Unveiling the Anatomical and Functional Attributes of Stipular Colleters in Palicourea tetraphylla Cham. & Schltdl. and Palicourea rudgeoides (Müll. Arg.) Standl. (Rubiaceae)
Abstract
:1. Introduction
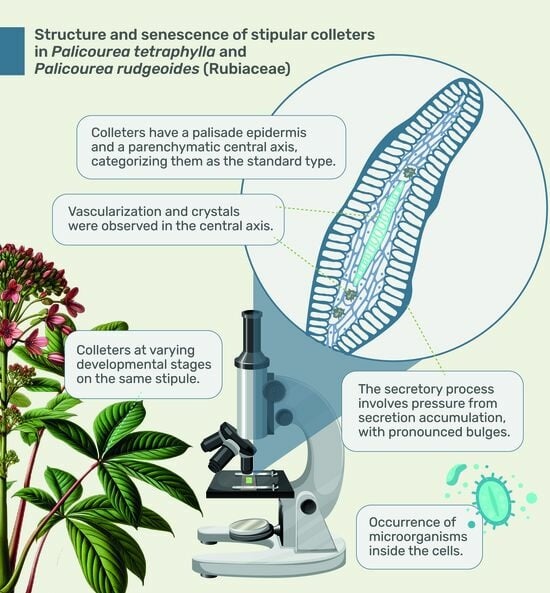
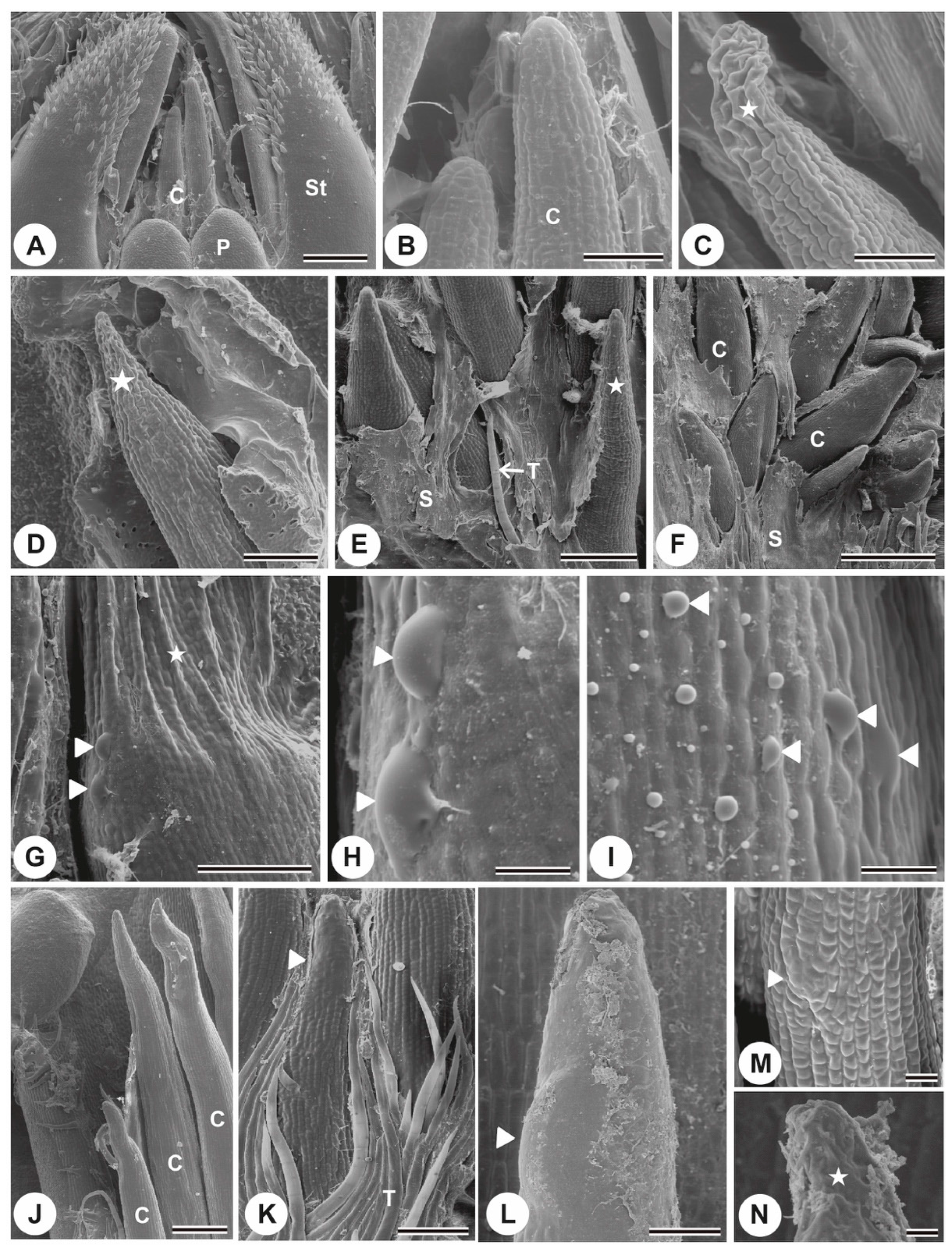
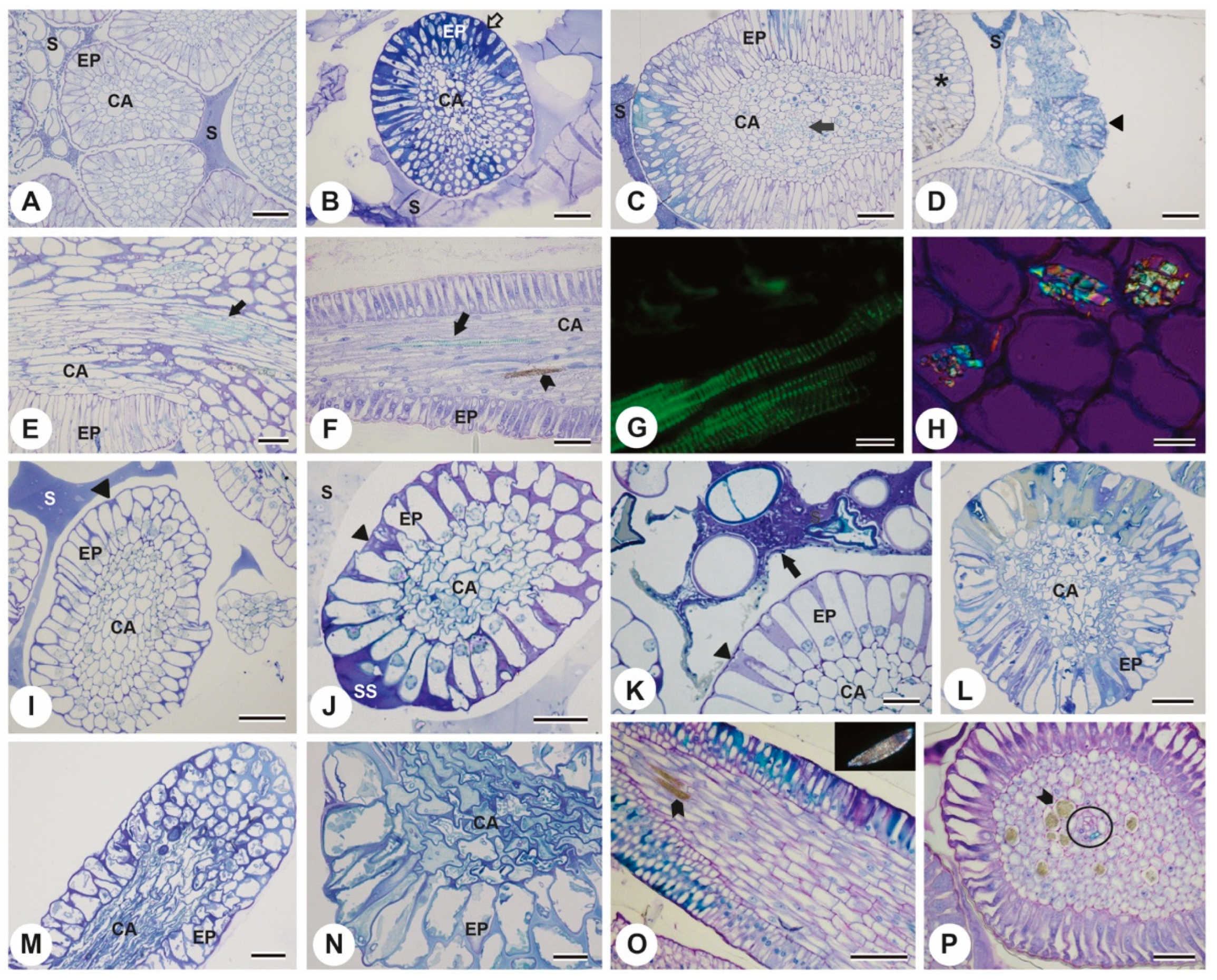
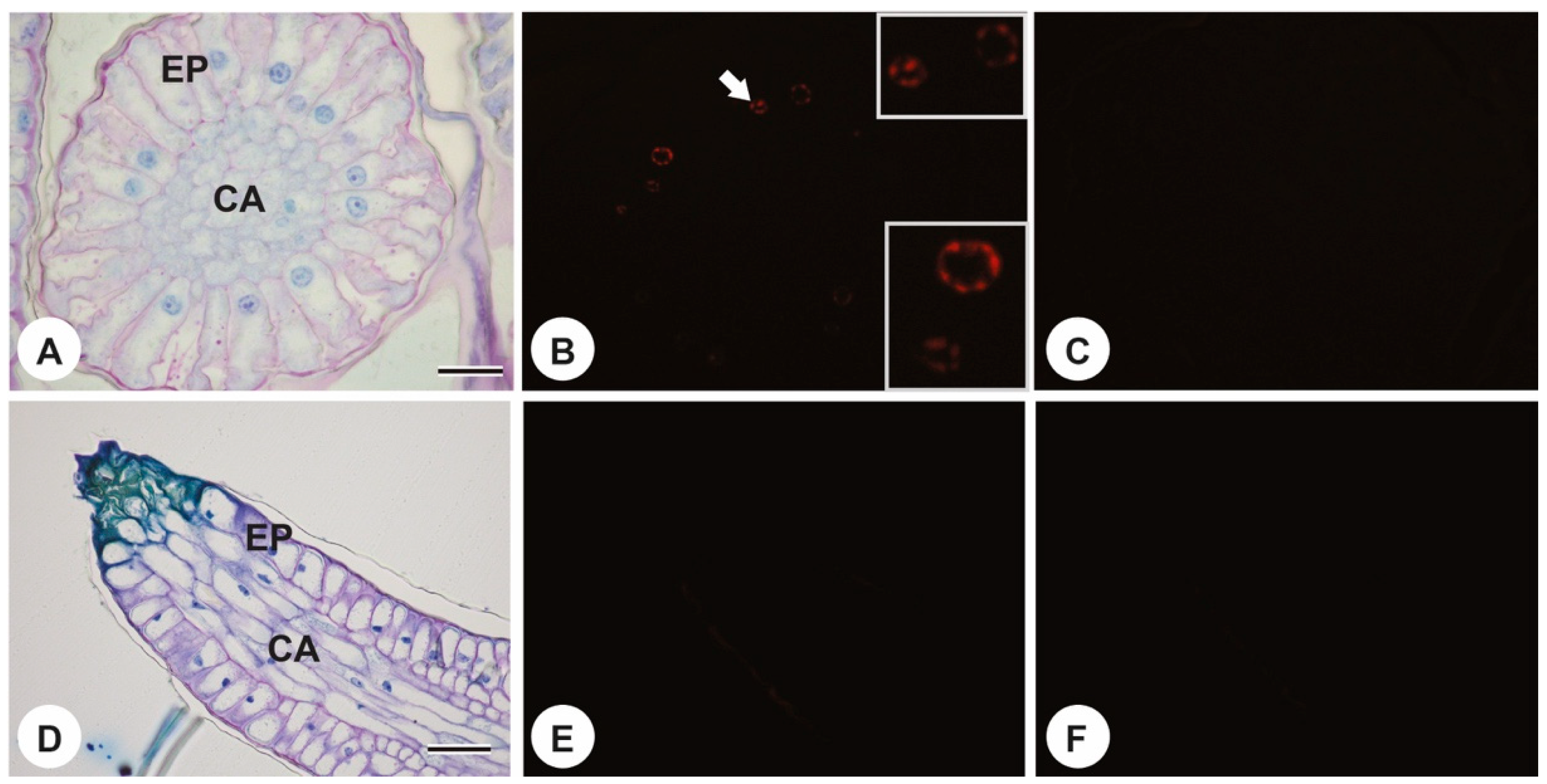
2. Results
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. Sample Preparation for Different Microscopy Techniques
4.3. DNA Fragmentation Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fahn, A. Structure and function of secretory cells. Adv. Bot. Res. 2000, 31, 37–75. [Google Scholar]
- Evert, R.F.; Eichhorn, S.E. Esau’s Plant Anatomy: Meristems, Cells, and Tissues of the Plant Body: Their Structure, Function, and Development, 3rd ed.; Wiley: Hoboken, NJ, USA, 2017; 624p. [Google Scholar]
- Thomas, V. Structural, functional and phylogenetic aspects of the colleter. Ann. Bot. 1991, 68, 287–305. [Google Scholar] [CrossRef]
- Miguel, E.C.; Gomes, V.M.; De Oliveira, M.A.; Da Cunha, M. Colleters in Bathysa nicholsonii K. Schum. (Rubiaceae): Ultrastructure, secretion protein composition and antifungal activity. Plant Biol. 2006, 8, 715–722. [Google Scholar] [CrossRef] [PubMed]
- Coelho, V.P.M.; Leite, J.P.V.; Fietto, L.G.; Ventrella, M.C. Colleters in Bathysa cuspidata (Rubiaceae): Development, ultrastructure and chemical composition of the secretion. Flora 2013, 208, 579–590. [Google Scholar] [CrossRef]
- Tullii, C.F.; Miguel, E.C.; Lima, N.B.; Fernandes, K.V.; Gomes, V.M.; Da Cunha, M. Characterization of stipular colleters of a Alseis pickelii. Botany 2013, 91, 403–413. [Google Scholar] [CrossRef]
- Miguel, E.C.; Da Cunha, M.; Miguel, T.B.A.R.; Barros, C.F. Ontogenesis secretion and senescence of Tocoyena bullata (V ell.) M art. (Rubiaceae) colleters. Plant Biol. 2016, 18, 851–858. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, J.C.; Tölke, E.D.; Demarco, D. Secretory patterns in colleters of Apocynaceae. Plants 2021, 10, 2770. [Google Scholar] [CrossRef] [PubMed]
- Silva, M.D.S.; Coutinho, Í.A.C.; Araújo, M.N.; Meira, R.M.S.A. Colleters in Chamaecrista (L.) Moench sect. Chamaecrista and sect. Caliciopsis (Leguminosae-Caesalpinioideae): Anatomy and taxonomic implications. Acta Bot. Bras. 2017, 31, 382–391. [Google Scholar]
- Almeida, A.L.; Paiva, E.A.S. Colleters in Mabea fistulifera Mart. (Euphorbiaceae): Anatomy and biology of the secretory process. Flora 2019, 258, 151439. [Google Scholar] [CrossRef]
- Cassola, F.; Nunes, C.E.P.; Lusa, M.G.; Garcia, V.L.; Mayer, J.L.S. Deep in the jelly: Histochemical and functional aspects of mucilage-secreting floral colleters in the orchids Elleanthus brasiliensis and E. crinipes. Front. Plant Sci. 2019, 10, 518. [Google Scholar] [CrossRef]
- Pinheiro, S.K.d.P.; Teófilo, F.B.S.; Lima, A.K.M.; Cordoba, B.V.; Miguel, T.B.A.R.; Miguel, E.d.C. Ontogenesis and secretion mechanism of Morinda citrifolia L. (Rubiaceae) colleters. S. Afr. J. Bot. 2019, 121, 26–33. [Google Scholar] [CrossRef]
- Thomas, V.; Dave, Y.; Menon, A.R.S. Anatomy and histochemistry of colleters in Roupelia grata Wall. (Apocynaceae). Nord. J. Bot. 1989, 8, 493–496. [Google Scholar] [CrossRef]
- Klein, D.E.; Gomes, V.M.; Silva-Neto, S.J.; Da Cunha, M. The structure of colleters in several species of Simira (Rubiaceae). Ann. Bot. 2004, 94, 733–740. [Google Scholar] [CrossRef] [PubMed]
- Tullii, C.F.; Alexandrino, C.R.; da Silva, N.D.; Olivares, F.L.; Zottich, U.; Gomes, V.M.; Da Cunha, M. Identifying bioactive compounds and beneficial microorganisms associated with colleters in Palicourea tetraphylla (Rubiaceae). S. Afr. J. Bot. 2023, 160, 328–337. [Google Scholar] [CrossRef]
- Tresmondi, F.; Canaveze, Y.; Guimarães, E.; Machado, S.R. Colleters in Rubiaceae from forest and savanna: The link between secretion and environment. Sci. Nat. 2017, 104, 17. [Google Scholar] [CrossRef] [PubMed]
- Costa, I.S.; Lucena, E.M.; Bonilla, O.H.; Guesdon, I.R.; Coutinho, I.A. Seasonal variation in colleter exudates in Myrcia splendens (Myrtaceae). Aust. J. Bot. 2020, 68, 403–412. [Google Scholar] [CrossRef]
- Robbrecht, E. Tropical Woody Rubiaceae. Characteristic Features and Progressions. Contributions to a New Subfamilial Classification; Opera Botanica Belgica; National Botanica Garden of Belgium: Meise, Belgium, 1988; p. 271. [Google Scholar]
- Romero, M.F.; Salas, R.; Gonzalez, A. Colleters in Cephalanthus (Rubiaceae) and reevaluation of occurrence of corolline colleters in Angiosperms. An. Acad. Bras. Cienc. 2022, 94, e20201975. [Google Scholar] [CrossRef] [PubMed]
- Robbrecht, E. The african genus Tricalysia A. Rich. (Rubiaceae). 4. A Revision of the species of section Tricalysia and section Rosea. Bull. Natl. Plantentuin Belg. 1987, 57, 39–208. [Google Scholar] [CrossRef]
- Dave, Y.; Kuriachen, P.M.; Thomas, V. Development, structure and senescence of colleters in Gardenia lucida Roxb. (Rubiaceae). Acta Soc. Bot. Pol. 1988, 57, 3–7. [Google Scholar] [CrossRef]
- Miguel, E.C.; Klein, D.E.; De Oliveira, M.A.; Da Cunha, M. Ultrastructure of secretory and senescence phase in colleters of Bathysa gymnocarpa K. Schum. and B. stipulata (Vell.) C. Presl. (Rubiaceae). Braz. J. Bot. 2010, 33, 455–466. [Google Scholar] [CrossRef]
- Faria, D.N.S.; Fernandes, V.F.; Marquete, R.; Meira, R.M.S.A. Morphology, anatomy, and exudates of stipular colleters in Casearia Jacq. (Salicaceae) across two tropical plant communities. Int. J. Plant Sci. 2019, 180, 141–152. [Google Scholar] [CrossRef]
- Moraes, R.R.; Lima, H.R.P.; Alexandrino, C.R.; Da Cunha, M. Structural and histochemical foliar traits assessing taxonomy of Rubiaceae species occurring in the Brazilian Atlantic Forest. Flora 2020, 268, 151625. [Google Scholar] [CrossRef]
- Berger, A.; Valant-Vetschera, K.; Schinnerl, J.; Brecker, L. Alkaloid diversification in the genus Palicourea (Rubiaceae: Palicoureeae) viewed from a (retro-) biogenetic perspective. Phytochem. Rev. 2022, 21, 915–939. [Google Scholar] [CrossRef]
- Taylor, C. Palicourea in Lista de Espécies da Flora do Brasil. Jardim Botânico do Rio de Janeiro. 2015. Available online: http://floradobrasil2015.jbrj.gov.br/FB14143 (accessed on 15 January 2023).
- Lersten, N.R. Morphology and distribution of colleters and crystals in relation to the taxonomy and bacterial leaf nodules in Psychotria (Rubiaceae). Am. J. Bot. 1974, 61, 973–981. [Google Scholar] [CrossRef]
- Da Cunha, M.; Vieira, R.C. Anatomia foliar de Psychotria vellosiana Benth. (Rubiaceae). Rodriguésia 1997, 49, 39–50. [Google Scholar]
- Thomas, V.; Dave, Y. Structure, origin, development and senescence of colleters in Nerium indicum Mill. (N. odorum Soland., Apocynaceae). Korean J. Bot. 1989, 32, 163–172. [Google Scholar]
- Vitarelli, N.C.; Santos, M. Anatomia de estípulas e coléteres de Psychotria carthagenensis Jacq. (Rubiaceae). Acta Bot. Bras. 2009, 23, 923–928. [Google Scholar] [CrossRef]
- Judkevich, M.D.; Salas, R.M.; Gonzalez, A.M. Colleters in american Spermacoceae genera (Rubiaceae): Morphoanatomical and evolutionary aspects. Int. J. Plant Sci. 2017, 178, 378–397. [Google Scholar] [CrossRef]
- Oliveira, P.H.F.; dos Santos, A.F.; Ramos, C.H.; de Souza, L.A. On the Floral Structure and Colleters and Their Bearing on the Systematics and Evolution in some species of Rubiaceae. RECIMA21 2023, 4, e412612. [Google Scholar]
- do Rio, M.C.S.; Castro, M.d.M.; Kinoshita, L.S. Distribuição e caracterização anatômica dos coléteres foliares de Prestonia coliata (Vell.) Woodson (Apocynaceae). Braz. J. Bot. 2002, 25, 339–349. [Google Scholar] [CrossRef]
- Paiva, É.A.S.; Rodrigues Machado, S. Ontogenesis, structure and ultrastructure of Hymenaea stigonocarpa (Fabaceae: Caesalpinioideae) colleters. Rev. Biol. Trop. 2006, 54, 943–950. [Google Scholar] [CrossRef]
- Miguel, E.C.; Moraes, D.G.; Da Cunha, M. Stipular colleters in Psychotria nuda (Cham. and Schltdl.) Wawra (Rubiaceae): Micromorphology, anatomy and crystals microanalysis. Acta Bot. Bras. 2009, 23, 1034–1039. [Google Scholar] [CrossRef]
- Muravnik, L.E.; Kostina, O.V.; Shavarda, A.L. Development, structure and secretion compounds of stipule colleters in Pentas lanceolata (Rubiaceae). S. Afr. J. Bot. 2014, 93, 27–36. [Google Scholar] [CrossRef]
- Fernandes, V.F.; Thadeo, M.; Dalvi, V.C.; Marquete, R.; Meira, R.M. Colleters in Casearia (Salicaceae): A new interpretation for the theoid teeth. Bot. J. Linn. Soc. 2016, 181, 682–691. [Google Scholar] [CrossRef]
- Paiva, É.A.S. Do calcium oxalate crystals protect against herbivory? Sci. Nat. 2021, 108, 24. [Google Scholar] [CrossRef]
- Kumachova, T.K.; Babosha, A.V.; Ryabchenko, A.S.; Voronkov, A.S. Colleters in leaves of Mespilus germanica L. (Rosaceae): Micromorphology, histochemistry and fluorescence. Micron 2023, 175, 103537. [Google Scholar] [CrossRef]
- Mohan, J.S.S.; Inamdar, J.R. Ultrastructure and secretion pf extrafloral nectarines of Plumeria rubra L. Ann. Bot. 1986, 57, 389–401. [Google Scholar] [CrossRef]
- Paiva, E.A.S. Occurrence, structure and functional aspects of the colleters of Copaifera langsdorffii Desf. (Fabaceae, Caesalpinioideae). C. R. Biol. 2009, 332, 1078–1084. [Google Scholar] [CrossRef]
- Paiva, E.A.S. Colleters in Cariniana estrellensis (Lecythidaceae): Structure, secretion and evidence for young leaf protection. J. Torrey Bot. Soc. 2012, 139, 1–8. [Google Scholar] [CrossRef]
- Miguel, E.C.; de Brito Campolina Marques, J.; de Paiva Pinheiro, S.K.; Alexandrino, C.R.; Miguel, T.B.d.A.R.; Da Cunha, M. Ontogeny, Secretory Process and Senescence of Colleters of Cnidoscolus pubescens (Euphorbiaceae). Trop. Plant Biol. 2023, 16, 135–145. [Google Scholar] [CrossRef]
- Pereira-Silva, C.G.; Ballego-Campos, I.; Sakuragui, C.M.; Gonçalves, E.G.; Paiva, E.A.S. First Report of Colleters in Araceae: A Case Study in Anthurium andraeanum Reveals Diverse Mucilage Glands Associated with the Developing Shoot. Plants 2023, 12, 2912. [Google Scholar] [CrossRef] [PubMed]
- Machado, S.R.; Paleari, L.M.; Paiva, É.A.; Rodrigues, T.M. Colleters on the inflorescence axis of Croton glandulosus (Euphorbiaceae): Structural and functional characterization. Int. J. Plant Sci. 2015, 176, 86–93. [Google Scholar] [CrossRef]
- Vieira, F.A.; Cunha, M.D.; Klein, D.E.; Carvalho, A.D.O.; Gomes, V.M. Purification and characterization of beta-1, 3-glucanase from the secretion of Simira glaziovii colleters (Rubiaceae). Braz. Arch. Biol. Technol. 2006, 49, 881–888. [Google Scholar] [CrossRef]
- Barreiro, D.P.; Machado, S.R. Dendroid colleters of Alibertia sessilis (Vell.) K. Schum., a non-nodulated Rubiaceae species. Braz. J. Bot. 2007, 30, 387–399. [Google Scholar] [CrossRef]
- Mercadante-Simões, M.O.; Paiva, E.A.S. Leaf colleters in Tontelea micrantha (Celastraceae, Salacioideae): Ecological, morphological and structural aspects. C. R. Biol. 2013, 336, 400–406. [Google Scholar] [CrossRef]
- Gagliardi, K.B.; Cordeiro, I.; Demarco, D. Protection and attraction: Bracts and secretory structures in reduced inflorescences of Malpighiales. Flora 2016, 220, 52–62. [Google Scholar] [CrossRef]
- Appezzato-da-Glória, B.; Estelita, M.E.M. Development, structure and distribution of colleters in Mandevilla illustris and M. Velutina (Apocynaceae). Braz. J. Bot. 2000, 23, 113–120. [Google Scholar] [CrossRef]
- Schwarz, E.A.; Furlan, A. Coléteres foliares de Oxypetalum R. Br. (Asclepiadoideae, Apocynaceae)—Aspectos ultraestruturais e anatômicos úteis à taxonomia das espécies do Paraná (Brasil). Acta Biol. Parana. 2002, 31, 79–97. [Google Scholar] [CrossRef]
- Demarco, D. Estruturas Secretoras Florais e Coléteres Foliares em Espécies de Cerrado de Aspidosperma Mart. E Blepharodon Decne (Apocynaceae s.l.). Master’s Thesis, Universidade Estadual de Campinas, Campinas, Brazil, 2005. [Google Scholar]
- Fahn, A. Plant Anatomy; Pergamon Press: Oxford, UK, 1990. [Google Scholar]
- Dexheimer, J.; Guenin, F. Étude des modalités de la sécrétion de mucilage par les trichomes stipulaires de Psychotria bacteriophila (Rubiacees). Cytologia 1981, 46, 731–747. [Google Scholar] [CrossRef]
- Brasil. Lei No 9.985 de 18 de julho de 2000. Institui o Sistema Nacional de Unidades de Conservação da Natureza e dá outras providências. Publicado no Diário Oficial da União em 19/07/2000. 2000. Available online: https://legis.senado.leg.br/norma/551861/publicacao/15716150 (accessed on 23 May 2023).
- Chamberlain, C.J. Methods in Plant Histology, 5th ed.; The University of Chicago Press: Chicago, IL, USA, 1932; 416p. [Google Scholar]
- O’Brien, T.P.; Feder, N.; McCully, M.E. Polychromatic staining of plant cell walls by toluidine blue O. Protoplasma 1964, 59, 368–373. [Google Scholar] [CrossRef]
- Pearse, A.G.E. Histochemistry, Theoretical and Applied: Preparative and Optical Technology, 4th ed.; Churchill Livingston: Edinburgh, UK, 1980. [Google Scholar]
- David, R.; Carde, J.P. Coloration différentielle dês inclusions lipidique et terpeniques dês pseudophylles du Pin maritime au moyen du reactif Nadi. Comptes Rendus Hebd. Séances L‘Académie Sci. 1964, 258, 1338–1340. [Google Scholar]
- Fischer, D.B. Protein staining of ribboned epon sections for light microscopy. Histochemie 1968, 16, 92–96. [Google Scholar] [CrossRef] [PubMed]
- Johansen, D. Plant Microtechnique; McGraw-Hill Book Company, Inc.: New York, NY, USA, 1940; 52p. [Google Scholar]
- Ganter, P.; Jollés, G. Hitologie Normale at Pathologique; Gauthier: Paris, France, 1970; Volume II. [Google Scholar]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bezerra, L.d.A.; Miguel, E.C.; Alexandrino, C.R.; de Azevedo Rangel Miguel, T.B.; Gomes, V.M.; Da Cunha, M. Unveiling the Anatomical and Functional Attributes of Stipular Colleters in Palicourea tetraphylla Cham. & Schltdl. and Palicourea rudgeoides (Müll. Arg.) Standl. (Rubiaceae). Plants 2024, 13, 1206. https://doi.org/10.3390/plants13091206
Bezerra LdA, Miguel EC, Alexandrino CR, de Azevedo Rangel Miguel TB, Gomes VM, Da Cunha M. Unveiling the Anatomical and Functional Attributes of Stipular Colleters in Palicourea tetraphylla Cham. & Schltdl. and Palicourea rudgeoides (Müll. Arg.) Standl. (Rubiaceae). Plants. 2024; 13(9):1206. https://doi.org/10.3390/plants13091206
Chicago/Turabian StyleBezerra, Laís de Almeida, Emilio Castro Miguel, Camilla Ribeiro Alexandrino, Thaiz Batista de Azevedo Rangel Miguel, Valdirene Moreira Gomes, and Maura Da Cunha. 2024. "Unveiling the Anatomical and Functional Attributes of Stipular Colleters in Palicourea tetraphylla Cham. & Schltdl. and Palicourea rudgeoides (Müll. Arg.) Standl. (Rubiaceae)" Plants 13, no. 9: 1206. https://doi.org/10.3390/plants13091206