Impacts of Soil Properties on Species Diversity and Structure in Alternanthera philoxeroides-Invaded and Native Plant Communities
Abstract
:1. Introduction
2. Results
2.1. Plant Species Composition and Invasion Trend
2.2. RDA Ordination of Soil Properties and Species Diversity
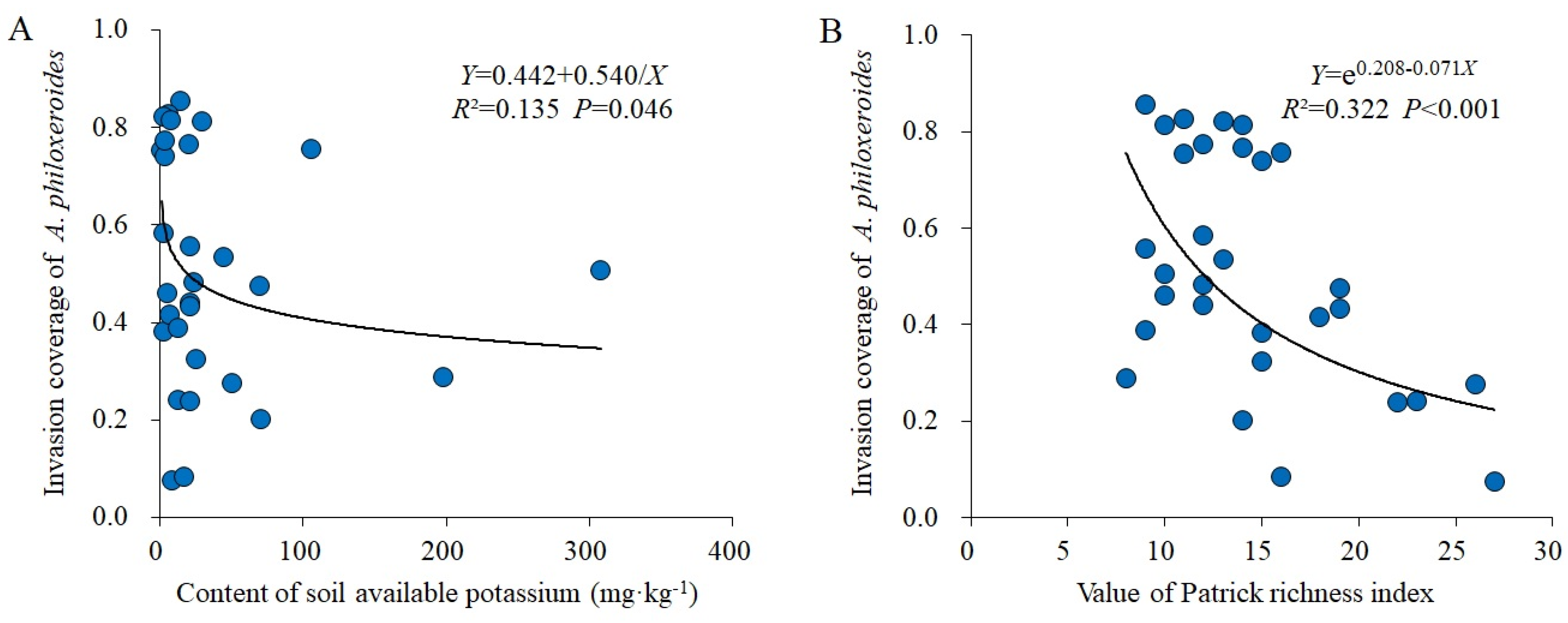
2.3. Regression Analysis of Soil Properties and Species Diversity
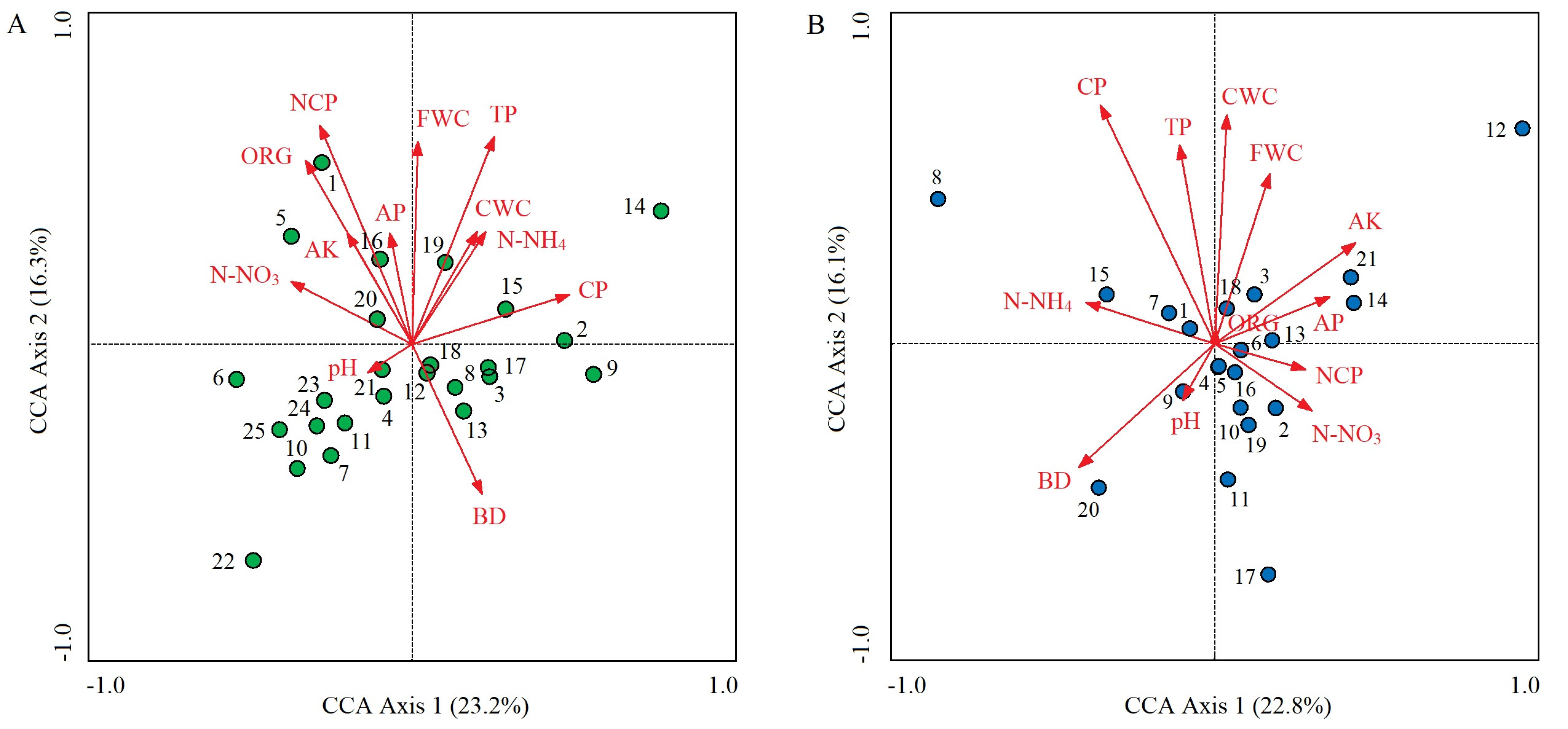
2.4. CCA Ordination of Soil Properties and Species Distribution
3. Discussion
3.1. Mechanism of Impact of Soil on Community Structure and Plant Invasions
3.2. Mechanism of Impact of Soil on Plant Diversity
3.3. Mechanism of Impact of Soil on Plant Species Distributions
4. Materials and Methods
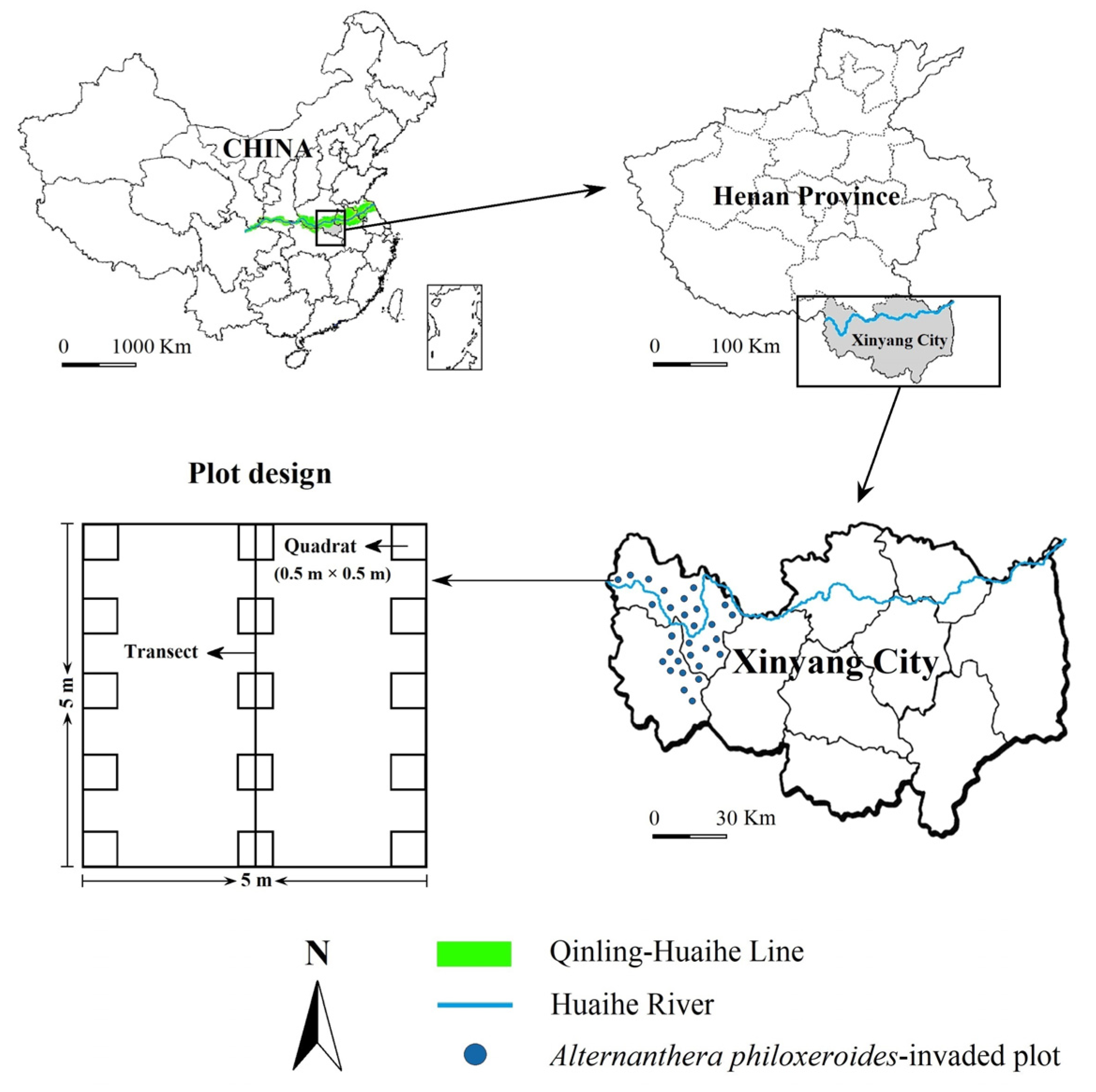
4.1. Field Survey
4.2. Indicator Measurement
4.2.1. Measurement of Soil Physical Properties
4.2.2. Measurement of Soil Chemical Properties
4.3. Data Calculation
4.3.1. Soil Physical Indicators
4.3.2. Importance Value
4.3.3. Species Diversity
4.4. Statistical Analyses
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Fernandez, R.D.; Castro-Diez, P.; Aragon, R.; Perez-Harguindeguy, N. Changes in community functional structure and ecosystem properties along an invasion gradient of Ligustrum lucidum. J. Veg. Sci. 2021, 32, e13098. [Google Scholar] [CrossRef]
- Fahey, C.; Angelini, C.; Flory, S.L. Grass invasion and drought interact to alter the diversity and structure of native plant communities. Ecology 2018, 99, 2692–2702. [Google Scholar] [CrossRef]
- Livingstone, S.W.; Isaac, M.E.; Cadotte, M.W. Invasive dominance and resident diversity: Unpacking the impact of plant invasion on biodiversity and ecosystem function. Ecol. Monogr. 2020, 90, e01425. [Google Scholar] [CrossRef]
- Carboni, M.; Livingstone, S.W.; Isaac, M.E.; Cadotte, M.W. Invasion drives plant diversity loss through competition and ecosystem modification. J. Ecol. 2021, 109, 3587–3601. [Google Scholar] [CrossRef]
- Wang, C.Y.; Wu, B.D.; Jiang, K.; Zhou, J.W.; Du, D.L. Canada goldenrod invasion affect taxonomic and functional diversity of plant communities in heterogeneous landscapes in urban ecosystems in East China. Urban For. Urban Green. 2019, 38, 145–156. [Google Scholar] [CrossRef]
- Balah, M.A.; Hassany, W.M. The relationship between Invasive Alien Solanum elaeagnifolium Cav. characters and impacts in different habitats. Biologia 2023, 78, 1253–1268. [Google Scholar] [CrossRef]
- Uddin, M.D.N.; Robinson, R.W. Responses of plant species diversity and soil physical-chemical-microbial properties to Phragmites australis invasion along a density gradient. Sci. Rep. 2017, 7, 11007. [Google Scholar] [CrossRef]
- Hansen, W.; Wollny, J.; Otte, A.; Eckstein, R.L.; Ludewig, K. Invasive legume affects species and functional composition of mountain meadow plant communities. Biol. Invasions 2021, 23, 281–296. [Google Scholar] [CrossRef]
- Ab Razak, N.; Gange, A.C.; Sutton, B.C.; Mansor, A. The invasive plant Impatiens glandulifera manipulates microbial associates of competing native species. Plants 2023, 12, 1552. [Google Scholar] [CrossRef]
- Süle, G.; Molnár, C.; Kovács-Hostyánszki, A.; Fenesi, A.; Bauer, N.; Szigeti, V. Escape from the garden: Spreading, effects and traits of a new risky invasive ornamental plant (Gaillardia aristata Pursh). NeoBiota 2023, 83, 43–69. [Google Scholar] [CrossRef]
- Yang, X.D.; Long, Y.X.; Sarkar, B.; Li, Y.; Lü, G.H.; Ali, A.; Yang, J.J.; Cao, Y.E. Influence of soil microorganisms and physicochemical properties on plant diversity in an arid desert of Western China. J. For. Res. 2021, 32, 2645–2659. [Google Scholar] [CrossRef]
- Liang, J.F.; Yuan, W.Y.; Gao, J.Q.; Roiloa, S.R.; Song, M.H.; Zhang, X.Y.; Yu, F.H. Soil resource heterogeneity competitively favors an invasive clonal plant over a native one. Oecologia 2020, 193, 155–165. [Google Scholar] [CrossRef]
- Gao, F.L.; He, Q.S.; Zhang, Y.D.; Yu, F.H.; Hou, J.H. Effects of soil nutrient heterogeneity on the growth and invasion success of alien plants: A multi-species study. Front. Ecol. Evol. 2021, 8, 619861. [Google Scholar] [CrossRef]
- Pan, J.; Bai, Z.K.; Cao, Y.G.; Zhou, W.; Wang, J.M. Influence of soil physical properties and vegetation coverage at different slope aspects in a reclaimed dump. Environ. Sci. Pollut. Res. 2017, 24, 23953–23965. [Google Scholar] [CrossRef]
- Tang, H.; Chen, M.; Wu, P.; Faheem, M.; Feng, Q.W.; Lee, X.Q.; Wang, S.S.; Wang, B. Engineered biochar effects on soil physicochemical properties and biota communities: A critical review. Chemosphere 2023, 311, 137025. [Google Scholar] [CrossRef]
- Li, W.T.; Bi, X.T.; Zheng, Y.L. Soil legacy effects on biomass allocation depend on native plant diversity in the invaded community. Sci. Prog. 2023, 106, 1–12. [Google Scholar] [CrossRef]
- Haury, J.; Druel, A.; Cabral, T.; Paulet, Y.; Bozec, M.; Coudreuse, J. Which adaptations of some invasive Ludwigia spp. (Rosidae, Onagraceae) populations occur in contrasting hydrological conditions in Western France? Hydrobiologia 2014, 737, 45–56. [Google Scholar] [CrossRef]
- Wang, C.Y.; Jiang, K.; Zhou, J.W.; Wu, B.D. Solidago canadensis invasion affects soil N-fixing bacterial communities in heterogeneous landscapes in urban ecosystems in East China. Sci. Total. Environ. 2018, 631–632, 702–713. [Google Scholar] [CrossRef]
- Yu, H.X.; Le Roux, J.J.; Jiang, Z.Y.; Sun, F.; Peng, C.L.; Li, W.H. Soil nitrogen dynamics and competition during plant invasion: Insights from Mikania micrantha invasions in China. New Phytol. 2021, 229, 3440–3452. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.X.; Lu, X.F.; Zhao, H.X.; Yang, Y.F.; Hale, L.; Gao, Q.; Liu, W.X.; Guo, J.Y.; Li, Q.; Zhou, J.Z.; et al. Ageratina adenophora invasions are associated with microbially mediated differences in biogeochemical cycles. Sci. Total. Environ. 2019, 677, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Hu, J.; Miao, L.; Yu, D.; Liu, C. The invasive stoloniferous clonal plant Alternanthera philoxeroides outperforms its co-occurring non-invasive functional counterparts in heterogeneous soil environments-invasion implications. Sci. Rep. 2016, 6, 38036. [Google Scholar] [CrossRef]
- Wu, H.; Carrillo, J.; Ding, J.Q. Species diversity and environmental determinants of aquatic and terrestrial communities invaded by Alternanthera philoxeroides. Sci. Total Environ. 2017, 581, 666–675. [Google Scholar] [CrossRef]
- Peng, X.M.; Yang, Y.F.; Yan, X.M.; Li, H.Y. The effects of water control on the survival and growth of Alternanthera philoxeroides in the vegetative reproduction and seedling stages. Sci. Rep. 2021, 11, 13556. [Google Scholar] [CrossRef]
- Wu, H.; Ismail, M.; Ding, J.Q. Global warming increases the interspecific competitiveness of the invasive plant alligator weed, Alternanthera philoxeroides. Sci. Total Environ. 2017, 575, 1415–1422. [Google Scholar] [CrossRef]
- You, W.H.; Li, N.N.; Zhang, J.; Song, A.; Du, D.L. The plant invader Alternanthera philoxeroides benefits from clonal integration more than its native co-genus in response to patch contrast. Plants 2023, 12, 2371. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, C.; Xiong, Y.T.; Wang, Y.; Li, Q.J. Combination effects of heavy metal and inter-specific competition on the invasiveness of Alternanthera philoxeroides. Environ. Exp. Bot. 2021, 189, 104532. [Google Scholar] [CrossRef]
- Ge, Y.Y.; Wang, Q.L.; Wang, L.; Liu, W.X.; Liu, X.Y.; Huang, Y.J.; Christie, P. Response of soil enzymes and microbial communities to root extracts of the alien Alternanthera philoxeroides. Arch. Agron. Soil Sci. 2018, 64, 708–717. [Google Scholar] [CrossRef]
- Cao, Q.; Zhang, H.; Ma, W.; Wang, R.; Liu, J. Composition characteristics of organic matter and bacterial communities under the Alternanthera philoxeroide invasion in wetlands. Appl. Sci. 2020, 10, 5571. [Google Scholar] [CrossRef]
- Harms, N.E.; Knight, I.A.; DeRossette, A.B.; Williams, D.A. Intraspecific trait plasticity to N and P of the wetland invader, Alternanthera philoxeroides under flooded conditions. Ecol. Evol. 2023, 13, e9966. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.Y.; Gao, H.; Li, J.; Wang, Y.H.; Gao, A.G.; Wen, J.H.; Balah, M.A.; Wu, A.P. The latitudinal and longitudinal allelopathic patterns of an invasive alligator weed (Alternanthera philoxeroides) in China. PLoS ONE 2023, 18, e0280866. [Google Scholar] [CrossRef] [PubMed]
- He, M.Y.; Hua, Z.X.; Chen, H.Y.; Liu, Y.; Li, Y.; Zhang, Z. Effects of simulated acid rain on rhizosphere microorganisms of invasive Alternanthera philoxeroides and native Alternanthera sessilis. Front. Microbiol. 2022, 13, 993147. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.J.; Chang, R.Y.; Guo, X.; Liang, X.Q.; Wang, R.Q.; Liu, J. Shifts in growth and competitive dominance of the invasive plant Alternanthera philoxeroides under different nitrogen and phosphorus supply. Environ. Exp. Bot. 2017, 135, 118–125. [Google Scholar] [CrossRef]
- Yang, R.R.; Li, K.; Fang, J.H.; Quan, Q.; Zhang, C.; Liu, J. The invasion of Alternanthera philoxeroides increased soil organic carbon in a river and a constructed wetland with different mechanisms. Front. Ecol. Evol. 2020, 8, 574528. [Google Scholar] [CrossRef]
- Yan, Z.W.; Lv, T.; Liu, Y.; Xing, B.; Chao, C.X.; Li, Y.; Wu, L.; Wang, L.G.; Liu, C.H.; Yu, D. Responses of soil phosphorus cycling and bioavailability to plant invasion in river-lake ecotones. Ecol. Appl. 2023, 33, e2843. [Google Scholar] [CrossRef] [PubMed]
- McIntire, E.J.B.; Fajardo, A. Facilitation as a ubiquitous driver of biodiversity. New Phytol. 2014, 201, 403–416. [Google Scholar] [CrossRef] [PubMed]
- Shiraishi, S.; Watanabe, I.; Kuno, K.; Fujii, Y. Evaluation of the allelopathic activity of five Oxalidaceae cover plants and the demonstration of potent weed suppression by Oxalis species. Weed Biol. Manag. 2005, 5, 128–136. [Google Scholar] [CrossRef]
- Lorenzo, P.; González, L.; Ferrero, V. Effect of plant origin and phenological stage on the allelopathic activity of the invasive species Oxalis pescaprae. Am. J. Bot. 2021, 108, 971–979. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Carrillo, J.; Ding, J.Q. Invasion by alligator weed, Alternanthera philoxeroides, is associated with decreased species diversity across the latitudinal gradient in China. J. Plant Ecol. 2016, 9, 311–319. [Google Scholar] [CrossRef]
- Chen, H.; Qian, H.; Spyreas, G.; Crossland, M. Native-exotic species richness relationships across spatial scales and biotic homogenization in wetland plant communities of Illinois, USA. Divers. Distrib. 2010, 16, 737–743. [Google Scholar] [CrossRef]
- Mungi, N.A.; Qureshi, Q.; Jhala, Y.V. Role of species richness and human impacts in resisting invasive species in tropical forests. J. Ecol. 2021, 109, 3308–3321. [Google Scholar] [CrossRef]
- Yan, Y.Z.; Jarvie, S.; Zhang, Q.; Han, P.; Liu, Q.F.; Zhang, S.S.; Liu, P.T. Habitat heterogeneity determines species richness on small habitat islands in a fragmented landscape. J. Biogeogr. 2023, 50, 976–986. [Google Scholar] [CrossRef]
- Chen, D.; Kleunen, M.V. Invasional meltdown mediated by plant-soil feedbacks may depend on community diversity. New Phytol. 2022, 235, 1589–1598. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Wang, Q.; Yu, Q.G.; Ye, J.; Gao, J.W.; Liu, H.T.; Yong, J.W.H.; Yu, Y.J.; Liu, X.X.; Kong, H.M.; et al. Is the NH4+-induced growth inhibition caused by the NH4+ form of the nitrogen source or by soil acidification? Front. Plant Sci. 2022, 13, 968707. [Google Scholar] [CrossRef] [PubMed]
- Li, S.P.; Jia, P.; Fan, S.Y.; Wu, Y.T.; Liu, X.; Meng, Y.N.; Li, Y.; Shu, W.S.; Li, J.T.; Jiang, L. Functional traits explain the consistent resistance of biodiversity to plant invasion under nitrogen enrichment. Ecol. Lett. 2022, 25, 778–789. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q.; Qian, H.; Zhang, J. Does regional species diversity resist biotic invasions? Plant Divers. 2023, 45, 353–357. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Tang, Y.; Tian, J.; Wu, X. Correlation between plant diversity and the physicochemical properties of soil microbes. Appl. Ecol. Environ. Res. 2019, 17, 10371–10388. [Google Scholar] [CrossRef]
- Di, W.U.; Andales, A.A.; Yang, H.; Sun, Q.; Chen, S.; Guo, X.; Li, D.H.; Du, T.S. Linking crop water productivity to soil physical, chemical and microbial properties. Front. Agric. Sci. Eng. 2021, 8, 545–558. [Google Scholar]
- Crawford, K.M.; Knight, T.M. Competition overwhelms the positive plant-soil feedback generated by an invasive plant. Oecologia 2017, 183, 211–220. [Google Scholar] [CrossRef] [PubMed]
- Khatri, K.; Negi, B.; Bargali, K.; Bargali, S.S. Trait plasticity: A key attribute in the invasion success of Ageratina adenophora in different forest types of Kumaun Himalaya, India. Environ. Dev. Sustain. 2023. on line. [Google Scholar] [CrossRef]
- Xiao, C.B.; Fang, Y.; Wang, S.M.; He, K. The alleviation of ammonium toxicity in plants. J. Integr. Plant Biol. 2023, 65, 1362–1368. [Google Scholar] [CrossRef]
- Meier, I.C.; Finzi, A.C.; Phillips, R.P. Root exudates increase N availability by stimulating microbial turnover of fast-cycling N pools. Soil Biol. Biochem. 2017, 106, 119–128. [Google Scholar] [CrossRef]
- Luo, R.Y.; Fan, J.L.; Wang, W.J.; Luo, J.F.; Kuzyakov, Y.; He, J.S.; Chu, H.Y.; Ding, W.X. Nitrogen and phosphorus enrichment accelerates soil organic carbon loss in alpine grassland on the Qinghai-Tibetan Plateau. Sci. Total Environ. 2019, 650, 303–312. [Google Scholar] [CrossRef] [PubMed]
- Qiu, T.Y.; Yu, J.L.; He, L.Y.; Liu, J.; Cui, Q.L.; Cui, Y.X.; Duan, C.J.; Zhao, S.L.; Wang, Y.Q.; Fang, L.C. Slope position mediates the co-utilization of phosphorus by plants and microbes through rhizosphere processes in a phosphorus-limited forest. Catena 2023, 222, 106808. [Google Scholar] [CrossRef]
- Ji, S.W.; Jiang, L.M.; Hu, D.; Lv, G.H. Impacts of plant and soil stoichiometry on species diversity in a desert ecosystem. AoB Plants 2022, 14, plac034. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.P.; Li, W.T.; Li, J.; Feng, Y.L. Temporal dynamics of plant-soil feedback and related mechanisms depend on environmental context during invasion processes of a subtropical invader. Plant Soil 2024, 496, 539–554. [Google Scholar] [CrossRef]
- Feng, X.; Xia, X.; Chen, S.T.; Lin, Q.M.; Zhang, X.H.; Cheng, K.; Liu, X.Y.; Bian, R.J.; Zheng, J.F.; Li, L.Q.; et al. Amendment of crop residue in different forms shifted micro-pore system structure and potential functionality of macroaggregates while changed their mass proportion and carbon storage of paddy topsoil. Geoderma 2022, 409, 115643. [Google Scholar] [CrossRef]
- De Long, J.R.; Heinen, R.; Heinze, J.; Morrien, E.; Png, G.K.; Sapsford, S.J.; Teste, F.P.; Fry, E.L. Plant-soil feedback: Incorporating untested influential drivers and reconciling terminology. Plant Soil. 2023, 485, 7–43. [Google Scholar] [CrossRef]
- Wu, J.; Yu, F.H. Belowground bud bank of invasive plants contributes to their successful invasion in coastal wetlands. Restor. Ecol. 2023, 31, e13821. [Google Scholar] [CrossRef]
- Lone, P.A.; Dar, J.A.; Subashree, K.; Raha, D.; Pandey, P.K.; Ray, T.; Khare, P.K.; Khan, M.L. Impact of plant invasion on physical, chemical and biological aspects of ecosystems: A review. Trop. Plant Res. 2019, 6, 528–544. [Google Scholar] [CrossRef]
- Kou, Z.; Yao, Y.; Hu, Y.; Zhang, B. Discussion on position of China’s north-south transitional zone by comparative analysis of mountain altitudinal belts. J. Mt. Sci. 2020, 17, 1901–1915. [Google Scholar] [CrossRef]
- Maron, J.; Marler, M. Native plant diversity resists invasion at both low and high resource levels. Ecology 2007, 88, 2651–2661. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Jia, X.; Han, L.; Tian, G.; Kang, S.; Zhao, Y. Spatial characteristics of the dominant fungi and their driving factors in forest soils in the Qinling Mountains, China. Catena 2021, 206, 105504. [Google Scholar] [CrossRef]
- Wallingford, P.D.; Morelli, T.L.; Allen, J.M.; Beaury, E.M.; Blumenthal, D.M.; Bradley, B.A.; Dukes, J.S.; Early, R.; Fusco, E.J.; Goldberg, D.E.; et al. Adjusting the lens of invasion biology to focus on the impacts of climate-driven range shifts. Nat. Clim. Change 2020, 10, 398–405. [Google Scholar] [CrossRef]
- Yan, H.Y.; Feng, L.; Zhao, Y.F.; Feng, L.; Wu, D.; Zhu, C.P. Prediction of the spatial distribution of Alternanthera philoxeroides in China based on ArcGIS and MaxEnt. Glob. Ecol. Conserv. 2020, 21, e00856. [Google Scholar] [CrossRef]
- Gerlach, R.; Buhk, C.; Schirmel, J.; Jungkunst, H.F. Exploration of the response of soil properties and plant species composition to centuries-old traditional meadow irrigation. J. Plant Ecol. 2023, 16, 72. [Google Scholar] [CrossRef]
- Yu, B.Q.; Xie, C.K.; Cai, S.Z.; Chen, Y.; Lv, Y.P.; Mo, Z.L.; Liu, T.L.; Yang, Z.W. Effects of tree root density on soil total porosity and non-capillary porosity using a ground-penetrating tree radar unit in Shanghai, China. Sustainability 2018, 10, 4640. [Google Scholar] [CrossRef]
- Wu, W.J.; Chen, G.J.; Meng, T.F.; Li, C.; Feng, H.; Si, B.C.; Siddique, K.H.M. Effect of different vegetation restoration on soil properties in the semi-arid Loess Plateau of China. Catena 2023, 220, 106630. [Google Scholar] [CrossRef]
- Zhang, Y.H.; Niu, J.Z.; Yu, X.X.; Zhu, W.L.; Du, X.Q. Effects of fine root length density and root biomass on soil preferential flow in forest ecosystems. For. Ecosyst. 2015, 24, e012. [Google Scholar] [CrossRef]
- Lyu, Q.; Liu, J.L.; Liu, J.J.; Luo, Y.; Chen, L.M.; Chen, G.; Zhao, K.J.; Chen, Y.Q.; Fan, C.; Li, X.W. Response of plant diversity and soil physicochemical properties to different gap sizes in a Pinus massoniana plantation. PeerJ 2021, 9, e12222. [Google Scholar] [CrossRef]
- Ma, Q.; Li, Z.; Xiao, H.L.; Hu, Z.; Pung, L. Mechanical properties of clay reinforced with Bermuda grass root under drying-wetting cycles. Environ. Earth Sci. 2021, 80, 31. [Google Scholar] [CrossRef]
- Blagodatskaya, E.; Littschwager, J.; Lauerer, M.; Kuzyakov, Y. Plant traits regulating N capture define microbial competition in the rhizosphere. Eur. J. Soil Biol. 2014, 61, 41–48. [Google Scholar] [CrossRef]
- Shannon-Firestone, S.; Reynolds, H.L.; Phillips, R.P.; Flory, S.L.; Yannarell, A. The role of ammonium oxidizing communities in mediating effects of an invasive plant on soil nitrification. Soil Biol. Biochem. 2015, 90, 266–274. [Google Scholar] [CrossRef]
- Aiswarya, N.K.; Muthuraj, K.; Siva, P.K.T.; Krishnan, E.; Nagarajan, N. Studies on the arbuscular mycorrhizal fungal diversity of selected medicinal plant species from kodikuthimala, malappuram, kerala. Kongunadu Res. J. 2017, 4, 51–58. [Google Scholar]
- Daryanto, S.; Wang, L.; Gilhooly, W.P.; Jacinthe, P.A. Nitrogen preference across generations under changing ammonium nitrate ratios. J. Plant Ecol. 2019, 12, 235–244. [Google Scholar] [CrossRef]
- Yang, J.; Li, E.H.; Zhou, R.; Xia, Y.; Yang, C.; Zhang, Y.Y. The effects of edaphic factors on riparian plants in the middle and lower reaches of the Hanjiang River, China. Plants 2022, 11, 531. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Dong, S.J.; Wang, L.; Zhu, Y.Y.; Jia, S.Q.; Rao, B.Q. Nitrogen enrichment alters the resistance of a noninvasive alien plant species to Alternanthera philoxeroides invasion. Front. Ecol. Evol. 2023, 11, 1215191. [Google Scholar] [CrossRef]
- Xie, H.T.; Tang, Y.; Yu, M.K.; Wang, G.G. The effects of afforestation tree species mixing on soil organic carbon stock, nutrients accumulation, and understory vegetation diversity on reclaimed coastal lands in Eastern China. Glob. Ecol. Conserv. 2021, 26, e01478. [Google Scholar] [CrossRef]
- Lei, Y.; Liao, R.W.; Su, Y.M.; Zhang, X.; Liu, D.Y.; Zhang, L. Variation characteristics of temperature and rainfall and their relationship with geographical factors in the Qinling Mountains. Atmosphere 2023, 14, 696. [Google Scholar] [CrossRef]
- Shi, S.L.; Zhao, F.Y.; Ren, X.M.; Meng, Z.J.; Dang, X.H.; Wu, X.L. Soil Infiltration properties are affected by typical plant communities in a semi-arid desert grassland in China. Water 2022, 14, 3301. [Google Scholar] [CrossRef]
- Bi, Y.L.; Zou, H.; Zhu, C.W. Dynamic monitoring of soil bulk density and infiltration rate during coal mining in sandy land with different vegetation. Int. J. Coal Sci. Technol. 2014, 1, 198–206. [Google Scholar] [CrossRef]



| Code | Plant Species | Total IV | Code | Plant Species | Total IV |
|---|---|---|---|---|---|
| Control communities | Invaded communities | ||||
| 1 | Oxalis articulata | 4.030 | 1 | Alternanthera philoxeroides | 15.309 |
| 2 | Cynodon dactylon | 3.817 | 2 | Cynodon dactylon | 2.266 |
| 3 | Eleusine indica | 3.533 | 3 | Galium odoratum | 1.917 |
| 4 | Galium odoratum | 2.862 | 4 | Oxalis articulata | 1.915 |
| 5 | Duchesnea indica | 1.645 | 5 | Eleusine indica | 1.758 |
| 6 | Dichondra micrantha | 1.614 | 6 | Rumex acetosa | 1.243 |
| 7 | Axonopus compressus | 1.376 | 7 | Medicago sativa | 1.116 |
| 8 | Lolium perenne | 1.318 | 8 | Paspalum paspaloides | 1.078 |
| 9 | Digitaria sanguinalis | 1.234 | 9 | Erigeron annuus | 0.993 |
| 10 | Medicago sativa | 1.119 | 10 | Duchesnea indica | 0.978 |
| 11 | Poa annua | 1.057 | 11 | Artemisia argyi | 0.956 |
| 12 | Erigeron annuus | 1.029 | 12 | Hydrocotyle sibthorpioides | 0.824 |
| 13 | Vicia hirsuta | 0.823 | 13 | Poa annua | 0.822 |
| 14 | Kyllinga brevifolia | 0.765 | 14 | Ophiopogon bodinieri | 0.822 |
| 15 | Imperata cylindrica | 0.760 | 15 | Cyperus rotundus | 0.710 |
| 16 | Ophiopogon bodinieri | 0.732 | 16 | Lolium perenne | 0.655 |
| 17 | Cayratia japonica | 0.721 | 17 | Dichondra micrantha | 0.646 |
| 18 | Mazus japonicus | 0.711 | 18 | Cayratia japonica | 0.622 |
| 19 | Stellaria media | 0.700 | 19 | Imperata cylindrica | 0.543 |
| 20 | Veronica polita | 0.648 | 20 | Beckmannia syzigachne | 0.532 |
| 21 | Sedum sarmentosum | 0.616 | 21 | Mazus japonicus | 0.512 |
| 22 | Kummerowia striata | 0.529 | |||
| 23 | Rumex acetosa | 0.526 | |||
| 24 | Plantago asiatica | 0.518 | |||
| 25 | Clinopodium chinense | 0.512 | |||
| Soil Factors | Control Communities | Invaded Communities | ||
|---|---|---|---|---|
| Axis 1 | Axis 2 | Axis 1 | Axis 2 | |
| BD | 0.081 | −0.064 | −0.107 | −0.047 |
| FWC | −0.290 | 0.041 | 0.009 | 0.011 |
| CWC | −0.121 | −0.273 | −0.054 | −0.333 |
| NCP | −0.321 | 0.404 * | −0.150 | 0.629 ** |
| CP | −0.064 | −0.402 * | −0.097 | −0.594 ** |
| TP | −0.196 | 0.007 | −0.162 | −0.280 |
| N-NH4 | −0.379 * | 0.388 * | 0.162 | −0.140 |
| N-NO3 | −0.241 | −0.025 | 0.001 | 0.047 |
| AP | −0.307 | 0.634 ** | −0.329 | 0.235 |
| AK | −0.418 * | 0.426 * | −0.271 | 0.380 * |
| ORG | −0.400 * | 0.289 | −0.309 | 0.325 |
| pH | −0.433 * | 0.135 | −0.273 | 0.677 ** |
| Soil Factors | Control Communities | Invaded Communities | ||
|---|---|---|---|---|
| Axis 1 | Axis 2 | Axis 1 | Axis 2 | |
| BD | 0.267 | −0.573 ** | −0.419 * | −0.383 * |
| FWC | 0.022 | 0.772 ** | 0.171 | 0.525 ** |
| CWC | 0.248 | 0.429 * | 0.038 | 0.706 ** |
| NCP | −0.352 | 0.835 ** | 0.280 | −0.080 |
| CP | 0.601 ** | 0.189 | −0.352 | 0.737 ** |
| TP | 0.314 | 0.791 ** | −0.109 | 0.614 ** |
| N-NH4 | 0.281 | 0.428 * | −0.398 * | 0.127 |
| N-NO3 | −0.463 ** | 0.238 | 0.300 | −0.207 |
| AP | −0.084 | 0.422 * | 0.355 | 0.145 |
| AK | −0.247 | 0.423 * | 0.435 * | 0.311 |
| ORG | −0.406 * | 0.701 ** | 0.004 | 0.046 |
| pH | −0.168 | −0.108 | −0.098 | −0.172 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, H.; Liu, Y.; Zhang, T.; Xu, M.; Rao, B. Impacts of Soil Properties on Species Diversity and Structure in Alternanthera philoxeroides-Invaded and Native Plant Communities. Plants 2024, 13, 1196. https://doi.org/10.3390/plants13091196
Wu H, Liu Y, Zhang T, Xu M, Rao B. Impacts of Soil Properties on Species Diversity and Structure in Alternanthera philoxeroides-Invaded and Native Plant Communities. Plants. 2024; 13(9):1196. https://doi.org/10.3390/plants13091196
Chicago/Turabian StyleWu, Hao, Yuxin Liu, Tiantian Zhang, Mingxia Xu, and Benqiang Rao. 2024. "Impacts of Soil Properties on Species Diversity and Structure in Alternanthera philoxeroides-Invaded and Native Plant Communities" Plants 13, no. 9: 1196. https://doi.org/10.3390/plants13091196





