Variation among Soybean Cultivars in Mesophyll Conductance and Leaf Water Use Efficiency
Abstract
:1. Introduction
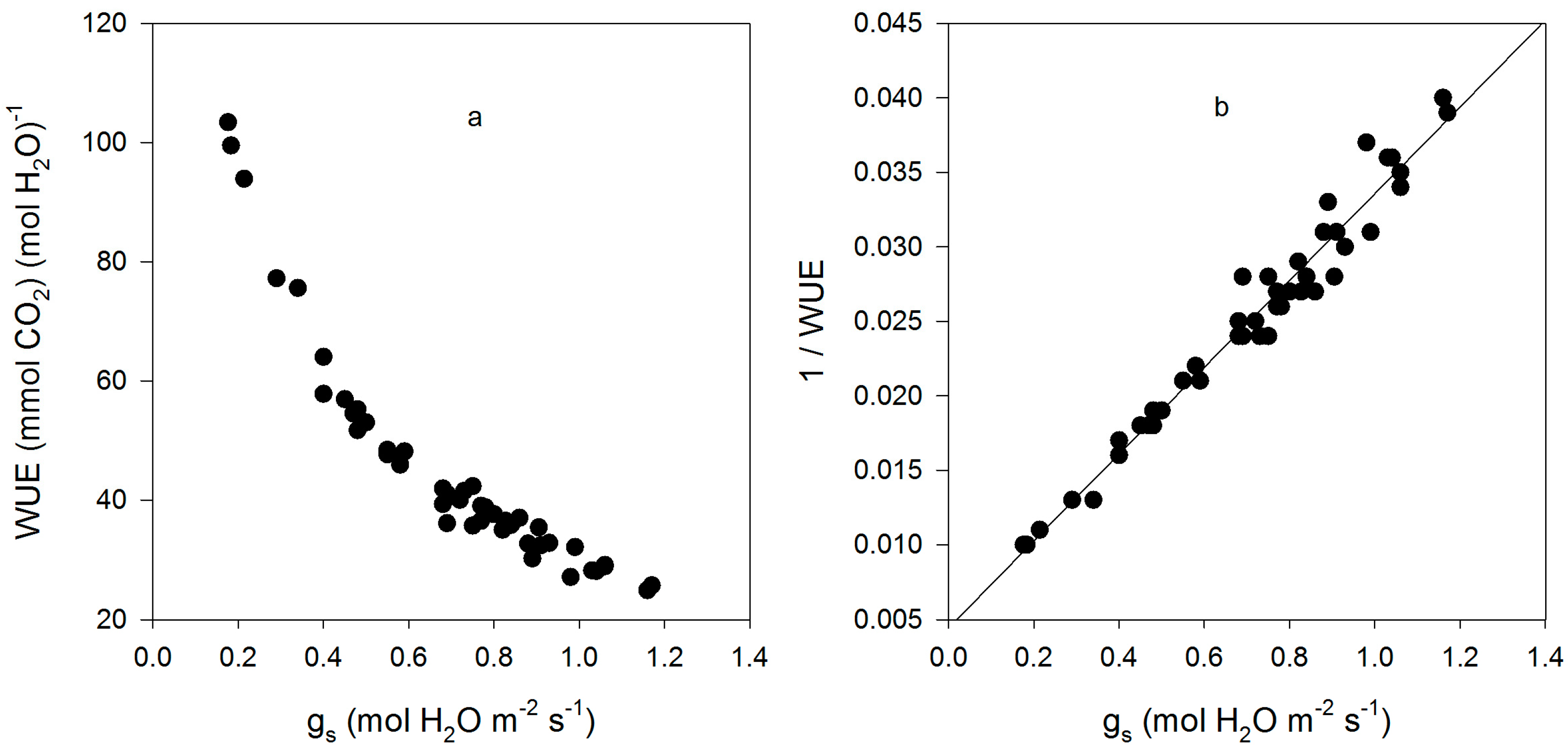
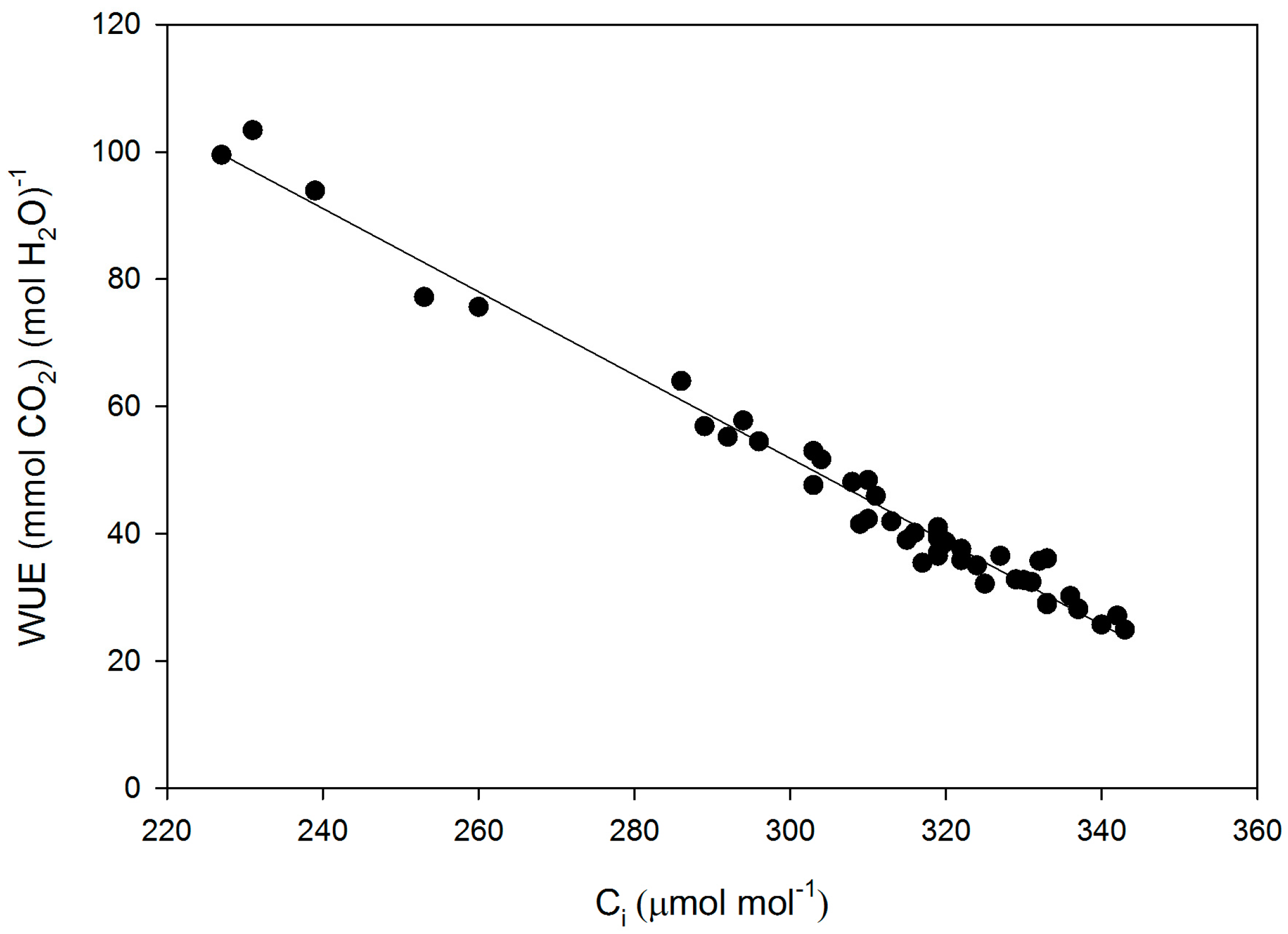
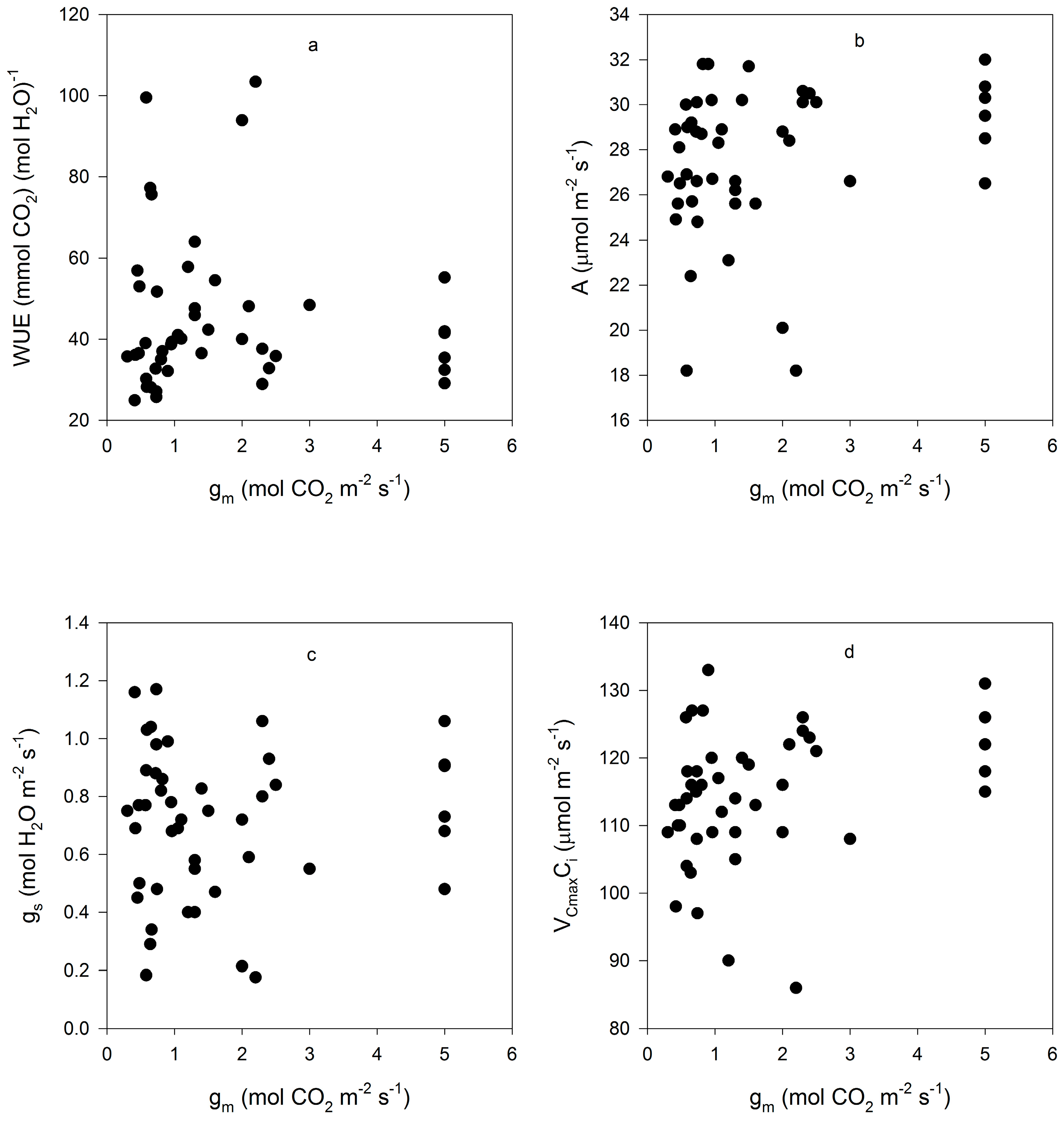
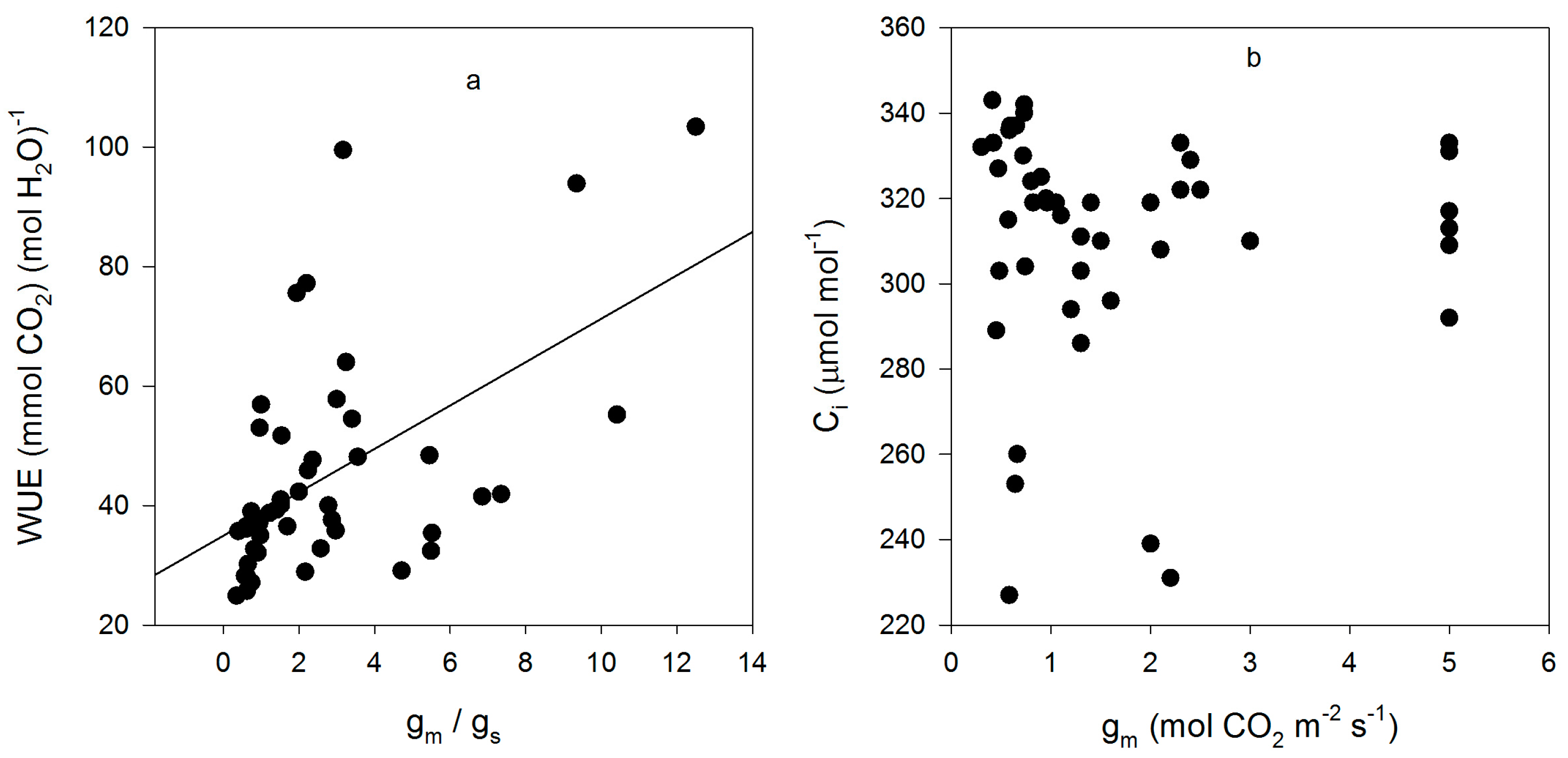
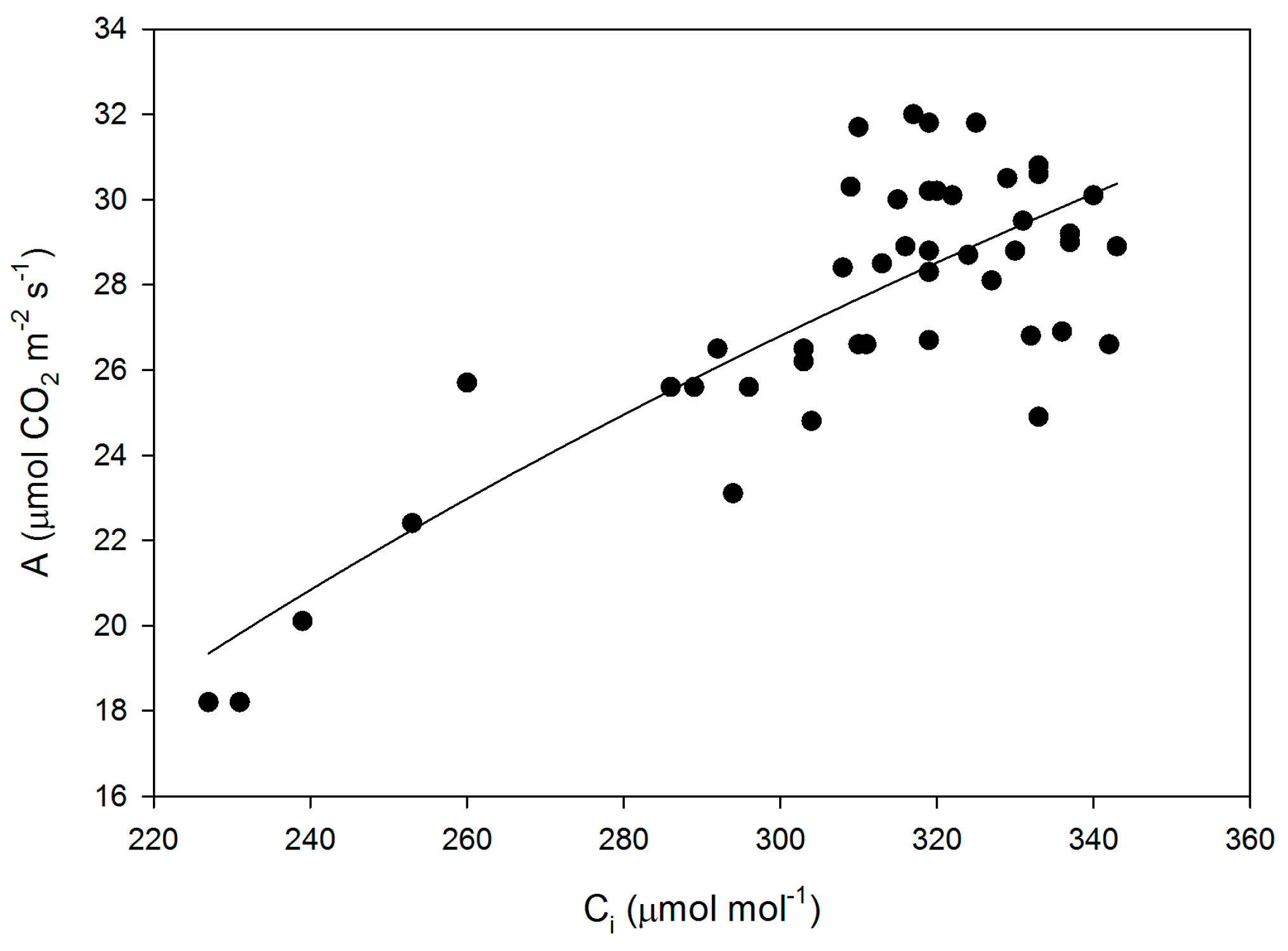
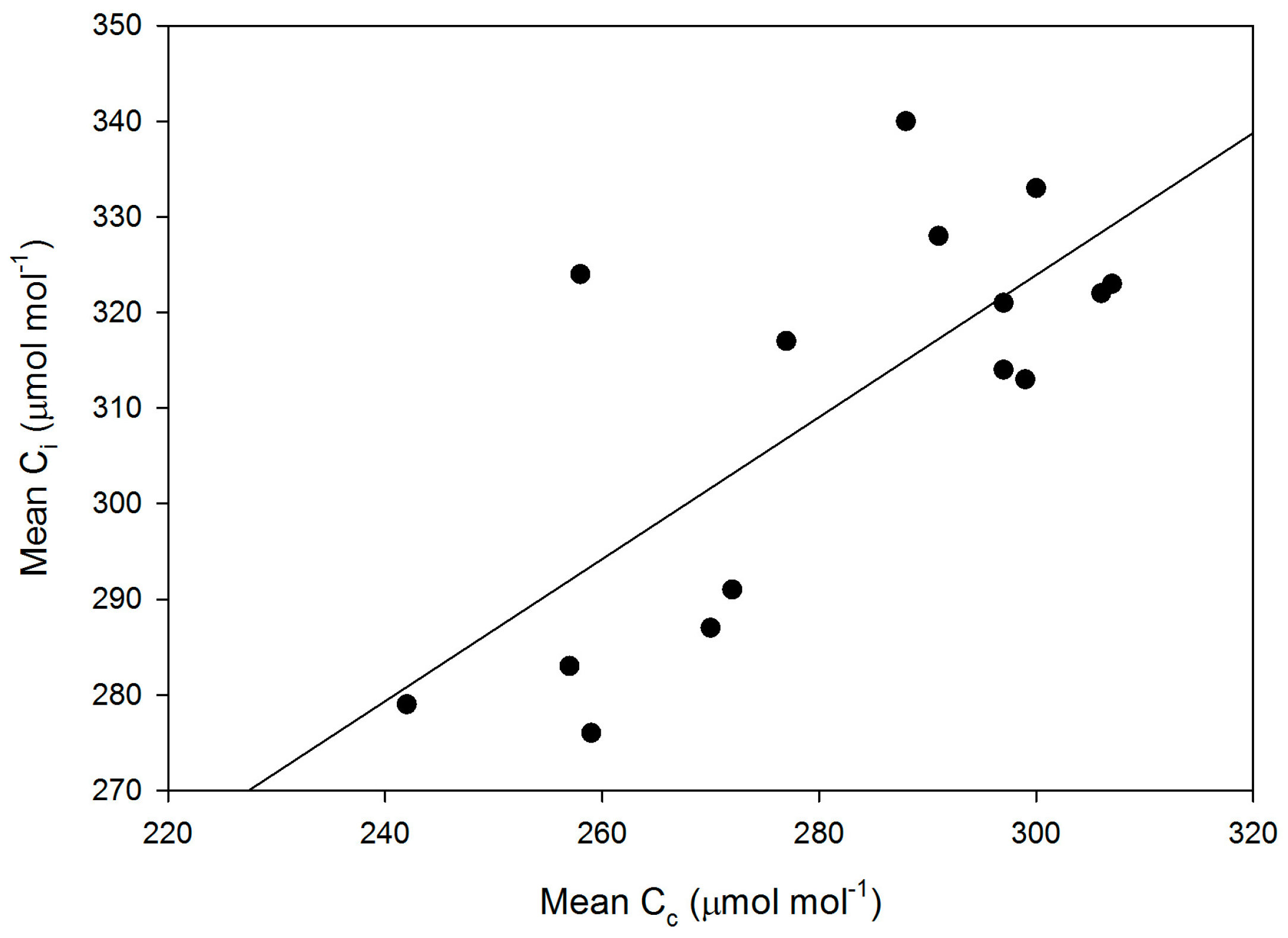
2. Results
3. Discussion
4. Materials and Methods
Conflicts of Interest
References
- Condon, A.G.; Richards, R.A.; Rebetzke, G.L.; Farquhar, G.D. Breeding for high water-use efficiency. J. Exp. Bot. 2004, 55, 2447–2460. [Google Scholar] [CrossRef] [PubMed]
- Seibt, U.; Rajabi, A.; Griffiths, H.; Berry, J.A. Carbon isotopes and water use efficiency: Sense and sensitivity. Oecologia 2008, 155, 441–454. [Google Scholar] [CrossRef] [PubMed]
- Farquhar, G.D.; von Caemmerer, S.; Berry, J.A. A biochemical model of photosynthetic CO2 assimilation in leaves of C3 species. Planta 1980, 149, 78–90. [Google Scholar] [CrossRef] [PubMed]
- Warren, C.R. Stand aside stomata, another actor deserves centre stage: The forgotten role of the internal conductance to CO2 transfer. J. Exp. Bot. 2008, 59, 1475–1487. [Google Scholar] [CrossRef] [PubMed]
- Evans, J.R.; Kaldenhoff, R.; Genty, B.; Terashima, I. Resistances along the CO2 diffusion pathway inside leaves. J. Exp. Bot. 2009, 21, 475–495. [Google Scholar] [CrossRef] [PubMed]
- Barbour, M.M.; Warren, C.R.; Farquhar, G.D.; Forrester, G.; Brown, H. Variability in mesophyll conductance between barley genotypes, and effects on transpiration efficiency and carbon isotype discrimination. Plant Cell Environ. 2010, 33, 1176–1185. [Google Scholar] [PubMed]
- Tomas, M.; Medrano, H.; Brugnoli, E.; Escalona, J.M.; Martorell, S.; Pou, A.; Ribas-Carbo, M.; Flexas, J. Variability of mesophyll conductance in grapevine cultivars under water stress conditions in relation to leaf anatomy and water use efficiency. Aust. J. Grape Wine Res. 2014, 20, 272–280. [Google Scholar] [CrossRef]
- Galmes, J.; Conesa, M.A.; Ochogavia, J.M.; Perdomo, J.A.; Francis, D.M.; Ribas-Carbo, M.; Save, R.; Flexas, J.; Medrano, H.; Cifre, J. Physiological and morphological adaptations in relation to water use efficiency in Mediterranean accessions of Solanum lycopersicum. Plant Cell Environ. 2011, 34, 245–260. [Google Scholar] [CrossRef] [PubMed]
- Jahan, E.; Amthor, J.S.; Farquhar, G.D.; Trethowan, R.; Barbour, M.M. Variation in mesophyll conductance among Australian wheat genotypes. Funct. Plant Biol. 2014, 41, 568–580. [Google Scholar] [CrossRef]
- Gioliani, R.; Koteyeva, N.; Voznesenskaya, E.; Evans, M.A.; Cousins, A.B.; Edwards, G.E. Coordination of leaf photosynthesis, transpiration, and structural traits in rice and wild relatives (genus Oryza). Plant Physiol. 2013, 162, 1632–1651. [Google Scholar] [CrossRef] [PubMed]
- Pons, T.L.; Flexas, J.; von Caemmeer, S.; Evans, J.R.; Genty, B.; Ribas-Carbo, M.; Brugnoli, E. Estimating mesophyll conducgtance to CO2: Methodology, potential errors, and recommendations. J. Exp. Bot. 2009, 60, 2217–2234. [Google Scholar] [CrossRef] [PubMed]
- Tholen, D.; Ethier, G.; Genty, B.; Pepin, S.; Zhu, X.-G. Variable mesophyll conductance revisited: Theoretical background and experimental implications. Plant Cell Environ. 2012, 35, 2087–2103. [Google Scholar] [CrossRef] [PubMed]
- Gu, L.; Sun, Y. Artefactual responses of mesophyll conductance to CO2 and irradiance estimated with the variable J and online isotope discrimination methods. Plant Cell Environ. 2014, 37, 1231–1249. [Google Scholar] [CrossRef] [PubMed]
- Bunce, J.A. Use of the response of photosynthesis to oxygen to estimate mesophyll conductance to carbon dioxide in water-stressed soybean leaves. Plant Cell Environ. 2009, 32, 875–881. [Google Scholar] [CrossRef] [PubMed]
- Blum, A. Effective use of water (EUW) and not water-use efficiency (WUE) is the target of crop yield improvement under drought stress. Field Crops Res. 2009, 112, 119–123. [Google Scholar] [CrossRef]
- Buckley, T.N.; Warren, C.R. The role of mesophyll conductance in the economics of nitrogen and water use in photosynthesis. Photosynth. Res. 2014, 119, 77–88. [Google Scholar] [CrossRef] [PubMed]
- Wilson, K.B.; Carlson, T.N.; Bunce, J.A. Feedback significantly influences the simulated effect of CO2 on seasonal evapotranspiration from two agricultural species. Glob. Chang. Biol. 1999, 5, 903–917. [Google Scholar] [CrossRef]
- Bunce, J.A. Use of a minimally invasive method of measuring leaf stomatal conductance to examine stomatal responses to water vapor pressure under field conditions. Agric. Meteorol. 2006, 139, 335–343. [Google Scholar] [CrossRef]
- Bunce, J.A. Identifying soybean lines differing in gas exchange sensitivity to humidity. Ann. Appl. Biol. 1984, 105, 313–318. [Google Scholar] [CrossRef]
- Fletcher, A.L.; Sinclair, T.R.; Allen, L.H., Jr. Transpiration responses to vapor pressure deficit in well water “slow-wilting” and commercial soybean. Environ. Exp. Bot. 2007, 61, 145–151. [Google Scholar] [CrossRef]
- Gilbert, M.E.; Holbrook, N.M.; Zwieniecki, M.A.; Sadok, W.; Sinclair, T.R. Field confirmation of genetic variation in soybean transpiration response to vapor pressure deficit and photosynthetic compensation. Field Crops Res. 2011, 124, 85–92. [Google Scholar] [CrossRef]
- Singh, S.K.; Reddy, V.R. Methods of mesophyll conductance estimation: Its impact on key biochemical parameters and photosynthetic limitations in phosphorus-stressed soybean across CO2. Physiol. Plant. 2016, 157, 234–254. [Google Scholar] [CrossRef] [PubMed]
- Sharkey, T.D.; Bernacchi, C.J.; Farquhar, G.D.; Singsaas, E.L. Fitting photosynthetic carbon dioxide response curves for C3 leaves. Plant Cell Environ. 2007, 30, 1035–1040. [Google Scholar] [CrossRef] [PubMed]

| Cultivar | gs | gm | A | WUE | VCmCi | VCmCc | Ci | Cc |
|---|---|---|---|---|---|---|---|---|
| A5959 | 703 | 2.63 | 27.7 | 43.1 | 113 | 118 | 313 | 299 |
| Biloxi | 803 | 3.60 | 28.9 | 36.4 | 116 | 123 | 323 | 307 |
| Chief | 878 | 2.28 | 31.1 | 35.9 | 127 | 139 | 321 | 297 |
| Clark | 677 | 0.42 | 26.6 | 40.3 | 108 | 138 | 324 | 258 |
| Essex | 587 | 1.50 | 26.4 | 56.9 | 114 | 121 | 291 | 272 |
| Fiskeby V | 418 | 2.56 | 24.4 | 67.6 | 114 | 122 | 276 | 259 |
| Ford | 1123 | 0.60 | 29.4 | 26.2 | 116 | 138 | 340 | 288 |
| Holt | 502 | 2.05 | 25.0 | 63.5 | 105 | 112 | 287 | 270 |
| Kent | 820 | 0.78 | 28.0 | 34.7 | 115 | 132 | 328 | 291 |
| Lincoln | 725 | 0.77 | 28.4 | 39.1 | 118 | 137 | 317 | 277 |
| Perry | 883 | 2.10 | 30.8 | 35.7 | 121 | 127 | 322 | 306 |
| PI-41 | 430 | 1.09 | 25.8 | 62.0 | 117 | 130 | 283 | 257 |
| Ripley | 380 | 0.76 | 23.7 | 64.0 | 101 | 118 | 279 | 242 |
| Spencer | 728 | 2.68 | 29.0 | 41.6 | 118 | 124 | 314 | 297 |
| Wabash | 910 | 1.11 | 28.1 | 31.8 | 114 | 128 | 333 | 300 |
| Probability | 0.001 | 0.016 | 0.081 | 0.029 | 0.097 | 0.279 | 0.019 | 0.036 |
| HSD | 592 | 2.79 | 31.1 | 56 | 65 |
© 2016 by the author; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bunce, J. Variation among Soybean Cultivars in Mesophyll Conductance and Leaf Water Use Efficiency. Plants 2016, 5, 44. https://doi.org/10.3390/plants5040044
Bunce J. Variation among Soybean Cultivars in Mesophyll Conductance and Leaf Water Use Efficiency. Plants. 2016; 5(4):44. https://doi.org/10.3390/plants5040044
Chicago/Turabian StyleBunce, James. 2016. "Variation among Soybean Cultivars in Mesophyll Conductance and Leaf Water Use Efficiency" Plants 5, no. 4: 44. https://doi.org/10.3390/plants5040044






