Expression of miR159 Is Altered in Tomato Plants Undergoing Drought Stress
Abstract
:1. Introduction
2. Results and Discussion
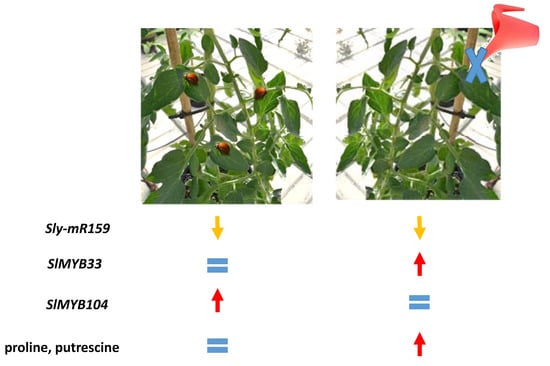
2.1. Expression of miR159 in Tomato Plants Undergoing Drought Stress
2.2. Assessment of sly-miR159 Stress-Specific Targeting of SlMYB33
3. Conclusions
4. Materials and Methods
4.1. Plants
4.2. Total RNA Isolation and RT-qPCR Analysis
4.3. Small RNA Isolation and RT-PCR Analysis
4.4. Amino Acids and Polyamines Quantification
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Swann, A.L.S. Plants and drought in a changing climate. Curr. Clim. Chang. Rep. 2018, 4, 192–201. [Google Scholar] [CrossRef]
- Hossain, M.A.; Wani, S.H.; Bhattacharjee, S.; Burritt, D.J.; Tran, L.-S.P. Drought Stress Tolerance in Plants, 1st ed.; Springer International Publishing: Basel, Switzerland, 2016; Volume 2, ISBN 978-3-319-32421-0. [Google Scholar]
- Cutler, S.R.; Rodriguez, P.R.; Finkelstein, R.R.; Abrams, S.R. Abscisic acid: Emergence of a core signaling network. Annu. Rev. Plant Biol. 2010, 61, 651–679. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Tao, Y.; Zhu, C. Emerging roles of microRNAs in the mediation of drought stress response in plants. J. Exp. Bot. 2013, 64, 3077–3086. [Google Scholar] [CrossRef] [PubMed]
- Reyes, J.L.; Chua, N.-H. ABA induction of miR159 controls transcript levels of two MYB factors during Arabidopsis seed germination. Plant J. 2007, 49, 592–606. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.-H.; Tian, X.; Li, Y.-J.; Wu, C.-A.; Zheng, C.-C. Microarray-based analysis of stress-regulated microRNAs in Arabidopsis thaliana. RNA 2008, 14, 836–843. [Google Scholar] [CrossRef]
- Wei, L.; Zhang, D.; Xiang, F.; Zhang, Z. Differentially expressed miRNAs potentially involved in the regulation of defense mechanism to drought stress in maize seedlings. Int. J. Plant Sci. 2009, 170, 979–989. [Google Scholar] [CrossRef]
- Xie, F.; Wang, Q.; Sun, R.; Zhang, B. Deep sequencing reveals important roles of microRNAs in response to drought and salinity stress in cotton. J. Exp. Bot. 2015, 66, 789–804. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, N.; Mi, X.; Wu, L.; Ma, R.; Zhu, X.; Yao, L.; Jin, X.; Si, H.; Wang, D. Identification of miR159s and their target genes and expression analysis under drought stress in potato. Comput. Biol. Chem. 2014, 53, 204–213. [Google Scholar] [CrossRef]
- Hackenberg, M.; Gustafson, P.; Langridge, P.; Shi, B.-J. Differential expression of microRNAs and other small RNAs in barley between water and drought conditions. Plant Biotechnol. J. 2015, 13, 2–13. [Google Scholar] [CrossRef]
- Li, Y.; Wan, L.; Bi, S.; Wan, X.; Li, Z.; Cao, J.; Tong, Z.; Xu, H.; He, F.; Li, X. Identification of drought-responsive microRNAs from roots and leaves of alfalfa by high-throughput sequencing. Genes 2017, 8, 119. [Google Scholar] [CrossRef]
- Pegler, J.L.; Grof, C.P.L.; Eamens, A.L. Profiling of the differential abundance of drought and salt stress-responsive microRNAs across grass crop and genetic model plant species. Agronomy 2018, 8, 118. [Google Scholar] [CrossRef]
- López-Galiano, M.J.; González-Hernández, A.I.; Crespo-Salvador, O.; Rausell, C.; Real, M.D.; Escamilla, M.; Camañes, G.; García-Agustín, P.; González-Bosch, C.; García-Robles, I. Epigenetic regulation of the expression of WRKY75 transcription factor in response to biotic and abiotic stresses in Solanaceae plants. Plant Cell Rep. 2018, 37, 167–176. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Yu, H.; Zhao, G.; Huang, Q.; Lu, Y.; Ouyang, B. Profiling of drought-responsive microRNA and mRNA in tomato using high-throughput sequencing. BMC Genom. 2017, 18, 481. [Google Scholar] [CrossRef]
- Liu, M.; Yu, H.; Zhao, G.; Huang, Q.; Lu, Y.; Ouyang, B. Identification of drought-responsive microRNAs in tomato using high-throughput sequencing. Funct. Integr. Genom. 2018, 18, 67–78. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B. MicroRNA: A new target for improving plant tolerance to abiotic stress. J. Exp. Bot. 2015, 66, 1749–1761. [Google Scholar] [CrossRef]
- Allen, R.S.; Li, J.; Stahle, M.I.; Dubroué, A.; Gubler, F.; Millar, A.A. Genetic analysis reveals functional redundancy and the major target genes of the Arabidopsis miR159 family. Proc. Natl. Acad. Sci. USA 2007, 104, 16371–16376. [Google Scholar] [CrossRef]
- Woodger, F.J.; Millar, A.; Murray, F.; Jacobsen, J.V.; Gubler, F. The role of GAMYB transcription factors in GA-regulated gene expression. J. Plant Growth Regul. 2003, 22, 176–184. [Google Scholar] [CrossRef]
- Dai, X.; Zhao, P.X. psRNATarget: A plant small RNA target analysis server. Nucleic Acids Res. 2011, 39, W155–W159. [Google Scholar] [CrossRef]
- Zheng, Z.; Reichel, M.; Deveson, I.; Wong, G.; Li, J.; Millar, A.A. Target RNA secondary structure is a major determinant of miR159 efficacy. Plant Physiol. 2017, 174, 1764–1778. [Google Scholar] [CrossRef]
- Li, Z.; Peng, R.; Tian, Y.; Han, H.; Xu, J.; Yao, Q. Genome-wide identification and analysis of the MYB transcription factor superfamily in Solanum lycopersicum. Plant Cell Physiol. 2016, 57, 1657–1677. [Google Scholar] [CrossRef]
- Pieczynski, M.; Marczewski, W.; Hennig, J.; Dolata, J.; Bielewicz, D.; Piontek, P.; Wyrzykowska, A.; Krusiewicz, D.; Strzelczyk-Zyta, D.; Konopka-Postupolska, D.; et al. Down-regulation of CBP80 gene expression as a strategy to engineer a drought-tolerant potato. Plant Biotechnol. J. 2013, 11, 459–469. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.; Wang, M.; Tian, Y.; He, W.; Han, L.; Xia, G. Over-expression of TaMYB33 encoding a novel wheat MYB transcription factor increases salt and drought tolerance in Arabidopsis. Mol. Biol. Rep. 2012, 39, 7183–7192. [Google Scholar] [CrossRef] [PubMed]
- Tonon, G.; Kevers, C.; Faivre-Rampant, O.; Graziani, M.; Gaspar, T. Effect of NaCl and mannitol iso-osmotic stresses on proline and free polyamine levels in embryogenic Fraxinus angustifolia callus. J. Plant Physiol. 2004, 161, 701–708. [Google Scholar] [CrossRef] [PubMed]
- Alcázar, R.; Cuevas, J.C.; Patron, M.; Altabella, T.; Tiburcio, A.F. Abscisic acid modulates polyamine metabolism under water stress in Arabidopsis thaliana. Physiol. Plant 2006, 128, 448–455. [Google Scholar] [CrossRef]
- Pál, M.; Tajti, J.; Szalai, G.; Peeva, V.; Végh, B.; Janda, T. Interaction of polyamines, abscisic acid and proline under osmotic stress in the leaves of wheat plants. Sci. Rep. 2018, 8, 12839. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Reichel, M.; Li, Y.; Millar, A.A. The functional scope of plant microRNA-mediated silencing. Trends Plant Sci. 2014, 19, 750–756. [Google Scholar] [CrossRef] [PubMed]
- Hoagland, D.R.; Arnon, D.I. The water-culture method for growing plants without soil. Circ. Calif. Agric. Exp. Sta. 1950, 347, 1–32. [Google Scholar]
- Balcells, I.; Cirera, S.; Busk, P.K. Specific and sensitive quantitative RT-PCR of miRNAs with DNA primers. BMC Biotechnol. 2011, 11, 70. [Google Scholar] [CrossRef] [PubMed]
- Untergasser, A.; Nijveen, H.; Rao, X.; Bisseling, T.; Geurts, R.; Leunissen, J.A.M. Primer3Plus, an enhanced web interface to Primer3. Nucleic Acids Res. 2007, 35, W71–W74. [Google Scholar] [CrossRef] [Green Version]
- Ruijter, J.M.; Ramakers, C.; Hoogaars, W.M.H.; Karlen, Y.; Bakker, O.; van den Hoff, M.J.B.; Moorman, A.F.M. Amplification efficiency: Linking baseline and bias in the analysis of quantitative PCR data. Nucleic Acids Res. 2009, 37, e45. [Google Scholar] [CrossRef]
- Sánchez-López, J.; Camañes, G.; Flors, V.; Vicent, C.; Pastor, V.; Vicedo, B.; Cerezo, M.; García-Agustín, P. Underivatized polyamine analysis in plant samples by ion pair LC coupled with electrospray tandem mass spectrometry. Plant Physiol. Biochem. 2009, 47, 592–598. [Google Scholar] [CrossRef]





| Gene | Forward Primer (5′-3′) | Reverse Primer (5′-3′) | Product Size (bp) |
|---|---|---|---|
| sly-miR159 | CGCAGTTTGGATTGAAGGGAG | CAGGTCCAGTTTTTTTTTTTTTTTTAGAG | 50 |
| SlMYB33 | TATGGGCATCCAGTCTCTCC | TGGGACTGGAAAAGATCGTC | 199 |
| SlMYB65 | TCTGCTGCATCGGTGTTTAG | TCTGGCCTGGGACAGATAAG | 164 |
| SlMYB104 | TTTCGGAATTGTTTGGAAGC | TGAAGAAGTTGCCGACAATG | 110 |
| SlMYB97 | CATGTCCCCTTGGAAGATTTAG | CTAGTGGCAAAGCAAAGTCATC | 181 |
| SlMYB120 | CACATTCCAGTCCAAACCAAC | CCTAGGTCGGAAGCACTGAG | 116 |
| SlP5CS | TGCTCAACAGGCCGGATATG | AAAGTGTGACCAAGGGGCTC | 126 |
| U6 snRNA | GGGGACATCCGATAAAATTGGAAC | TGGACCATTTCTCGATTTGTGC | 88 |
| RPS18 | GGGCATTCGTATTTCATAGTCAGAG | CGGTTCTTGATTAATGAAAACATCCT | 105 |
| Primer Pair | Forward Primer (5′-3′) | Reverse Primer (5′-3′) | Product Size (bp) |
|---|---|---|---|
| OFw, ORv | TATGGGCATCCAGTCTCTCC | TGGGACTGGAAAAGATCGTC | 199 |
| FFw, FRv | ATGACGGTTCTTTGCTTGCT | CTGTCTGGTTTTGGAGTGAAGG | 200 |
| RPS18FW, RPS18RV | GGGCATTCGTATTTCATAGTCAGAG | CGGTTCTTGATTAATGAAAACATCCT | 105 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
López-Galiano, M.J.; García-Robles, I.; González-Hernández, A.I.; Camañes, G.; Vicedo, B.; Real, M.D.; Rausell, C. Expression of miR159 Is Altered in Tomato Plants Undergoing Drought Stress. Plants 2019, 8, 201. https://doi.org/10.3390/plants8070201
López-Galiano MJ, García-Robles I, González-Hernández AI, Camañes G, Vicedo B, Real MD, Rausell C. Expression of miR159 Is Altered in Tomato Plants Undergoing Drought Stress. Plants. 2019; 8(7):201. https://doi.org/10.3390/plants8070201
Chicago/Turabian StyleLópez-Galiano, María José, Inmaculada García-Robles, Ana I. González-Hernández, Gemma Camañes, Begonya Vicedo, M. Dolores Real, and Carolina Rausell. 2019. "Expression of miR159 Is Altered in Tomato Plants Undergoing Drought Stress" Plants 8, no. 7: 201. https://doi.org/10.3390/plants8070201