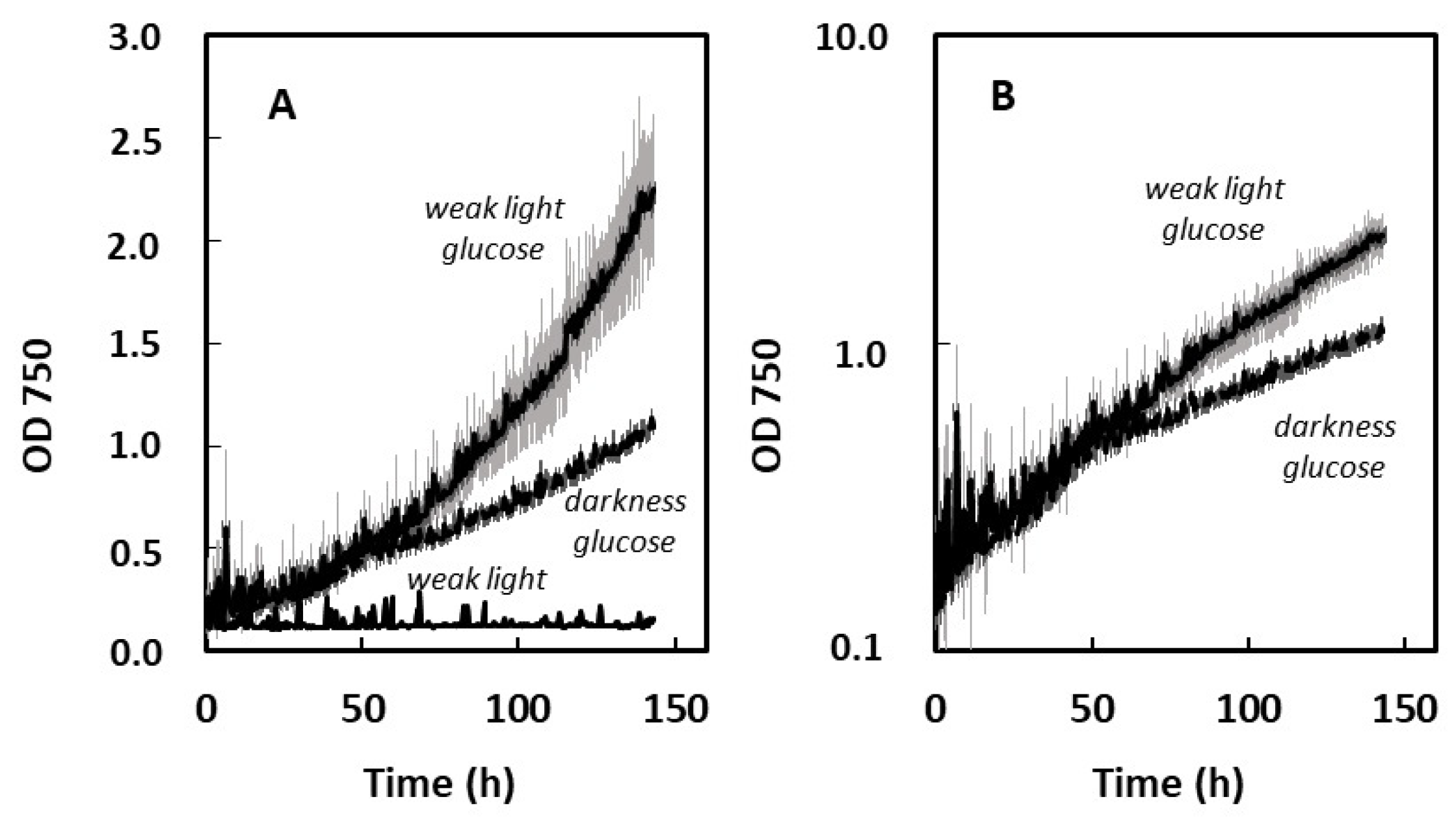

| Species | YX/glucose | YX/acetate |
|---|---|---|
| Chlorella sorokiniana | 0.55 ± 0.02 | 0.31 ± 0.03 |
| Scenedesmus vacuolatus | 0.51 ± 0.01 | 0.32 ± 0.01 |
| Chlamydomonas reinhardtii | - | 0.32 ± 0.01 |
| Species | Substrate | Carotene | Lutein | Chl (a + b) | |||
|---|---|---|---|---|---|---|---|
| dark | w.l. | dark | w.l. | dark | w.l. | ||
| C. sorokiniana | Acetate | 23 | 28 | 21 | 29 | 28 | 33 |
| Glucose | 10 | 17 | 19 | 23 | 12 | 21 | |
| S. vacuolatus | Acetate | 14 | 26 | 18 | 26 | 11 | 22 |
| Glucose | 08 | 12 | 10 | 14 | 07 | 10 | |
| S. acutus | Acetate | 56 | 90 | 31 | 65 | 35 | 68 |
| Glucose | 53 | 112 | 33 | 76 | 39 | 97 | |
| C. reinhardtii | Acetate | 47 | 78 | 16 | 41 | 40 | 80 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Le, T.T.; Corato, A.; Gerards, T.; Gérin, S.; Remacle, C.; Franck, F. Heterotrophy Compared to Photoautotrophy for Growth Characteristics and Pigment Compositions in Batch Cultures of Four Green Microalgae. Plants 2024, 13, 1182. https://doi.org/10.3390/plants13091182
Le TT, Corato A, Gerards T, Gérin S, Remacle C, Franck F. Heterotrophy Compared to Photoautotrophy for Growth Characteristics and Pigment Compositions in Batch Cultures of Four Green Microalgae. Plants. 2024; 13(9):1182. https://doi.org/10.3390/plants13091182
Chicago/Turabian StyleLe, Thanh Tung, Amélie Corato, Thomas Gerards, Stéphanie Gérin, Claire Remacle, and Fabrice Franck. 2024. "Heterotrophy Compared to Photoautotrophy for Growth Characteristics and Pigment Compositions in Batch Cultures of Four Green Microalgae" Plants 13, no. 9: 1182. https://doi.org/10.3390/plants13091182





