Understanding of Leaf Development—the Science of Complexity
Abstract
:1. Introduction
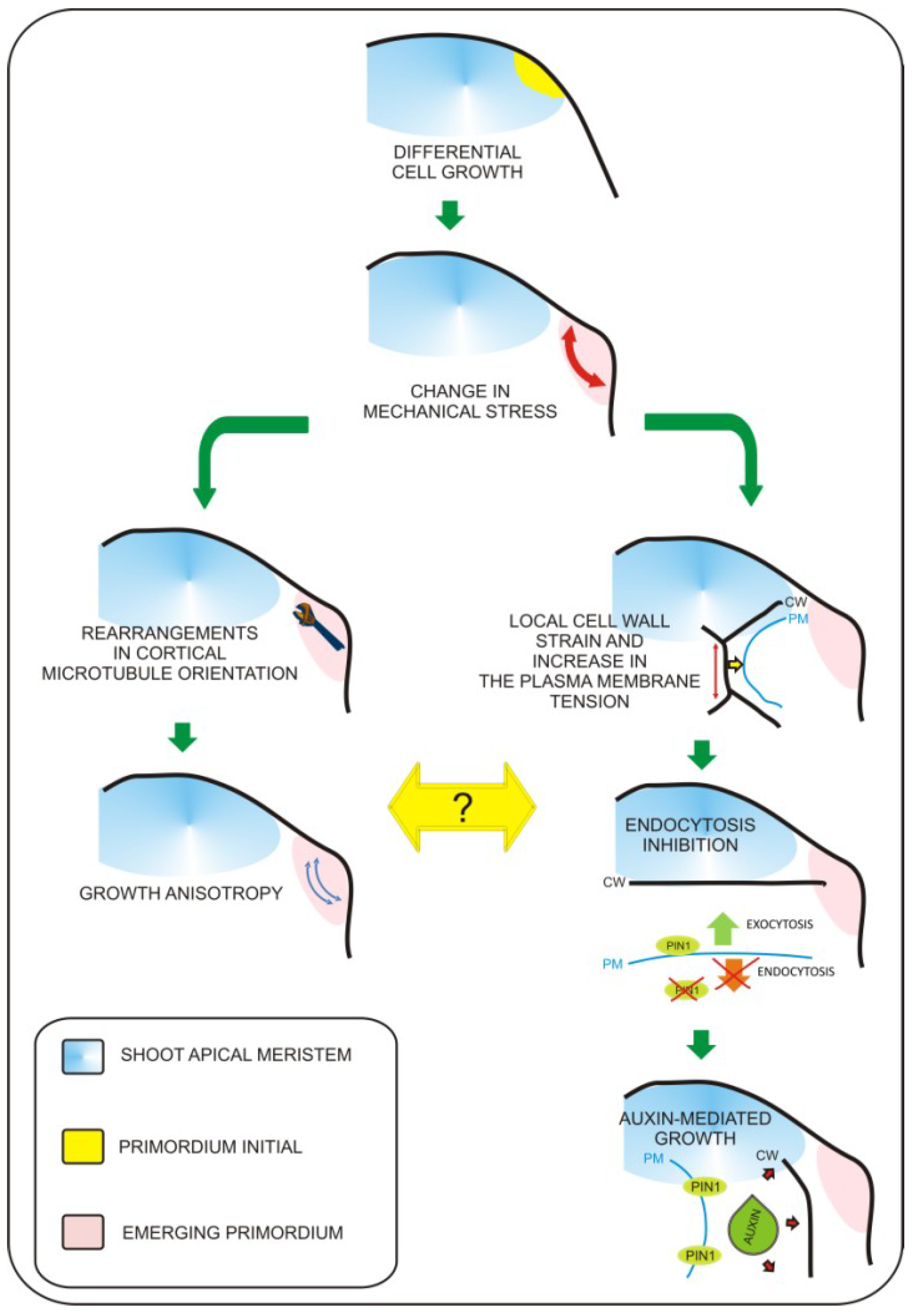
2. Leaf Primordium Formation—The Biomechanical Perspective
3. Light Directed Leaf Morphogenesis—The Cellular Response
4. Epidermis and Mechanical Regulation of Turgor-Driven Leaf Expansion
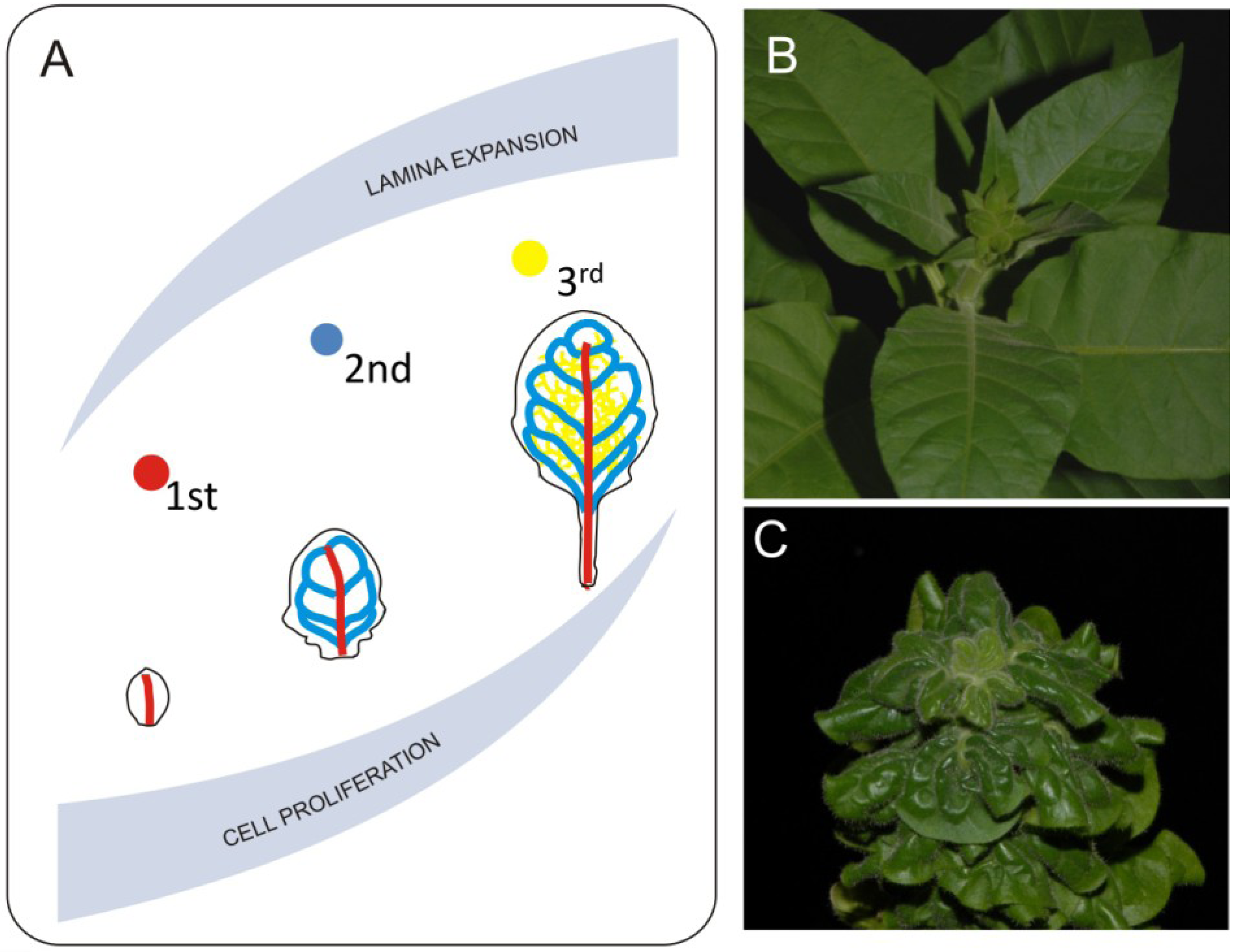
5. Proliferation/Differentiation Balance within Leaf Epidermis
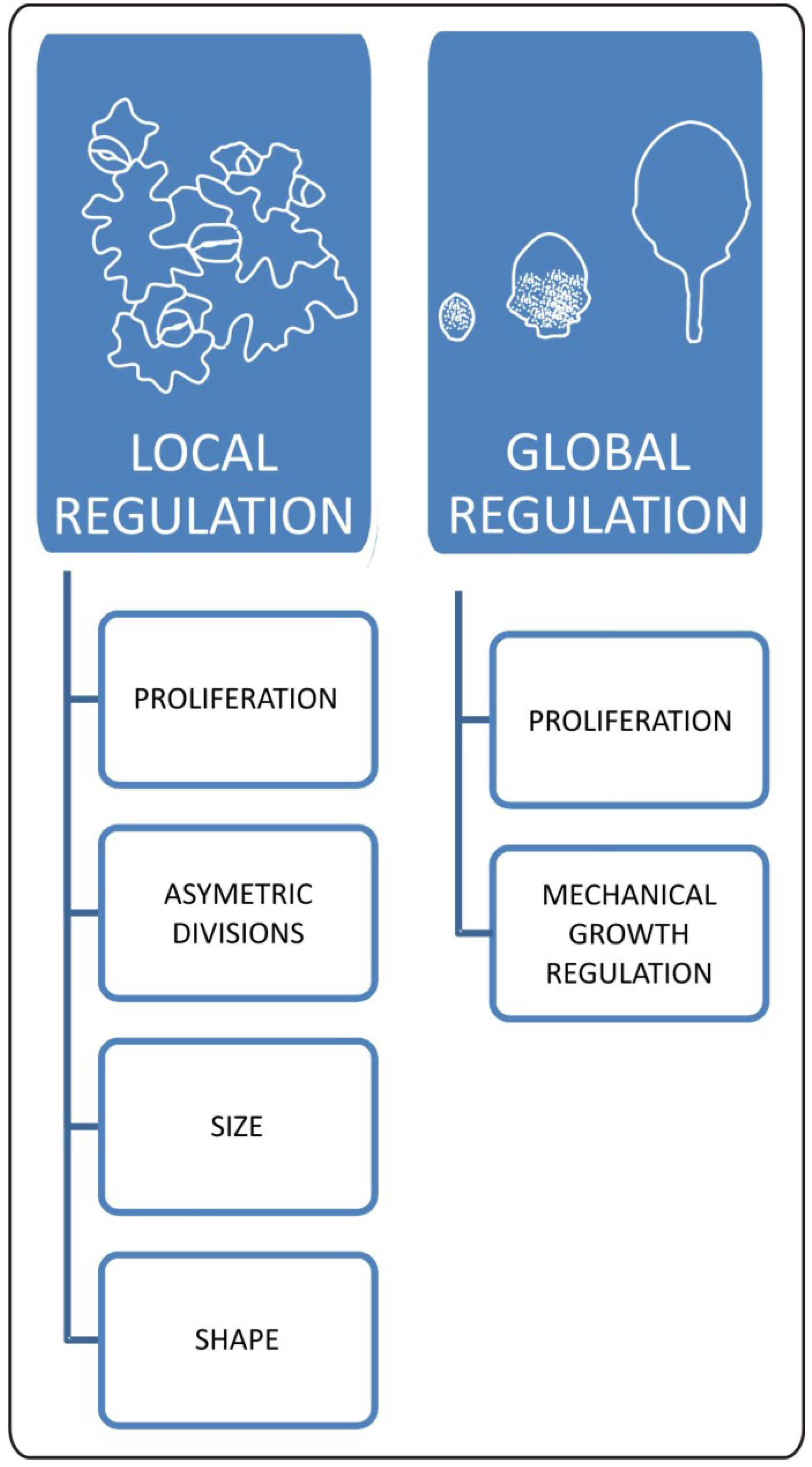
6. Integrating Cellular Balance with General Patterns of Leaf Development
7. Effects of Vasculature on Leaf Expansion and Final Form Acquisition
8. Gradients of Growth and Leaf Shape Formation
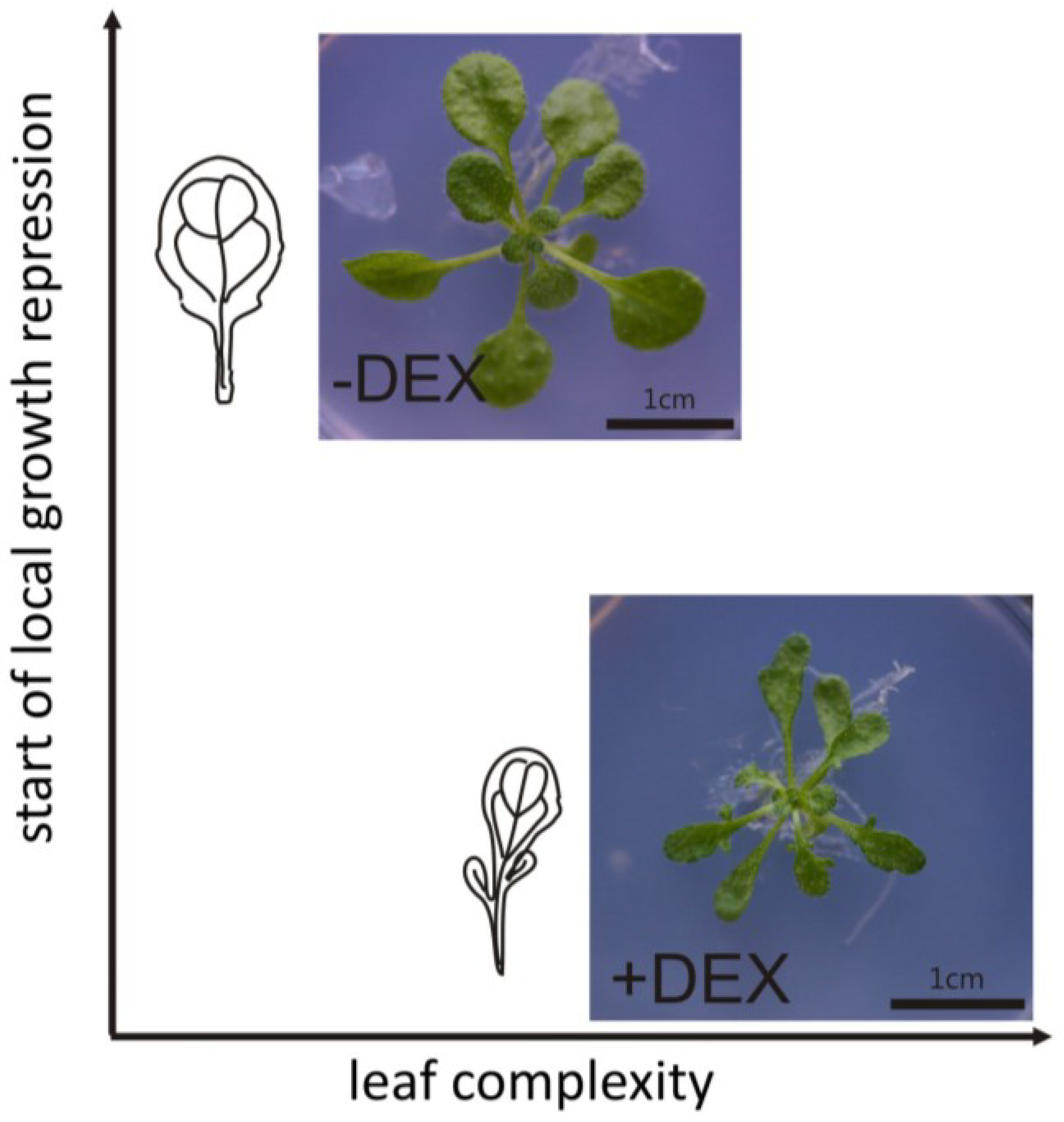
9. Concluding Remarks
Acknowledgments
Conflict of Interest
References
- Beerling, D. The Emerald Planet: How Plants Changed Earth’s History; Oxford University Press: New York, NY, USA, 2007. [Google Scholar]
- Boudaoud, A. An introduction to the mechanics of morphogenesis for plant biologists. Trends Plant Sci. 2010, 15, 353–360. [Google Scholar] [CrossRef]
- Gonzalez, N.; Vanhaeren, H.; Inzé, D. Leaf size control: Complex coordination of cell division and expansion. Trends Plant Sci. 2012, 17, 332–340. [Google Scholar] [CrossRef]
- Efroni, I.; Yuval, E.; Lifschitz, E. Morphogenesis of simple and compound leaves: A critical review. Plant Cell 2010, 22, 1019–1032. [Google Scholar] [CrossRef]
- Nakayama, N.; Kuhlemeier, C. Leaf development: Untangling the spirals. Curr. Biol. 2009, 2, R71–R74. [Google Scholar] [CrossRef]
- Byrne, M.E. Making leaf. Curr. Opin. Plant Biol. 2012, 15, 24–30. [Google Scholar] [CrossRef]
- Fleming, A.; McQueen-Mason, S.; Mandel, T.; Kuhlemeier, C. Induction of leaf primordia by the cell wall protein expansin. Science 1997, 276, 1415–1418. [Google Scholar] [CrossRef]
- Pien, S.; Wyrzykowska, J.; McQueen-Mason, S.; Smart, C.; Fleming, A. Local expression of expansin induces the entire process of leaf development and modifies leaf shape. Proc. Natl. Acad. Sci. USA 2001, 98, 11812–11817. [Google Scholar] [CrossRef]
- Peaucelle, A.; Louvet, R.; Johansen, J.N.; Höfte, H.; Laufs, P.; Pelloux, J.; Mouille, G. Arabidopsis phyllotaxis is controlled by the methyl-esterification status of cell-wall pectins. Curr. Biol. 2008, 18, 1943–1948. [Google Scholar] [CrossRef]
- Hamant, O.; Heisler, M.G.; Jonsson, H.; Krupinski, P.; Uyttewaal, M.; Bokov, P.; Corson, F.; Sahlin, P.; Boudaoud, A.; Meyerowitz, E.M.; et al. Developmental patterning by mechanical signals in Arabidopsis. Science 2008, 322, 1650–1655. [Google Scholar] [CrossRef]
- Heisler, M.G.; Hamant, O.; Krupinski, P.; Uyttewaal, M.; Ohno, C.; Jönsson, H.; Traas, J.; Meyerowitz, E.M. Alignment between PIN1 polarity and microtubule orientation in the shoot apical meristem reveals a tight coupling between morphogenesis and auxin transport. PLoS Biol. 2010, 8, e1000516. [Google Scholar] [CrossRef]
- Kierzkowski, D.; Nakayama, N.; Routier-Kierzkowska, A.-L.; Weber, A.; Bayer, E.; Schorderet, M.; Reinhardt, D.; Kuhlemeier, C.; Smith, R.S. Elastic domains regulate growth and organogenesis in the plant shoot apical meristem. Science 2012, 335, 1096–1099. [Google Scholar] [CrossRef]
- Peaucelle, A.; Braybrook, S.A.; Le Guillou, L.; Bron, E.; Kuhlemeier, C.; Hofte, H. Pectin-induced changes in cell wall mechanics underlie organ initiation in Arabidopsis. Curr. Biol. 2011, 21, 1720–1726. [Google Scholar] [CrossRef]
- Uyttewaal, M.; Burian, A.; Alim, K.; Landrein, B.; Borowska-Wykret, D.; Dedieu, A.; Peaucelle, A.; Ludynia, M.; Traas, J.; Boudaoud, A.; et al. Mechanical stress acts via katanin to amplify differences in growth rate between adjacent cells in Arabidopsis. Cell 2012, 149, 439–451. [Google Scholar] [CrossRef]
- Nakayama, N.; Smith, R.S.; Mandel, T.; Robinson, S.; Kimoura, S.; Boudaoud, A.; Kuhlemeier, C. Mechanical regulation of auxin-mediated growth. Curr. Biol. 2012, 22, 1468–1476. [Google Scholar] [CrossRef]
- Pien, S.; Wyrzykowska, J.; Fleming, A.J. Novel marker genes for early leaf development indicate spatial regulation of carbohydrate metabolism within the apical meristem. Plant J. 2001, 25, 663–674. [Google Scholar]
- Yoshida, S.; Mandel, T.; Kuhlemeier, C. Stem cell activation by light guides plant organogenesis. Genes Dev. 2011, 25, 1439–1450. [Google Scholar] [CrossRef]
- Reddy, G.V.; Heisler, M.G.; Ehrhardt, D.W.; Meyerowitz, E.M. Real-time lineage analysis reveals oriented cell divisions associated with morphogenesis at the shoot apex of Arabidopsis thaliana. Development 2004, 131, 4225–4237. [Google Scholar] [CrossRef]
- Wyrzykowska, J.; Schorderet, M.; Pien, S.; Gruissem, W.; Fleming, A.J. Induction of differentiation in the shoot apical meristem by transient overexpression of a retinoblastoma-related protein. Plant Physiol. 2006, 141, 1338–1348. [Google Scholar] [CrossRef]
- Borghi, L.; Gutzat, R.; Fütterer, J.; Laizet, Y.; Hennig, L.; Gruissem, W. Arabidopsis retinoblastoma-related is required for stem cell maintenance, cell differentiation, and lateral organ production. Plant Cell 2010, 22, 1792–1811. [Google Scholar] [CrossRef]
- Wyrzykowska, J.; Pien, S.; Shen, W.H.; Fleming, A.J. Manipulation of leaf shape by modulation of cell division. Development 2002, 129, 957–964. [Google Scholar]
- Savaldi-Goldstein, S.; Peto, C.; Chory, J. The epidermis both drives and restricts plant shoot growth. Nature 2007, 446, 199–202. [Google Scholar] [CrossRef]
- Wang, Z.Y.; Seto, H.; Fujioka, S.; Yoshida, S.; Chory, J. BRI1 is a critical component of a plasma-membrane receptor for plant steroids. Nature 2001, 410, 380–383. [Google Scholar] [CrossRef]
- Clouse, S.D.; Langford, M.; McMorris, T.C. A brassinosteroid-insensitive mutant in Arabidopsis thaliana exhibits multiple defects in growth and development. Plant Physiol. 1986, 111, 671–678. [Google Scholar]
- Bemis, S.M.; Torii, K.U. Autonomy of cell proliferation and developmental programs during Arabidopsis aboveground organ morphogenesis. Dev. Biol. 2007, 304, 367–381. [Google Scholar] [CrossRef]
- Wang, H.; Fowke, L.C.; Crosby, W.L. A plant cyclindependent kinase inhibitor gene. Nature 1997, 386, 451–452. [Google Scholar] [CrossRef]
- Weinl, C.; Marquardt, S.; Kuijt, S.J.H.; Nowack, M.K.; Jakoby, M.J.; Hülskamp, M.; Schnittger, A. Novel functions of plant cyclin-dependent kinase inhibitors, ICK1/KRP1, can act non-cell-autonomously and inhibit entry into mitosis. Plant Cell 2005, 17, 1704–1722. [Google Scholar] [CrossRef]
- De Veylder, L.; Beeckman, T.; Beemster, G.T.S.; Krols, L.; Terras, F.; Landrieu, I.; van der Schueren, E.; Maes, S.; Naudts, M.; Inzé, D.L. Functional analysis of cyclin-dependent kinase inhibitors of Arabidopsis. Plant Cell 2001, 13, 1653–1668. [Google Scholar]
- Kuwabara, A.; Backhaus, A.; Malinowski, R.; Bauch, M.; Hunt, L.; Nagata, T.; Monk, N.; Sanguinetti, G.; Fleming, A. A shift towards smaller cell size via manipulation of cell cycle gene expression acts to smoothen Arabidopsis leaf shape. Plant Physiol. 2011, 156, 2196–2206. [Google Scholar]
- Schnittger, A.; Weinl, C.; Bouyer, D.; Schobinger, U.; Hulskamp, M. Misexpression of the cyclin-dependent kinase inhibitor ICK1/KRP1 in single-celled Arabidopsis trichomes reduces endoreduplication and cell size and induces cell death. Plant Cell 2003, 15, 303–315. [Google Scholar] [CrossRef]
- Dong, J.; Bergmann, D.C. Stomatal Patterning and Development. Curr. Top. Dev. Biol. 2010, 91, 267–297. [Google Scholar] [CrossRef]
- Robinson, S.; de Reuille, P.B.; Chan, J.; Bergmann, D.; Prusinkiewicz, P.; Coen, E. Generation of Spatial Patterns Through Cell Polarity Switching. Science 2011, 333, 1436–1440. [Google Scholar]
- Pillitteri, L.J.; Peterson, K.M.; Horst, R.J.; Torii, K.U. Molecular Profiling of stomatal meristemoids reveals new component of asymmetric cell division and commonalities among stem cell populations in Arabidopsis. Plant Cell 2011, 23, 3260–3275. [Google Scholar]
- Donnelly, P.M.; Bonetta, D.; Tsukaya, H.; Dengler, R.E.; Dengler, N.G. Cell cycling and cell enlargement in developing leaves of Arabidopsis. Dev. Biol. 1999, 215, 407–419. [Google Scholar] [CrossRef]
- Andriankaja, M.; Dhondt, S.; de Bodt, S.; Vanhaeren, H.; Coppens, F.; de Milde, L.; Muhlenbock, P.; Skirycz, A.; Gonzalez, N.; Beemster, G.T.S.; et al. Exit from proliferation during leaf development in Arabidopsis thaliana: A not-so-gradual process. Dev. Cell 2012, 22, 64–78. [Google Scholar]
- Asl, L.K.; Dhondt, S.; Boudolf, V.; Beemster, G.T.S.; Beeckman, T.; Inzé, D.; Govaerts, W.; de Veylder, L. Model-based analysis of Arabidopsis leaf epidermal cells reveals distinct division and expansion patterns for pavement and guard cells. Plant Physiol. 2011, 156, 2172–218. [Google Scholar]
- Elsner, J.; Michalski, M.; Kwiatkowska, D. Spatiotemporal variation of leaf epidermal cell growth: A quantitative analysis of Arabidopsis thaliana wild-type and triple cyclinD3 mutant plants. Ann. Bot. 2012, 109, 897–910. [Google Scholar]
- Ferjani, A.; Horiguchi, G.; Yano, S.; Tsukaya, H. Analysis of leaf development in fugu mutants of Arabidopsis reveals three compensation modes that modulate cell expansion in determinate organs. Plant Physiol. 2007, 144, 988–999. [Google Scholar]
- Dhondt, S.; Coppens, F.; de Winter, F.; Swarup, K.; Merks, R.M.H.; Inze, D.; Bennett, M.J.; Beemster, G.T.S. SHORT-ROOT and SCARECROW regulate leaf growth in Arabidopsis by stimulating S-phase progression of the cell cycle. Plant Physiol. 2010, 154, 1183–1195. [Google Scholar] [CrossRef]
- Lin, L.; Zhong, S.-H.; Cui, X.-F.; Li, J.; He, Z.-H. Characterization of temperature-sensitive mutants reveals a role for receptor-like kinase SCRAMBLED/STRUBBELIG in coordinating cell proliferation and differentiation during Arabidopsis leaf development. Plant J. 2012, 72, 707–720. [Google Scholar]
- Nakata, M.; Matsumoto, N.; Tsugeki, R.; Rikirsch, E.; Laux, T.; Okada, K. Roles of the middle domain-specific WUSCHEL-RELATED HOMEOBOX genes in early development of leaves in Arabidopsis. Plant Cell 2012, 24, 519–535. [Google Scholar]
- Anastasiou, E.; Kenz, S.; Gerstung, M.; MacLean, D.; Timmer, J.; Fleck, C.; Lenhard, M. Control of plant organ size by KLUH/CYP78A5-dependent intercellular signalling. Dev. Cell 2007, 13, 843–856. [Google Scholar] [CrossRef]
- Wuyts, N.; Palauqui, J.C.; Conejero, G.; Verdeil, J.L.; Granier, C.; Massonnetm, C. High-contrast three-dimensional imaging of the Arabidopsis leaf enables the analysis of cell dimensions in the epidermis and mesophyll. Plant Methods 2010, 6, 17. [Google Scholar] [CrossRef]
- Wuyts, N.; Massonnet, C.; Dauzat, M.; Granier, C. Structural assessment of the impact of environmental constraints on Arabidopsis thaliana leaf growth: A 3D approach. Plant Cell Environ. 2012, 35, 1631–1646. [Google Scholar] [CrossRef]
- Scarpella, E.; Barkoulas, M.; Tsiantis, M. Control of leaf and vein development by auxin. Cold Spring Harb. Perspect. Biol. 2010, 2, a001511. [Google Scholar] [CrossRef]
- Dhondt, S.; van Haerenborgh, D.; van Cauwenbergh, C.; Merks, R.M.H.; Philips, W.; Beemster, G.T.S.; Inze, D. Quantitative analysis of venation patterns of Arabidopsis leaves by supervised image analysis. Plant J. 2012, 69, 553–563. [Google Scholar] [CrossRef]
- Malinowski, R.; Kasprzewska, A.; Fleming, A.J. Targetted manipulation of leaf form via local growth repression. Plant J. 2011, 66, 941–952. [Google Scholar] [CrossRef]
- Yang, L.; Conway, S.R.; Poethig, R.S. Vegetative phase change is mediated by a leaf-derived signal that represses the transcription of miR156. Development 2011, 138, 245–249. [Google Scholar] [CrossRef]
- Yang, L.; Wu, G.; Poethig, R.S. Mutations in the GW-repeat protein SUO reveal a developmental function for microRNA-mediated translational repression in Arabidopsis. Proc. Natl. Acad. Sci. USA 2012, 109, 315–320. [Google Scholar] [CrossRef]
- Nakaya, M.; Tsukaya, H.; Murakami, N.; Kato, M. Brassinosteroids control the proliferation of leaf cells in Arabidopsis thaliana. Plant Cell Physiol. 2002, 43, 239–244. [Google Scholar] [CrossRef]
- Malinowski, R.; Higgins, R.; Luo, Y.; Piper, L.; Nazir, A.; Bajwa, V.S.; Clouse, S.D.; Thompson, P.R.; Stratmann, J.W. The tomato brassinosteroid receptor BRI1 increases binding of systemin to tobacco plasma membranes, but is not involved in systemin signaling. Plant Mol. Biol. 2009, 70, 603–616. [Google Scholar] [CrossRef]
- Laguna, M.F.; Bohn, S.; Jagla, E.A. The role of elastic stresses on leaf venation morphogenesis. PLoS Comput. Biol. 2008, 4, e1000055. [Google Scholar] [CrossRef]
- Corson, F.; Hamant, O.; Bohn, S.; Traas, J.; Boudaoud, A.; Couder, Y. Turning a plant tissue into a living cell froth through isotropic growth. Proc. Natl. Acad. Sci. USA 2009, 106, 8453–8458. [Google Scholar]
- Scoffoni, C.; Rawls, M.; McKown, A.; Cochard, H.; Sack, L. Decline of leaf hydraulic conductance with dehydration: relationship to leaf size and venation architecture. Plant Physiol. 2011, 156, 832–843. [Google Scholar] [CrossRef]
- Sack, L.; Scoffoni, C.; McKown, A.D.; Frole, K.; Rawls, M.; Havran, J.C.; Tran, H.; Tran, T. Developmentally based scaling of leaf venation architecture explains global ecological patterns. Nat. Commun. 2012, 3, 837. [Google Scholar] [CrossRef]
- Nicotra, A.; Leigh, A.; Boyce, C.; Jones, C.S.; Niklas, C.J.; Royer, D.L.; Tsukaya, H. The evolution and functional significance of leaf shape in the angiosperms. Funct. Plant Biol. 2011, 38, 535–552. [Google Scholar] [CrossRef]
- Blein, T.; Pulido, A.; Vialette-Guiraud, A.; Nikovics, K.; Morin, H.; Hay, A.; Johansen, I.E.; Tsiantis, M.; Laufs, P. A conserved molecular framework for compound leaf development. Science 2008, 322, 1835–1839. [Google Scholar] [CrossRef]
- Bilsborough, G.D.; Runions, A.; Barkoulas, M.; Jenkins, H.W.; Hasson, A.; Galinha, C.; Laufs, P.; Hay, A.; Prusinkiewicz, P.; Tsiantis, M. Model for the regulation of Arabidopsis thaliana leaf margin development. Proc. Natl. Acad. Sci. USA 2011, 108, 3424–3429. [Google Scholar] [CrossRef]
- Kawamura, E.; Horiguchi, G.; Tsukaya, H. Mechanisms of leaf tooth formation in Arabidopsis. Plant J. 2010, 62, 429–441. [Google Scholar] [CrossRef]
- Nath, U.; Crawford, B.C.; Carpenter, R.; Coen, E. Genetic control of surface curvature. Science 2003, 299, 1404–1407. [Google Scholar] [CrossRef]
- Koyama, T.; Mitsuda, N.; Seki, M.; Shinozaki, K.; Ohme-Takagi, M. TCP transcription factors regulate the activities of ASYMMETRIC LEAVES1 and miR164, as well as the auxin response, during differentiation of leaves in Arabidopsis. Plant Cell 2010, 22, 3574–3588. [Google Scholar] [CrossRef]
- Hay, A.; Tsiantis, M. The genetic basis for differences in leaf form between Arabidopsis thaliana and its wild relative Cardamine hirsuta. Nat. Genet. 2006, 38, 942–947. [Google Scholar] [CrossRef]
- Barkoulas, M.; Hay, A.; Kougioumoutzi, E.; Tsiantis, M. A developmental framework for dissected leaf formation in the Arabidopsis relative Cardamine hirsuta. Nat. Genet. 2008, 40, 1136–1141. [Google Scholar] [CrossRef]
- Piazza, P.; Bailey, D.; Cartolano, M.; Krieger, J.; Cao, J.; Ossowski, S.; Schneeberger, K.; He, F.; de Meaux, J.; Hall, M.N.; et al. Arabidopsis thaliana leaf form evolved via loss of knox expression in leaves in association with a selective sweep. Curr. Biol. 2010, 21, 2223–2228. [Google Scholar]
- Shani, E.; Ben-Gera, H.; Shleizer-Burko, S.; Burko, Y.; Weiss, D.; Ori, N. Cytokinin regulates compound leaf development in tomato. Plant Cell 2010, 22, 3206–3217. [Google Scholar] [CrossRef]
- Kaplan, D.R. Fundamental concepts of leaf morphology and morphogenesis: A contribution to the interpretation of molecular genetic mutants. Int. J. Plant Sci. 2001, 162, 465–474. [Google Scholar] [CrossRef]
- Kuchen, E.E.; Fox, S.; Barbierde Reuille, P.; Kennaway, R.; Bensmihen, S.; Avondo, J.; Calder, G.M.; Southam, P.; Robinson, S.; Bangham, A.; et al. Generation of leaf shape through early patterns of growth and tissue polarity. Science 2012, 335, 1092–1096. [Google Scholar] [CrossRef]
- Pajor, R.; Fleming, A.; Osborne, C.P.; Rolfe, S.A.; Sturrock, C.J.; Mooney, S.J. Seeing space: Visualization and quantification of plant leaf structure using X-ray micro-computed tomography. J. Exp. Bot. 2013, 64, 385–390. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Malinowski, R. Understanding of Leaf Development—the Science of Complexity. Plants 2013, 2, 396-415. https://doi.org/10.3390/plants2030396
Malinowski R. Understanding of Leaf Development—the Science of Complexity. Plants. 2013; 2(3):396-415. https://doi.org/10.3390/plants2030396
Chicago/Turabian StyleMalinowski, Robert. 2013. "Understanding of Leaf Development—the Science of Complexity" Plants 2, no. 3: 396-415. https://doi.org/10.3390/plants2030396