Chromosome Location Contributing to Ozone Tolerance in Wheat
Abstract
:1. Introduction
2. Results
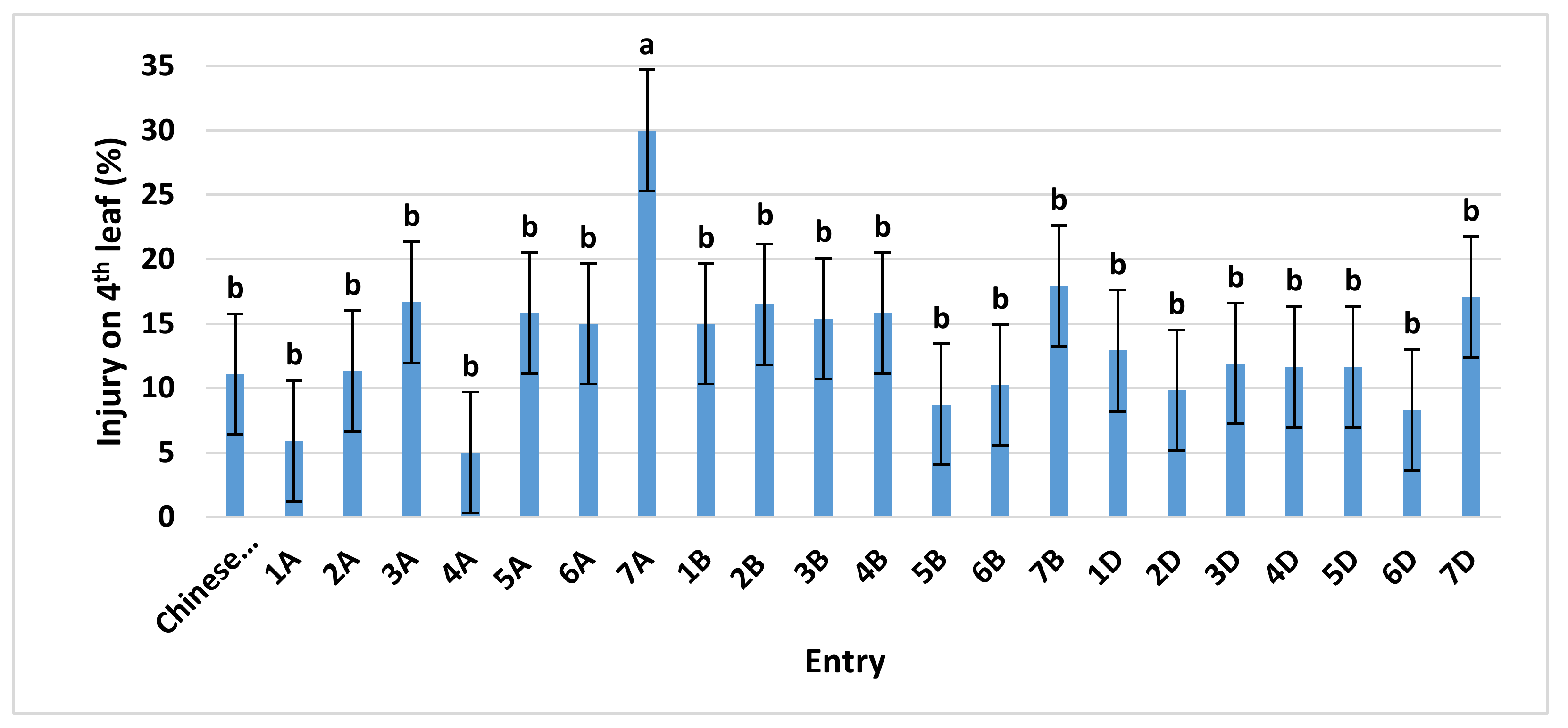
2.1. Ozone Injury to ‘Chinese Spring’ Monosomics Using Continuous Stirred Tank Reactors
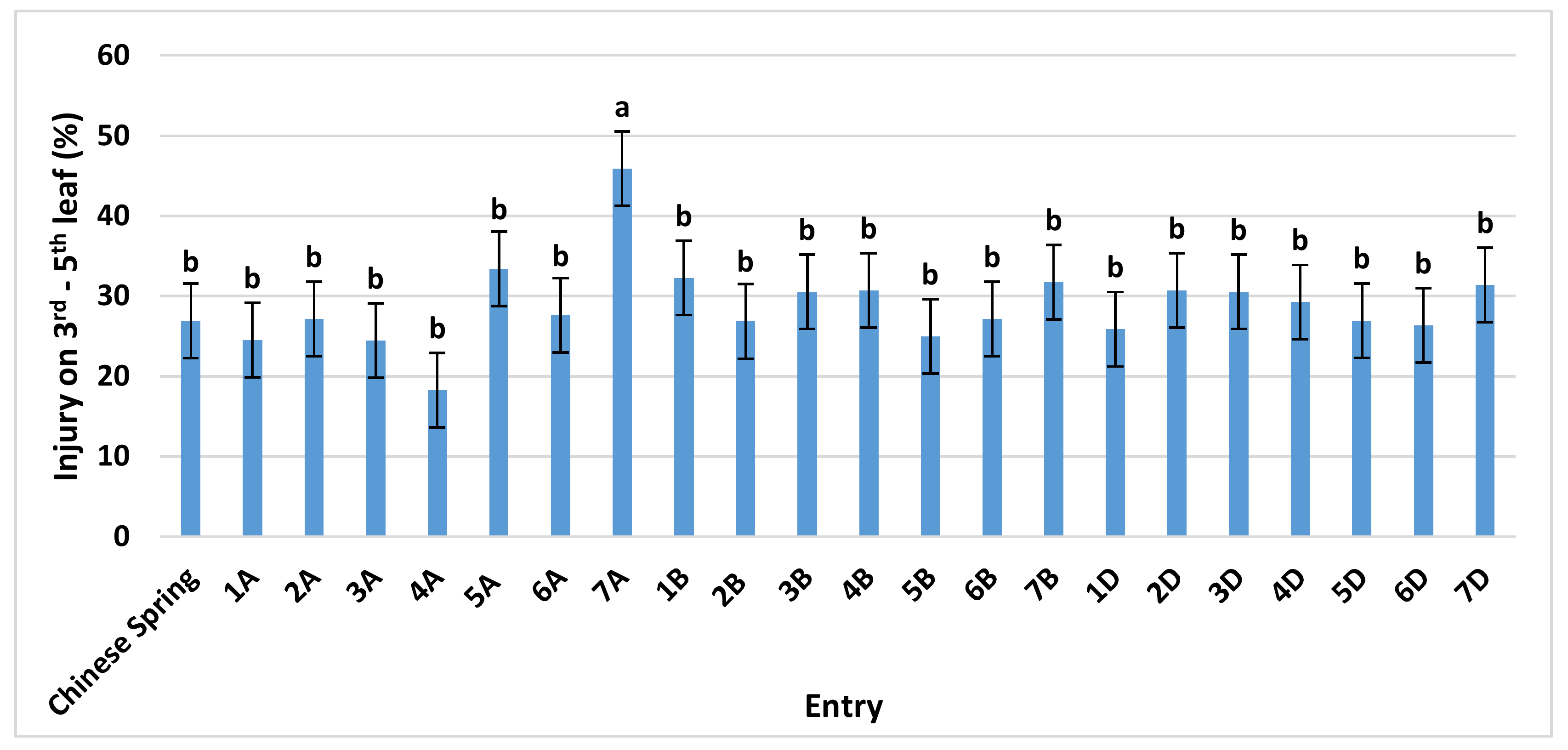
2.2. Ozone Injury to ‘Chinese Spring’ Monosomics Using Outdoor Plant Environment Chambers (OPECs)
3. Discussion
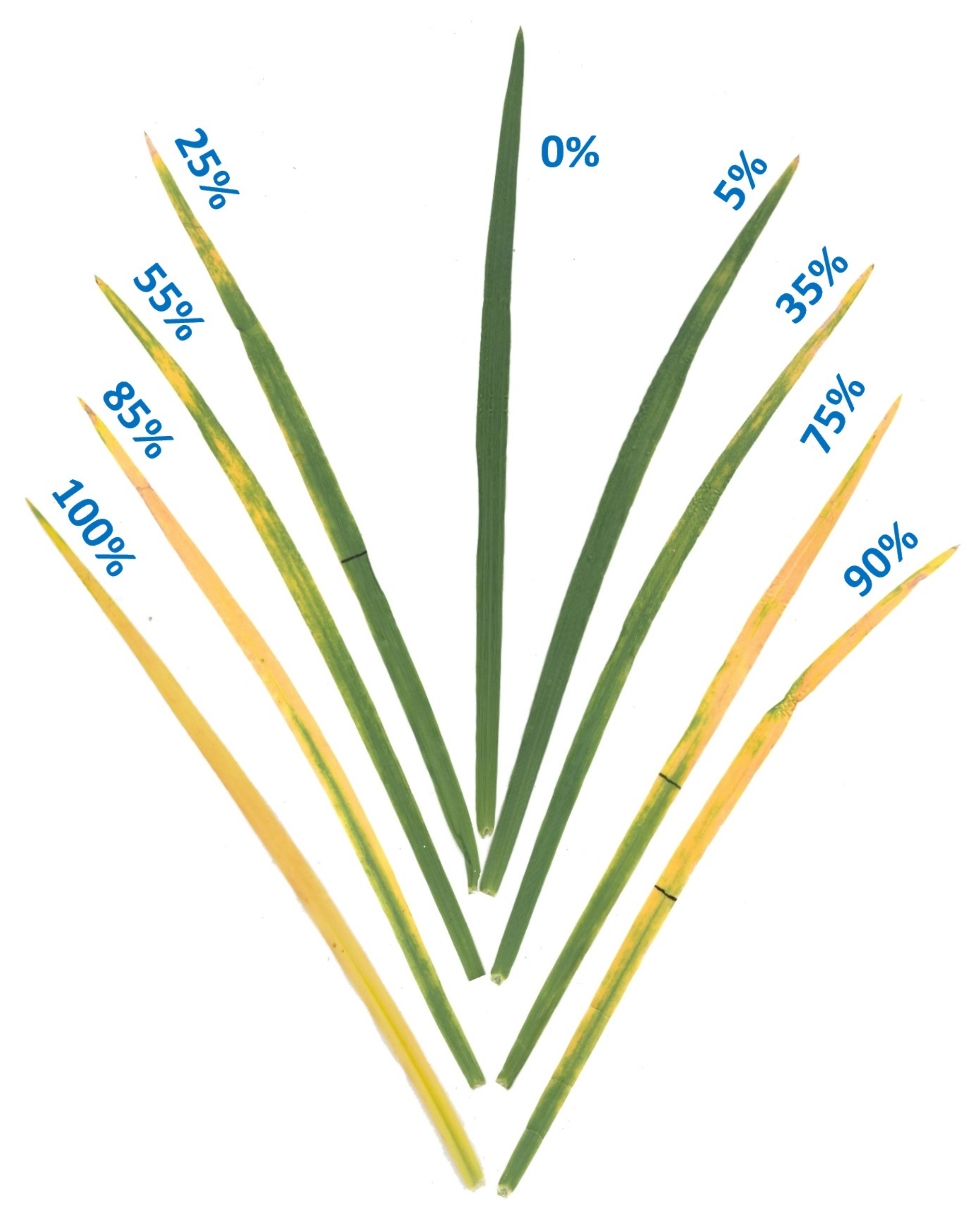
4. Materials and Methods
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- IPCC. Climate Change 2014: Impacts, Adaptation, and Vulnerability. Part A: Global and Sectoral Aspects. In Contribution of Working Group II to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Field, C.B., Barros, V.R., Dokken, D.J., Mach, K.J., Mastrandrea, M.D., Bilir, T.E., Chatterjee, M., Ebi, K.L., Estrada, Y.O., Genova, R.C., et al., Eds.; Cambridge University Press: New York, NY, USA, 2014; p. 1132. [Google Scholar]
- Sandermann, H.; Ernst, D.; Heller, W.; Langebartels, C. Ozone: An abiotic elicitor of plant defence reactions. Trends Plant Sci. 1998, 3, 47–50. [Google Scholar] [CrossRef]
- Vaultier, M.N.; Jolivet, Y. Ozone sensing and early signaling in plants: An outline from the cloud. Environ. Exp. Bot. 2015, 114, 144–152. [Google Scholar] [CrossRef]
- Vahisalu, T.; Puzorjova, I.; Brosche, M.; Valk, E.; Lepiku, M.; Moldau, H.; Pechter, P.; Wang, Y.S.; Lindgren, O.; Salojarvi, J.; et al. Ozone-triggered rapid stomatal response involves the production of reactive oxygen species, and is controlled by SLAC1 and OST1. Plant J. 2010, 62, 442–453. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Agrawal, S.B. Impact of tropospheric ozone on wheat (Triticum aestivum L.) in the eastern Gangetic plains of India as assessed by ethylenediurea (EDU) application during different developmental stages. Agric. Ecosyst. Environ. 2010, 138, 214–221. [Google Scholar] [CrossRef]
- Avnery, S.; Mauzerall, D.L.; Liu, J.; Horowitz, L.W. Global crop yield reductions due to surface ozone exposure: 1. Year 2000 crop production losses and economic damage. Atmos. Environ. 2011, 45, 2284–2296. [Google Scholar] [CrossRef]
- Avnery, S.; Mauzerall, D.L.; Liu, J.; Horowitz, L.W. Global crop yield reductions due to surface ozone exposure: 2. Year 2030 potential crop production losses and economic damage under two scenarios of O3 pollution. Atmos. Environ. 2011, 45, 2297–2309. [Google Scholar] [CrossRef]
- Mills, G.; Sharps, K.; Simpson, D.; Pleijel, H.; Broberg, M.; Uddling, J.; Jaramillo, F.; Davies, W.J.; Dentener, F.; Van den Berg, M.; et al. Ozone pollution will compromise efforts to increase global wheat production. Glob. Chang. Biol. 2018, 24, 3560–3574. [Google Scholar] [CrossRef]
- Mills, G.; Sharps, K.; Simpson, D.; Pleijel, H.; Frei, M.; Burkey, K.; Emberson, L.; Uddling, J.; Broberg, M.; Feng, Z.; et al. Closing the global ozone yield gap: Quantification and co-benefits for multi-stress tolerance. Glob. Chang. Biol. 2018, 24, 4869–4893. [Google Scholar] [CrossRef]
- Shiferaw, B.; Smale, M.; Braun, H.; Duveiller, E.; Reynolds, M.; Muricho, G. Crops that feed the world 10. Past successes and future challenges to the role played by wheat in global food security. Food Sec. 2013, 5, 291–317. [Google Scholar] [CrossRef] [Green Version]
- Ainsworth, E.A. Understanding and improving global crop response to ozone pollution. Plant J. 2017, 90, 886–897. [Google Scholar] [CrossRef]
- Frei, M. Breeding of ozone resistant rice: Relevance, approaches and challenges. Environ. Pollut. 2015, 197, 144–155. [Google Scholar] [CrossRef] [PubMed]
- Sears, E.R. The Aneuploids of Common Wheat; University of Missouri Agricultural Experiment Station Research Bulletin: Columbia, MO, USA, 1954. [Google Scholar]
- Mashaheet, A.M.S. Effects of Near-Ambient O3 and CO2 on Wheat Performance and Interactions with Leaf and Stem Rust Pathogens. Ph.D. Thesis, North Carolina State University, Raleigh, NC, USA, 2016. [Google Scholar]
- Appels, R.; Eversole, K.; Stein, N.; Feuillet, C.; Keller, B.; Rogers, J.; Pozniak, C.J.; Choulet, F.; Distelfeld, A.; Poland, J.; et al. Shifting the limits in wheat research and breeding using a fully annotated reference genome. Science 2018, 361, eaar7191. [Google Scholar] [CrossRef] [PubMed]
- Zadoks, J.C.; Chang, T.T.; Konzak, C.F. A decimal code for the growth stages of cereals. Weed Res. 1974, 14, 415–421. [Google Scholar] [CrossRef]
- Biswas, D.K.; Xu, H.; Li, Y.G.; Liu, M.Z.; Chen, Y.H.; Sun, J.Z.; Jiang, G.M. Assessing the genetic relatedness of higher ozone sensitivity of modern wheat to its wild and cultivated progenitors/relatives. J. Exp. Bot. 2008, 59, 951–963. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davydov, V.A. Characterization of the stomatal apparatus in monosomic lines of ‘Chinese Spring’ wheat. Russ. J. Genet. 1999, 35, 458–461. [Google Scholar]
- Worthington, M.; Lyerly, J.; Petersen, S.; Brown-Guedira, G.; Marshall, D.; Cowger, C.; Parks, R.; Murphy, J.P. MlUM15: An Aegilops neglecta-derived powdery mildew resistance gene in common wheat. Crop Sci. 2014, 54, 1397–1406. [Google Scholar] [CrossRef]
- Shavrukov, Y.; Shamaya, N.; Baho, M.; Edwards, J.; Ramsey, C.; Nevo, E.; Langridge, P.; Tester, M. Salinity tolerance and Na+ exclusion in wheat: Variability, genetics, mapping populations and QTL analysis. Czech J. Genet. Plant Breed. 2011, 47, 85–93. [Google Scholar] [CrossRef]
- Kumar, S.; Stack, R.W.; Friesen, T.L.; Faris, J.D. Identification of a novel Fusarium head blight resistance quantitative trait locus on chromosome 7A in tetraploid wheat. Phytopathology 2007, 97, 592–597. [Google Scholar] [CrossRef]
- Burton, A.L.; Burkey, K.O.; Carter, T.E.; Orf, J.; Cregan, P.B. Phenotypic variation and identification of quantitative trait loci for ozone tolerance in a Fiskeby III × Mandarin (Ottawa) soybean population. Theor. Appl. Genet. 2016, 129, 1113–1125. [Google Scholar] [CrossRef]
- Heck, W.W.; Philbeck, R.B.; Dunning, J.A. A Continuous Stirred Tank Reactor (CSTR) System for Exposing Plants to Gaseous Air Contaminants: Principles, Specifications, Construction, and Operation; USDA Agricultural Research Service: Washington, DC, USA, 1978; p. 32.
- Rogers, H.H.; Jeffries, H.E.; Stahel, E.P.; Heck, W.W.; Ripperton, L.A.; Witherspoon, A.M. Measureing air pollutant uptake by plants: Direct kinetic technique. J. Air Pollut. Control Assoc. 1977, 27, 1192–1197. [Google Scholar] [CrossRef]
- Flowers, M.D.; Fiscus, E.L.; Burkey, K.O.; Booker, F.L.; Dubois, J.J.B. Photosynthesis, chlorophyll fluorescence, and yield of snap bean (Phaseolus vulgaris L.). J. Exp. Bot. 2007, 61, 190–198. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mashaheet, A.M.; Burkey, K.O.; Marshall, D.S. Chromosome Location Contributing to Ozone Tolerance in Wheat. Plants 2019, 8, 261. https://doi.org/10.3390/plants8080261
Mashaheet AM, Burkey KO, Marshall DS. Chromosome Location Contributing to Ozone Tolerance in Wheat. Plants. 2019; 8(8):261. https://doi.org/10.3390/plants8080261
Chicago/Turabian StyleMashaheet, Alsayed M., Kent O. Burkey, and David S. Marshall. 2019. "Chromosome Location Contributing to Ozone Tolerance in Wheat" Plants 8, no. 8: 261. https://doi.org/10.3390/plants8080261





