Zinc Oxide Nanoparticles and Zinc Sulfate Alleviate Boron Toxicity in Cotton (Gossypium hirsutum L.)
Abstract
:1. Introduction
2. Material and Methods
2.1. Nanoparticle Preparation
2.2. Plant Experiment Setup
2.3. Measurement of Seedlings’ Growth and Biomass
2.4. Photosynthetic Parameters
2.5. Quantification of Chlorophyll Content
2.6. Estimation of Boron Content and Elemental Analysis
2.7. Determination of Antioxidant Enzymes
2.8. Determination of MDA, H2O2, and O2− Contents
2.9. Transcriptome Sequencing
2.10. Statistical Analysis
3. Results
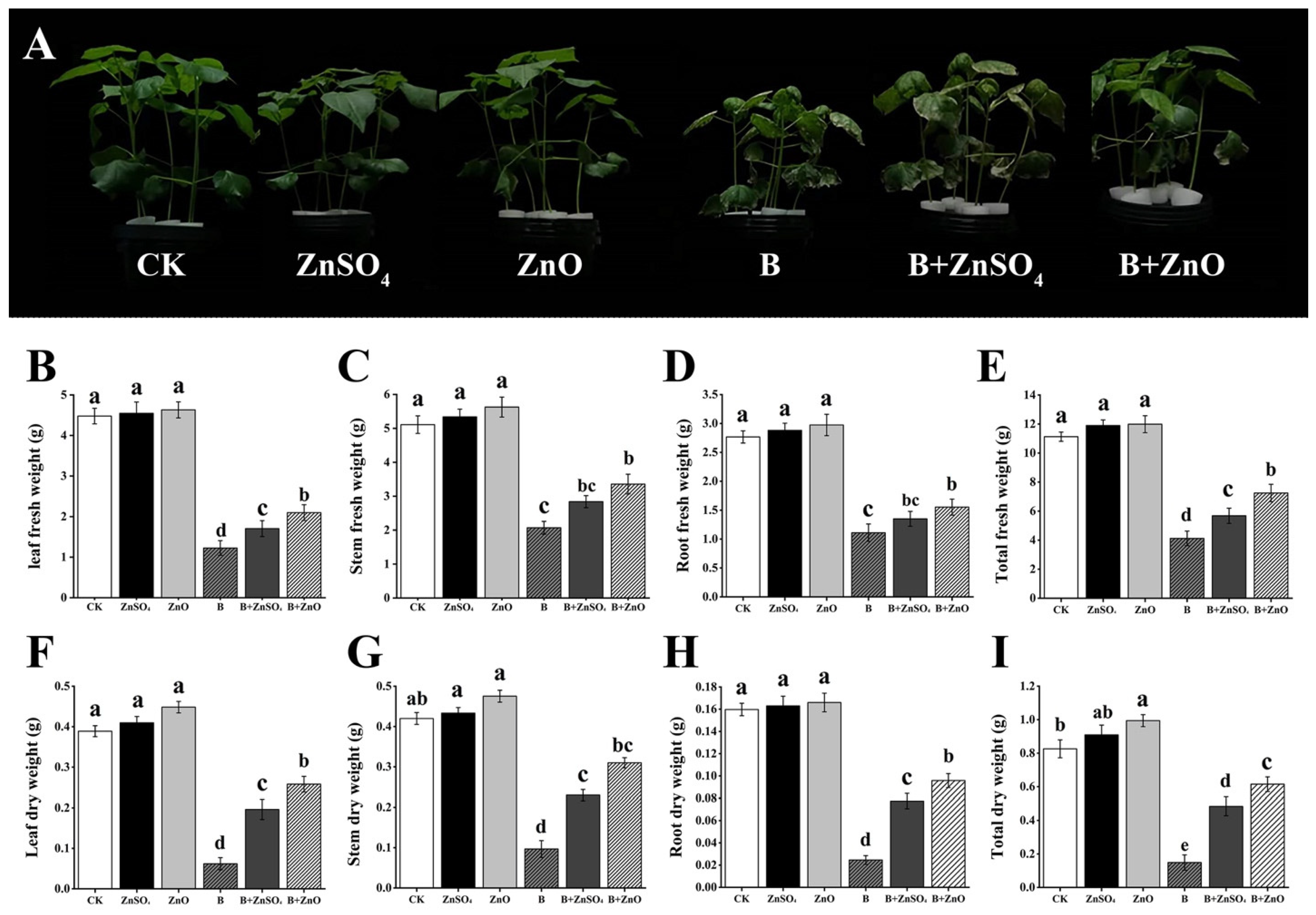
3.1. Effect of ZnO NPs and ZnSO4 on the Fresh Weight and Dry Weight of Cotton Seedlings under Boron Toxicity
3.2. RNA-Seq and Differentially Expressed Genes (DEGs) Analysis
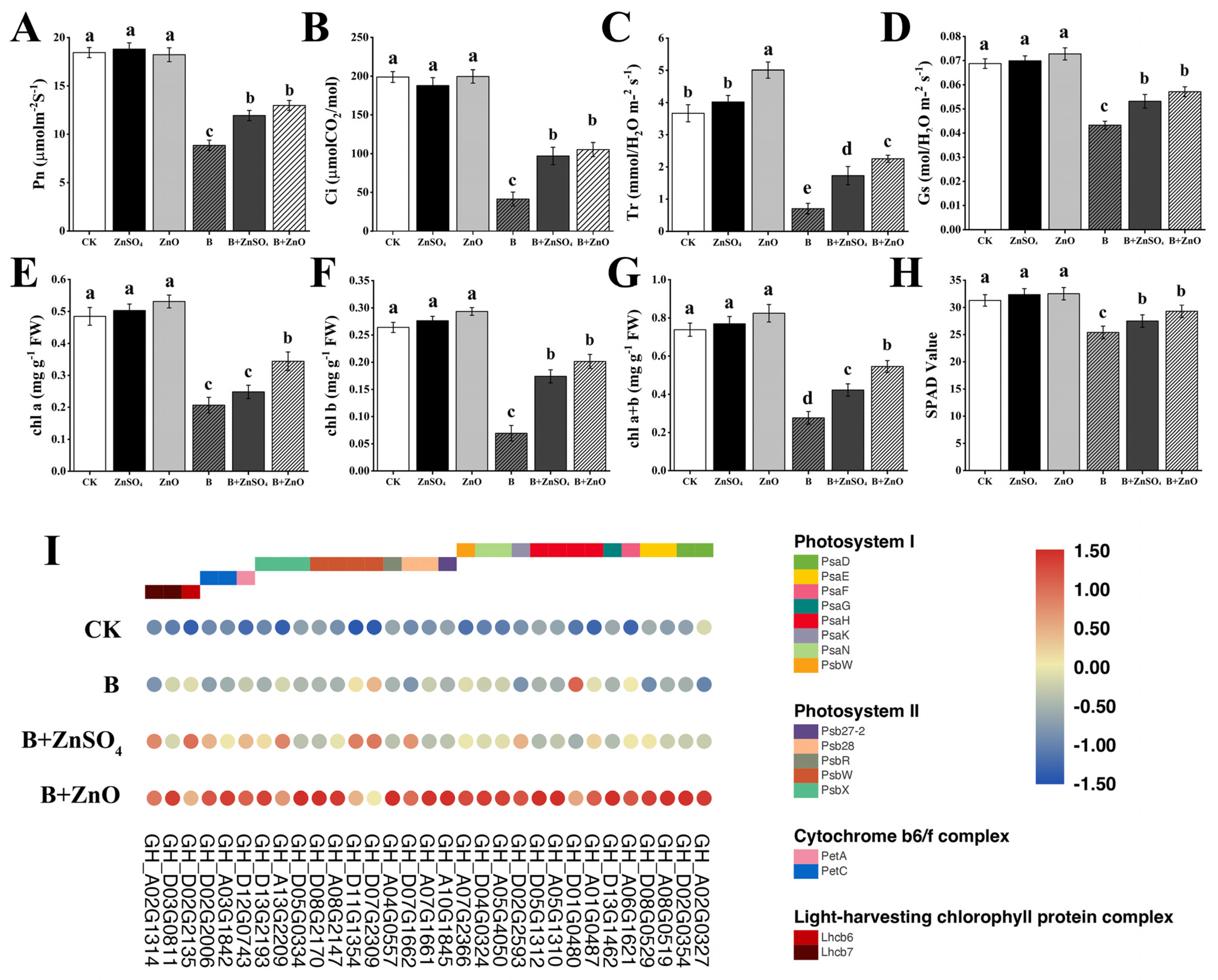
3.3. ZnO NPs and ZnSO4 Application Enhances Photosynthetic Parameters and Chlorophyll Contents of Cotton Seedlings under Boron Toxicity
3.4. ZnO NPs and ZnSO4 Application Regulates Mineral Nutrients and Reduced Boron Contents in a Boron-Stressed Cotton Seedlings
3.5. ZnO NPs and ZnSO4 Application Enhances the Antioxidant Activity and Reduces Oxidative Stress in the Root of the Cotton Seedlings under Boron Toxicity
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- González-Fontes, A. Why boron is an essential element for vascular plants: A comment on Lewis (2019) “Boron: The essential element for vascular plants that never was. New Phytol. 2020, 226, 1228–1230. [Google Scholar] [CrossRef] [PubMed]
- Warington, K. The effect of boric acid and borax on the broad bean and certain other plants. Ann. Bot. 1923, 37, 629–672. [Google Scholar] [CrossRef]
- Pandey, A.; Khan, M.K.; Hakki, E.E.; Gezgin, S.; Hamurcu, M. Combined boron toxicity and salinity stress—An insight into its interaction in plants. Plants 2019, 8, 364. [Google Scholar] [CrossRef] [PubMed]
- Gupta, U.C.; Jame, Y.W.; Campbell, C.A.; Leyshon, A.J.; Nicholaichuk, W. Boron toxicity and deficiency: A review. Can. J. Soil Sci. 1985, 65, 381–409. [Google Scholar] [CrossRef]
- Landi, M.; Margaritopoulou, T.; Papadakis, I.E.; Araniti, F. Boron toxicity in higher plants: An update. Planta 2019, 250, 1011–1032. [Google Scholar] [CrossRef] [PubMed]
- Shireen, F.; Nawaz, M.A.; Chen, C.; Zhang, Q.; Zheng, Z.; Sohail, H.; Sun, J.; Cao, H.; Huang, Y.; Bie, Z. Boron: Functions and Approaches to Enhance Its Availability in Plants for Sustainable Agriculture. Int. J. Mol. Sci. 2018, 19, 1856. [Google Scholar] [CrossRef] [PubMed]
- Wimmer, M.A.; Abreu, I.; Bell, R.W.; Bienert, M.D.; Brown, P.H.; Dell, B.; Fujiwara, T.; Goldbach, H.E.; Lehto, T.; Mock, H.P.; et al. Boron: An essential element for vascular plants: A comment on Lewis (2019) “Boron: The essential element for vascular plants that never was”. New Phytol. 2020, 226, 1232–1237. [Google Scholar] [CrossRef] [PubMed]
- El-Shazoly, R.M.; Metwally, A.A.; Hamada, A.M. Salicylic acid or thiamin increases tolerance to boron toxicity stress in wheat. J. Plant Nutr. 2019, 42, 702–722. [Google Scholar] [CrossRef]
- Liu, C.; Lu, W.; Ma, Q.; Ma, C. Effect of silicon on the alleviation of boron toxicity in wheat growth, boron accumulation, photosynthesis activities, and oxidative responses. J. Plant Nutr. 2017, 40, 2458–2467. [Google Scholar] [CrossRef]
- Naz, T.; Akhtar, J.; Iqbal, M.M.; Anwar-ul-Haq, M.; Murtaza, G.; Niazi, N.K.; Farooq, O.; Ali, M.; Dell, B. Assessment of gas exchange attributes, chlorophyll contents, ionic composition and antioxidant enzymes of bread wheat genotypes in boron toxic, saline and boron. Int. J. Agric. Biol. 2019, 21, 1271–1278. [Google Scholar]
- Zulfiqar, F.; Ashraf, M. Nanoparticles potentially mediate salt stress tolerance in plants. Plant Physiol. Biochem. 2021, 160, 257–268. [Google Scholar] [CrossRef] [PubMed]
- Tolay, I. The impact of different Zinc (Zn) levels on growth and nutrient uptake of Basil (Ocimum basilicum L.) grown under salinity stress. PLoS ONE 2021, 16, e0246493. [Google Scholar] [CrossRef] [PubMed]
- Zahedi, S.M.; Abdelrahman, M.; Hosseini, M.S.; Hoveizeh, N.F.; Tran, L.S.P. Alleviation of the effect of salinity on growth and yield of strawberry by foliar spray of selenium-nanoparticles. Environ. Pollut. 2020, 263, 114369. [Google Scholar] [CrossRef] [PubMed]
- Prasad, R.; Bhattacharyya, A.; Nguyen, Q.D. Nanotechnology in Sustainable Agriculture: Recent Developments, Challenges, and Perspectives. Front. Microbiol. 2017, 8, 1014. [Google Scholar] [CrossRef]
- Khan, M.N.; Mobin, M.; Abbas, Z.K.; AlMutairi, K.A.; Siddiqui, Z.H. Role of nanomaterials in plants under challenging environments. Plant Physiol. Biochem. 2017, 110, 194–209. [Google Scholar] [CrossRef] [PubMed]
- Alabdallah, N.M.; Alzahrani, H.S. The potential mitigation effect of ZnONPs on [Abelmoschus esculentus, L. Moench] metabolism under salt stress conditions. Saudi J. Biol. Sci. 2020, 27, 3132–3137. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Sengar, R.S.; Shahi, U.P.; Rajput, V.D.; Minkina, T.; Ghazaryan, K.A. Prominent Effects of Zinc Oxide Nanoparticles on Roots of Rice (Oryza sativa L.) Grown under Salinity Stress. Stresses 2022, 3, 33–46. [Google Scholar] [CrossRef]
- Zulfiqar, F.; Navarro, M.; Ashraf, M.; Akram, N.A.; Munné-Bosch, S. Nanofertilizer use for sustainable agriculture: Advantages and limitations. Plant Sci. 2019, 289, 110270. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.K.; Niu, Y.P.; Ma, H.; Hafeez, A.; Luo, H.H.; Zhang, W.F. Photosynthesis and biomass allocation of cotton as affected by deep-layer water and fertilizer application depth. Photosynthetica 2017, 55, 638–647. [Google Scholar] [CrossRef]
- Kolodziejczak-Radzimska, A.; Jesionowski, T. Zinc oxide—From synthesis to application: A review. Materials 2014, 7, 2833–2881. [Google Scholar] [CrossRef] [PubMed]
- Ong, C.B.; Ng, L.Y.; Mohammad, A.W. A review of ZnO nanoparticles as solar photocatalysts: Synthesis, mechanisms and applications. Renew. Sustain. Energy Rev. 2018, 81, 536–551. [Google Scholar] [CrossRef]
- Mahendra, C.; Murali, M.; Manasa, G.; Ponnamma, P.; Abhilash, M.R.; Lakshmeesha, T.R.; Satish, S. Antibacterial and antimitotic potential of bio-fabricated zinc oxide nanoparticles of Cochlospermum religiosum (L.). Microb. Pathog. 2017, 110, 620–629. [Google Scholar] [CrossRef]
- Altieri, M.; Nicholls, C.I. Sustainable Agriculture Reviews; Springer: Dordrecht, The Netherlands, 2012; Volume 11, pp. 1–29. [Google Scholar]
- O’Carrigan, A.; Babla, M.; Wang, F.; Liu, X.; Mak, M.; Thomas, R.; Bellotti, B.; Chen, Z.H. Analysis of gas exchange, stomatal behaviour and micronutrients uncovers dynamic response and adaptation of tomato plants to monochromatic light treatments. Plant Physiol. Biochem. 2014, 82, 105–115. [Google Scholar] [CrossRef] [PubMed]
- Ling, Q.; Huang, W.; Jarvis, P. Use of a SPAD-502 meter to measure leaf chlorophyll concentration in Arabidopsis thaliana. Photosynth. Res. 2011, 107, 209–214. [Google Scholar] [CrossRef] [PubMed]
- Hussain, J.; Khan, A.L.; Rehman, N.; Hamayun, M.; Shinwari, Z.K.; Ullah, W.; Lee, I.J. Assessment of herbal products and their composite medicinal plants through proximate and micronutrients analyses. J. Med. Plants Res. 2009, 3, 1072–1077. [Google Scholar]
- Zhang, W.F.; Zhang, F.; Raziuddin, R.; Gong, H.J.; Yang, Z.M.; Lu, L.; Ye, Q.F.; Zhou, W.J. Effects of 5-aminolevulinic acid on oilseed rape seedling growth under herbicide toxicity stress. J. Plant Growth Regul. 2008, 27, 159–169. [Google Scholar] [CrossRef]
- Kadhum, M.A.; Hadwan, M.H. A precise and simple method for measuring catalase activity in biological samples. Chem. Pap. 2021, 75, 1669–1678. [Google Scholar] [CrossRef]
- Shannon, L.M.; Kay, E.; Lew, J.Y. Peroxidase Isozymes from Horseradish Roots. J. Biol. Chem. 1966, 241, 2166–2172. [Google Scholar] [CrossRef] [PubMed]
- Diao, M.; Ma, L.; Wang, J.; Cui, J.; Fu, A.; Liu, H.Y. Selenium Promotes the Growth and Photosynthesis of Tomato Seedlings Under Salt Stress by Enhancing Chloroplast Antioxidant Defense System. J. Plant Growth Regul. 2014, 33, 671–682. [Google Scholar] [CrossRef]
- Jiang, M.; Liu, Y.; Li, R.; Li, S.; Tan, Y.; Huang, J.; Shu, Q. An inositol 1,3,4,5,6-pentakisphosphate 2-kinase 1 mutant with a 33-nt deletion showed enhanced tolerance to salt and drought stress in rice. Plants 2021, 10, 23. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Langmead, B.; Salzberg, S.L. HISAT: A fast spliced aligner with low memory requirements. Nat. Methods 2015, 12, 357–360. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Chen, J.; Fang, L.; Zhang, Z.; Ma, W.; Niu, Y.; Ju, L.; Deng, J.; Zhao, T.; Lian, J.; et al. Gossypium barbadense and Gossypium hirsutum genomes provide insights into the origin and evolution of allotetraploid cotton. Nat. Genet. 2019, 51, 739–748. [Google Scholar] [CrossRef] [PubMed]
- Trapnell, C.; Williams, B.A.; Pertea, G.; Mortazavi, A.; Kwan, G.; Van Baren, M.J.; Salzberg, S.L.; Wold, B.J.; Pachter, L. Transcript assembly and quantification by RNA-Seq reveals unannotated transcripts and isoform switching during cell differentiation. Nat. Biotechnol. 2010, 28, 511–515. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; He, Q.; Zhang, F.; Yu, J.; Li, C.; Zhao, T.; Zhang, Y.; Xie, Q.; Su, B.; Mei, L.; et al. Melatonin enhances cotton immunity to Verticillium wilt via manipulating lignin and gossypol biosynthesis. Plant J. 2019, 100, 784–800. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, D.K.; Singh, V.P.; Prasad, S.M.; Chauhan, D.K.; Dubey, N.K. Silicon nanoparticles (SiNp) alleviate chromium (VI) phytotoxicity in Pisum sativum (L.) seedlings. Plant Physiol. Biochem. 2015, 96, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Dubchak, S.; Ogar, A.; Mietelski, J.W.; Turnau, K. Influence of silver and titanium nanoparticles on arbuscular mycorrhiza colonization and accumulation of radiocaesium in Helianthus annuus. Span. J. Agric. Res. 2010, 8, 1200–1228. [Google Scholar] [CrossRef]
- Konate, A.; He, X.; Zhang, Z.; Ma, Y.; Zhang, P.; Alugongo, G.M.; Rui, Y. Magnetic (Fe3O4) nanoparticles reduce heavy metals uptake and mitigate their toxicity in wheat seedling. Sustainability 2017, 9, 790. [Google Scholar] [CrossRef]
- Kloubert, V.; Rink, L. Zinc as a micronutrient and its preventive role of oxidative damage in cells. Food Funct. 2015, 6, 3195–3204. [Google Scholar] [CrossRef]
- Ali, E.; Mahmoud, A.M. Effect of foliar spray by different salicylic acid and zinc concentrations on seed yield and yield components of mungbean in sandy soil. Asian J. Crop Sci. 2013, 5, 33–40. [Google Scholar] [CrossRef]
- Hua, T.; Zhang, R.; Sun, H.; Liu, C. Alleviation of boron toxicity in plants: Mechanisms and approaches. Crit. Rev. Environ. Sci. Technol. 2021, 51, 2975–3015. [Google Scholar] [CrossRef]
- Del Rio, D.; Stewart, A.J.; Pellegrini, N. A review of recent studies on malondialdehyde as toxic molecule and biological marker of oxidative stress. Nutr. Metab. Cardiovasc. Dis. 2005, 15, 316–328. [Google Scholar] [CrossRef] [PubMed]
- Arnao, M.B.; Hernández-Ruiz, J. Melatonin and its relationship to plant hormones. Ann. Bot. 2018, 121, 195–207. [Google Scholar] [CrossRef]
- Bharti, A.; Garg, N. SA and AM symbiosis modulate antioxidant defense mechanisms and asada pathway in chickpea genotypes under salt stress. Ecotoxicol. Environ. Saf. 2019, 178, 66–78. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Guo, Y.; Lan, Z.; Zhang, Z.; Ahammed, G.J.; Chang, J.; Zhang, Y.; Wei, C.; Zhang, X. Melatonin antagonizes ABA action to promote seed germination by regulating Ca2+ efflux and H2O2 accumulation. Plant Sci. 2021, 303, 110761. [Google Scholar] [CrossRef] [PubMed]
- Wen, D.; Gong, B.; Sun, S.; Liu, S.; Wang, X.; Wei, M.; Yang, F.; Li, Y.; Shi, Q. Promoting roles of melatonin in adventitious root development of Solanum lycopersicum L. By regulating auxin and nitric oxide signaling. Front. Plant Sci. 2016, 7, 718. [Google Scholar] [CrossRef] [PubMed]
- Arnao, M.B.; Hernández-Ruiz, J. Protective effect of melatonin against chlorophyll degradation during the senescence of barley leaves. J. Pineal Res. 2009, 46, 58–63. [Google Scholar] [CrossRef]
- Onik, J.C.; Wai, S.C.; Li, A.; Lin, Q.; Sun, Q.; Wang, Z.; Duan, Y. Melatonin treatment reduces ethylene production and maintains fruit quality in apple during postharvest storage. Food Chem. 2021, 337, 127753. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Zhang, H.-J.; Zhao, B.; Sun, Q.-Q.; Cao, Y.-Y.; Li, R.; Wu, X.-X.; Weeda, S.; Li, L.; Ren, S.; et al. The RNA-seq approach to discriminate gene expression profiles in response to melatonin on cucumber lateral root formation. J. Pineal Res. 2014, 56, 39–50. [Google Scholar] [CrossRef]
- Ali, M.S.; Baek, K.H. Jasmonic acid signaling pathway in response to abiotic stresses in plants. Int. J. Mol. Sci. 2020, 21, 621. [Google Scholar] [CrossRef] [PubMed]
- Farooq, M.A.; Detterbeck, A.; Clemens, S.; Dietz, K.J. Silicon-induced reversibility of cadmium toxicity in rice. J. Exp. Bot. 2016, 67, 3573–3585. [Google Scholar] [CrossRef]
- Rajaie, M.; Ejraie, A.K.; Owliaie, H.R.; Tavakoli, A.R. Effect of zinc and boron interaction on growth and mineral composition of lemon seedlings in a calcareous soil. Int. J. Plant Prod. 2009, 3, 39–50. [Google Scholar]
- Swietlik, D.; LaDuke, J.V. Productivity, growth, and leaf mineral composition of orange and grapefruit trees foliar-sprayed with zinc and manganese. J. Plant Nutr. 1991, 14, 129–142. [Google Scholar] [CrossRef]
- Hosseini, S.M.; Maftoun, M.; Karimian, N.; Ronaghi, A.; Emam, Y. Effect of zinc x boron interaction on plant growth and tissue nutrient concentration of corn. J. Plant Nutr. 2007, 30, 773–781. [Google Scholar] [CrossRef]
- Graham, R.D.; Welch, R.M.; Grunes, D.L.; Cary, E.E.; Norvell, W.A. Effect of Zinc Deficiency on the Accumulation of Boron and Other Mineral Nutrients in Barley. Soil Sci. Soc. Am. J. 1987, 51, 652–657. [Google Scholar] [CrossRef]
- Singh, J.P.; Dahiya, D.J.; Narwal, R.P. Boron uptake and toxicity in wheat in relation to zinc supply. Fertil. Res. 1990, 24, 105–110. [Google Scholar] [CrossRef]
- Swietlik, D. Interaction between zinc deficiency and boron toxicity on growth and mineral nutrition of sour orange seedlings. J. Plant Nutr. 1995, 18, 1191–1207. [Google Scholar] [CrossRef]
- Güneş, A.; Alpaslan, M.; Çikili, Y.; Özcan, H. The effect of zinc on alleviation of boron toxicity in tomato plants (Lycopersicon esculentum L.). Turk. J. Agric. For. 2000, 24, 505–509. [Google Scholar]
- Sinha, P.; Jain, R.; Chatterjee, C. Interactive effect of boron and zinc on growth and metabolism of mustard. Commun. Soil Sci. Plant Anal. 2000, 31, 41–49. [Google Scholar] [CrossRef]
- Sharma, P.N.; Kumar, N.; Bisht, S.S. Effect of zinc deficiency on chlorophyll content, photosynthesis and water relations of cauliflower plants. Photosynthetica 1994, 30, 353–359. [Google Scholar]
- Briat, J.F.; Curie, C.; Gaymard, F. Iron utilization and metabolism in plants. Curr. Opin. Plant Biol. 2007, 10, 276–282. [Google Scholar] [CrossRef]
- Kobayashi, T.; Nishizawa, N.K. Iron uptake, translocation, and regulation in higher plants. Annu. Rev. Plant Biol. 2012, 63, 131–152. [Google Scholar] [CrossRef] [PubMed]
- Sinclair, S.A.; Krämer, U. The zinc homeostasis network of land plants. Biochim. Biophys. Acta Mol. Cell Res. 2012, 1823, 1553–1567. [Google Scholar] [CrossRef] [PubMed]
- Tariq, M.; Mott, C.J.B. The Significance of Boron in Plant Nutrition and Environment—A Review. J. Agron. 2007, 6, 1–10. [Google Scholar]



| Comparison Group | Total DEGs | Up-Regulated Genes | Down-Regulated Genes |
|---|---|---|---|
| B vs. CK | 15,509 | 6261 | 9248 |
| B + ZnSO4 vs. B | 1086 | 915 | 171 |
| B + ZnO vs. B | 4000 | 2408 | 1592 |
| B + ZnO vs. B + ZnSO4 | 2144 | 1022 | 1122 |
| Treatments | Root | Stem | Leaf | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mg | K | Fe | Zn | Mg | K | Fe | Zn | Mg | K | Fe | Zn | |
| CK | 4.30 a | 36.34 b | 0.60 b | 0.55 d | 2.18 ab | 109.93 a | 0.14 b | 0.09 c | 6.09 a | 48.08 a | 0.15 a | 0.07 c |
| ZnSO4 | 1.89 b | 51.78 a | 1.38 a | 3.16 c | 2.61 a | 55.58 b | 0.09 b | 0.50 a | 6.08 a | 46.71 a | 0.09 b | 0.46 a |
| ZnO | 1.26 b | 18.23 c | 0.76 b | 13.95 a | 1.15 c | 30.79 b | 0.25 a | 0.55 b | 1.74 c | 14.15 d | 0.05 b | 0.15 b |
| B | 1.78 b | 20.22 c | 0.60 b | 0.47 d | 0.73 c | 45.52 b | 0.06 c | 0.09 c | 5.06 a | 35.14 ab | 0.06 b | 0.12 b |
| B + ZnSO4 | 2.06 b | 54.31 a | 1.35 a | 3.21 c | 1.77 bc | 57.24 b | 0.03 c | 0.45 a | 5.06 a | 40.15 a | 0.08 b | 0.18 b |
| B + ZnO | 1.46 b | 13.72 d | 0.47 c | 10.13 b | 0.56 d | 52.31 b | 0.03 c | 0.58 a | 3.00 b | 22.45 c | 0.05 b | 0.17 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nassarawa, I.S.; Li, Z.; Xue, L.; Li, H.; Muhammad, U.; Zhu, S.; Chen, J.; Zhao, T. Zinc Oxide Nanoparticles and Zinc Sulfate Alleviate Boron Toxicity in Cotton (Gossypium hirsutum L.). Plants 2024, 13, 1184. https://doi.org/10.3390/plants13091184
Nassarawa IS, Li Z, Xue L, Li H, Muhammad U, Zhu S, Chen J, Zhao T. Zinc Oxide Nanoparticles and Zinc Sulfate Alleviate Boron Toxicity in Cotton (Gossypium hirsutum L.). Plants. 2024; 13(9):1184. https://doi.org/10.3390/plants13091184
Chicago/Turabian StyleNassarawa, Ismail Sanusi, Zhuolin Li, Longshuo Xue, Huazu Li, Uzair Muhammad, Shuijin Zhu, Jinhong Chen, and Tianlun Zhao. 2024. "Zinc Oxide Nanoparticles and Zinc Sulfate Alleviate Boron Toxicity in Cotton (Gossypium hirsutum L.)" Plants 13, no. 9: 1184. https://doi.org/10.3390/plants13091184





