Biodegradable Polyhydroxyalkanoates Formed by 3- and 4-Hydroxybutyrate Monomers to Produce Nanomembranes Suitable for Drug Delivery and Cell Culture
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
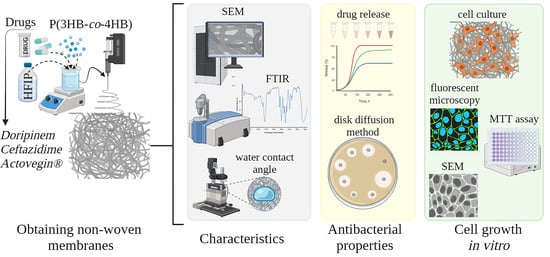
2.2. Obtaining Non-Woven Membranes by Electrostatic Molding of a Polymer Solution
2.3. Characteristics of Non-Woven Membranes P(3HB-co-4HB) Loaded with Drugs
2.4. Dynamics of Drug Release from Non-wovenP(3HB-co-4HB) Membranes In Vitro
2.5. Antibacterial Properties of Non-Woven Membranes P(3HB-co-4HB)/Antibiotics
2.6. Evaluation of Non-Woven Membranes as Scaffolds for Cell Growth In Vitro
2.7. Statistics
3. Results
3.1. Characterization of ESF Non-woven Membranes P(3HB-co-4HB)/Drug
3.2. Dynamics of Drug Release from ESF Membranes P(3HB-co-4HB)/Drug In Vitro
3.3. Antibacterial Activity of Non-Woven Membranes P(3HB-co-4HB)/Drug
3.4. Evaluation of Biocompatibility of ESFMembranes P(3HB-co-4HB)/Drug in Cell Culture
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gregory, D.A.; Fricker, A.T.R.; Mitrev, P.; Ray, M.; Asare, E.; Sim, D.; Larpnimitchai, S.; Zhang, Z.; Ma, J.; Tetali, S.S.V.; et al. Additive Manufacturing of Polyhydroxyalkanoate-Based Blends Using Fused Deposition Modelling for the Development of Biomedical Devices. J. Funct. Biomater. 2023, 14, 40. [Google Scholar] [CrossRef]
- Kalia, V.C.; Patel, S.K.S.; Lee, J.-K. Exploiting Polyhydroxyalkanoates for Biomedical Applications. Polymers 2023, 15, 1937. [Google Scholar] [CrossRef]
- Huang, Z.M.; Zhang, Y.Z.; Kotaki, M.; Ramakrishna, S. A review on polymer nanofibers by electrospinning and their applications in nanocomposites. Compos. Sci. Technol. 2003, 63, 2223–2253. [Google Scholar] [CrossRef]
- Smith, L.A.; Ma, P.X. Nano-fibrous scaffolds for tissue engineering. Colloids Surf. B Biointerf. 2004, 39, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Reneker, D.H.; Kataphinan, W.; Theron, A.; Zussman, E.; Yarin, A.L. Nanofiber garlands of polycaprolactone by electrospinning. Polymer 2002, 43, 6785–6794. [Google Scholar] [CrossRef]
- Yarin, A.L.; Zussman, E. Upward needleless electrospinning of multiple nanofibers. Polymer 2004, 45, 2977–2980. [Google Scholar] [CrossRef]
- Yener, F.; Jirsak, O. Comparison between the Needle and Roller Electrospinning of Polyvinylbutyral. J. Nanomater. 2012, 839317. [Google Scholar] [CrossRef] [Green Version]
- Zong, X.; Kim, K.; Fang, D.; Ran, S.; Hsiao, B.S.; Chu, B. Structure and process relationship of electrospun bioabsorbable nanofiber membranes. Polymer 2002, 43, 4403–4412. [Google Scholar] [CrossRef]
- Ding, B.; Wang, M.; Wang, X.; Yu, J.; Sun, G. Electrospun nanomaterials for ultrasensitive sensors. Mater. Today 2010, 13, 16–27. [Google Scholar] [CrossRef]
- Kenawy, E.R.; Layman, J.M.; Watkins, J.R.; Bowlin, G.L.; Matthews, J.A.; Simpson, D.G.; Wnek, G.E. Electrospinning of poly(ethylene-co-vinyl alcohol) fibers. Biomaterials 2003, 24, 907–913. [Google Scholar] [CrossRef]
- Kessing, R.; Fenn, J.; Tepper, G. The use of AC potentials in electrospraying and elecrospining processes. Polymer 2004, 45, 2981–2984. [Google Scholar] [CrossRef]
- Khil, M.S.; Kim, H.K.; Kim, M.S.; Park, S.Y.; Lee, D.R. Nanofibrous mats of poly(trimethylene terephthalate) via elecrospining. Polymer 2004, 45, 295–301. [Google Scholar] [CrossRef]
- Mo, X.M.; Xu, C.Y.; Kotaki, M.; Ramakrishna, S. Electrospun P(LLA-CL) nanofiber: A biomimetic extracellular matrix for smooth muscle cell and endothelial cell proliferation. Biomaterials 2004, 25, 1883–1890. [Google Scholar] [CrossRef] [PubMed]
- Theron, S.A.; Zussman, E.; Yarin, A.L. Experimental investigation of the govering parameters in the elecrospining of polymer solutions. Polymer 2004, 45, 2017–2030. [Google Scholar] [CrossRef]
- Riboldi, S.; Sampaolesi, M.; Neuenschwander, P.; Cossu, G.; Mantero, S. Elecrospun degradable polyesterurethane membranes: Potential scaffolds for skeletal muscle tissue. Biomaterials 2005, 26, 4606–4615. [Google Scholar] [CrossRef] [Green Version]
- Hu, S.; Liu, G.; Zhou, Y.; Huang, Z.; Xie, X.; Jing, X. Electrospinning of polymeric nanofibers for drug delivery applications. J. Control. Release 2014, 10, 12–21. [Google Scholar] [CrossRef]
- Kajdič, S.; Planinšek, O.; Gašperlin, M.; Kocbek, P. Electrospun nanofibers for customized drug-delivery systems. J. Drug Deliv. Sci. Technol. 2019, 51, 672–681. [Google Scholar] [CrossRef]
- Akhgari, A.; Shakib, Z.; Sanati, S. A review on electrospun nanofibers for oral drug delivery. Nanomed. J. 2017, 4, 197–207. [Google Scholar] [CrossRef]
- Kajdič, S.; Zupančič, Š.; Roškar, R.; Kocbek, P. The potential of nanofibers to increase solubility and dissolution rate of the poorly soluble and chemically unstable drug lovastatin. Int. J. Pharm. 2020, 573, 118809. [Google Scholar] [CrossRef]
- Carvalho, B.M.; Pellá, M.C.G.; Hardt, J.C.; de Souza Rossin, A.R.; Tonet, A.; Ilipronti, T.; Caetano, J.; Dragunksi, D.C. Ecovio®-based nanofibers as a potential fast transdermal releaser of aceclofenac. J. Mol. Liq. 2021, 325, 115206. [Google Scholar] [CrossRef]
- Celebioglu, A.; Uyar, T. Electrospun formulation of acyclovir/cyclodextrin nanofibers for fast-dissolving antiviral drug delivery. Mater. Sci. Eng. C 2021, 118, 111514. [Google Scholar] [CrossRef]
- Xu, H.; Zhang, F.; Wang, M.; Lv, H.; Yu, D.G.; Liu, X.; Shen, H. Electrospun hierarchical structural films for effective wound healing. Biomater. Adv. 2022, 136, 212795. [Google Scholar] [CrossRef] [PubMed]
- Jariwala, T.; Ico, G.; Tai, Y.; Park, H.; Myung, N.V.; Nam, J. Mechano-Responsive Piezoelectric Nanofiber as an On-Demand Drug Delivery Vehicle. ACS Appl. Bio. Mater. 2021, 4, 3706–3715. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Cheng, S.; Lu, W.; Wang, Y.; Zhang, P.; Yao, Q. Electrospun fibers and their application in drug controlled release, biological dressings, tissue repair, and enzyme immobilization. RSC Adv. 2019, 9, 25712–25729. [Google Scholar] [CrossRef] [PubMed]
- Abdalkarim, S.Y.H.; Yu, H.; Wang, C.; Chen, Y.; Zou, Z.; Han, L.; Yao, J.; Tamm, K.C. Thermo and light-responsive phase change nanofibers with high energy storage efficiency for energy storage and thermally regulated on–off drug release devices. Chem. Eng. J. 2019, 375, 121979. [Google Scholar] [CrossRef]
- Chen, G.-Q. Plastics Completely Synthesized by Bacteria: Polyhydroxyalkanoates. In Plastics from Bacteria; Chen, G.-Q., Ed.; Springer: Berlin/Heidelberg, Germany, 2010; pp. 17–37. [Google Scholar]
- Sudesh, K.; Abe, H.; Doi, Y. Synthesis, structure and properties of polyhydroxyalkanoates: Biological polyesters. Prog. Polym. Sci. 2000, 25, 1503–1555. [Google Scholar] [CrossRef]
- Chen, G.-Q.; Jiang, X.-R.; Guo, Y. Synthetic biology of microbes synthesizing polyhydroxyalkanoates (PHA). Synt. Syst. Biotech. 2016, 1, 236–242. [Google Scholar] [CrossRef] [Green Version]
- Koller, M. The Handbook of Polyhydroxyalkanoates, Postsynthetic Treatment, Processing and Applications; Taylor & Francis: Oxfordshire, UK, 2020. [Google Scholar]
- Koller, M. Polyhydroxyalkanoate biosynthesis at the edge of water activitiy haloarchaea as biopolyester factories. Bioengineering 2019, 6, 34. [Google Scholar] [CrossRef] [Green Version]
- Koller, M.; Mukherjee, A. Polyhydroxyalkanoates—linking properties, applications, and end-of-life options. Chem. Biochem. Eng. 2020, 34, 115–129. [Google Scholar] [CrossRef]
- Kalia, V.; Gogante, P.; Cinelli, P.; Seggiani, V.A.; Alaverex, A.; Lazzeri, A. Processing and thermomachanical properties of PHA. In The Handbook of Polyhydroxyalkanoates; Koller, M., Ed.; Taylor & Francis: Oxfordshire, UK, 2020; pp. 91–118. [Google Scholar]
- Lakshmi, S.; Laurencin, C. Biodegradable polymers as biomaterials. Prog. Polym. Sci. 2007, 32, 762–798. [Google Scholar] [CrossRef]
- Philip, S.; Keshavarz, T.; Roy, I. Polyhydroxyalkanoates: Biodegradable polymers with a range of applications. J. Chem. Technol. Biotechnol. 2007, 82, 233–247. [Google Scholar] [CrossRef]
- Kalia, V.; Kumar, S.; Patel, S.; Shanmugam, R.; Lee, J.-K. Polyhydroxyalkanoates: Trends and advances toward biotechnological applications. Bioresour. Technol. 2021, 326, 124737. [Google Scholar] [CrossRef] [PubMed]
- Volova, T.G.; Vinnik, Y.S.; Shishatskaya, E.I.; Markelova, N.M.; Zaikov, G.E. Natural Based Polymers for Biomedical Applications; Apple Academic Press: Point Pleasant, NJ, USA, 2017. [Google Scholar]
- Zhang, J.; Shishatskaya, E.I.; Volova, T.G.; Ferreira da Silva, L.; Chen, G.Q. Polyhydroxyalkanoates (PHA) for therapeutic applications. Mater. Sci. Eng. C 2018, 86, 144–150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Asare, E.; Gregory, D.A.; Frisker, A.; Marcello, E.A.; Paxinou, C.S.; Taylor, J.; Haucock, W.; Roy, I. Polyhydroxyalkanoates, Their Processing and Biomedical Application; Koller, M., Ed.; Taylor & Francis: Oxfordshire, UK, 2020; pp. 255–284. [Google Scholar]
- Williams, S.F.; Martin, D.P. Applications of polyhydroxyalkanoates (PHA) in medicine and pharmacy. In Biopolymers; Steinbüchel, A., Ed.; Wiley-VCH Verlag GmbH & Co. KgaA: Weinheim, Germany, 2005; pp. 91–103. [Google Scholar] [CrossRef]
- Chen, G.-Q.; Wu, Q. The application of polyhydroxyalkanoates as tissue engineering materials. Biomaterials 2005, 26, 6565–6578. [Google Scholar] [CrossRef]
- Zhao, X.; Niu, Y.; Mi, C.; Gong, H.; Yang, X.; Cheng, J.; Zhou, Z.; Liu, J.; Peng, X.; Wei, D. Electrospinning nanofibers of microbial polyhydroxyalkanoates for applications in medical tissue engineering. J. Appl. Polym. Sci. 2021, 59, 1994–2013. [Google Scholar] [CrossRef]
- Prakash, P.; Lee, W.-H.; Loo, C.-Y.; Wong, H.S.J.; Parumasivam, T. Advances in Polyhydroxyalkanoate Nanocarriers for Effective Drug Delivery: An Overview and Challenges. Nanomaterials 2022, 12, 175. [Google Scholar] [CrossRef]
- Williams, S.F.; Martin, D.P.; Moses, A.C. The history of GalaFLEX P4HB scaffold. Aesthetic Surg. J. 2016, 36, 33–42. [Google Scholar] [CrossRef] [Green Version]
- Utsunomia, C.; Ren, Q.; Zinn, M. Poly(4-Hydroxybutyrate): Current state and perspectives. Front. Bioeng. Biotechnol. 2020, 8, 257. [Google Scholar] [CrossRef] [Green Version]
- Gordeev, S.A.; Shishatskaya, E.I.; Volova, T.G. Production and study of foreign-oriented poly(hydroxybutyrate/hydroxyvalerate) copolymers. Perspect. Mater. 2005, 3, 50–55. (In Russian) [Google Scholar]
- Gordeev, S.A.; Shishatskaya, E.I.; Volova, T.G. Production of ultrathin fibers from polyhydroxyalkanoates by electrostatic spinning. Plast. Masses 2006, 4, 49–52. (In Russian) [Google Scholar]
- Sombatmankhong, K.; Suwantong, O.; Waleetorncheepsawat, S.; Supaphol, P. Electrospun fiber mats of poly(3-hydroxybutyrate), poly(3-hydroxybutyrate-co-3-hydroxyvalerate), and their blends. J. Polym. Sci. Part B Polym. Phys. 2006, 44, 2923–2933. [Google Scholar] [CrossRef]
- Cheng, M.L.; Lin, C.C.; Su, H.L.; Chen, P.Y.; Sun, Y.M. Processing and characterization of electrospun poly(3-hydroxybutyrate-co-3-hydroxyhexanoate) nanofibrous membranes. Polymer 2008, 49, 546–553. [Google Scholar] [CrossRef]
- Wang, Y.; Gao, R.; Wang, P.P.; Jian, J.; Jiang, X.L.; Yan, C.; Lin, X.; Wu, L.; Chen, G.Q.; Wu, Q. The differential effects of aligned electrospunPHBHHx fibers on adipogenic and asteogenic potential of MSCs through the regeneration of PPAR signaling. Biomaterials 2012, 3, 485–493. [Google Scholar] [CrossRef]
- Wang, Z.; Ma, K.; Jiang, X.; Xie, J.; Cai, P.; Li, F.; Liang, R.; Zhao, J.; Zheng, L. Electrospun poly(3-hydroxybutyrate-co-4-hydroxybutyrate)/Octacalcium phosphate Nanofibrous membranes for effective guided bone regeneration. Mater. Sci. Eng. C 2020, 112, 110763. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Lv, H.; Cao, X.; Liu, Y.; Yu, D. Recent progress of the preparation and application of electrospun porous nanofibers. Polymers 2023, 15, 921. [Google Scholar] [CrossRef] [PubMed]
- Volova, T.G.; Goncharov, D.B.; Sukovatyi, A.G.; Shabanov, A.; Nikolaeva, E.D.; Shishatskaya, E.I. Electrospinning of polyhydroxyalkanoate fibrous scaffolds:effect on electrospinning parameters on structure and properties. J. Biomater. Sci. Polym. Edit. 2014, 25, 370–393. [Google Scholar] [CrossRef]
- Sombatmankhong, K.; Sanchavanakit, N.; Pavasant, P.; Supaphol, P. Bone scaffolds from electrospun fiber mats of poly(3-hydroxybutyrate), poly(3-hydroxybutyrate-co-3-hydroxyvalerate) and their blend. Polymer 2007, 48, 1419–1427. [Google Scholar] [CrossRef]
- Tong, H.W.; Wang, M. Electrospinning of poly(hydroxybutyrate-co-hydroxyvalerate) fibrous scaffolds for tissue engineering applications: Effects of electrospinning parameters and solution properties. J. Macromol. Sci. 2011, 50, 1535–1558. [Google Scholar] [CrossRef]
- Tong, H.W.; Wang, M.; Lu, W.L. Electrospinning and evaluation of PHBV-based tissue engineering scaffolds with different fiber diameters, surface topography and compositions. J. Biomater. Sci. 2012, 23, 779–806. [Google Scholar] [CrossRef]
- Yu, B.Y.; Chen, P.Y.; Sun, Y.M.; Lee, Y.T.; Young, T.H. Response of human mesenchymal stem cells (hmsc) to the topographic variation of poly(3-hydroxybutyrate-co-3-hydroxyhexanoate) (phbhhx) films. J. Biomater. Sci. 2012, 23, 1–26. [Google Scholar] [CrossRef]
- Li, X.T.; Zhang, Y.; Chen, G.Q. Nanofibrous polyhydroxyalkanoates matrices as cell growth supporting materials. Biomaterials 2008, 29, 3720–3728. [Google Scholar] [CrossRef]
- Bashur, C.A.; Dahlgren, L.A.; Goldstein, A.S. Effect of fiber diameter and orientation on fibroblast morphology and proliferation on electrospun poly(D, L-lactic-co-glycolic acid) meshes. Biomaterials 2006, 27, 5681–5688. [Google Scholar] [CrossRef]
- Pham, Q.P.; Sharma, U.; Mikos, A.G. Electrospinning of polymeric nanofibers for tissue engineering applications: A review. Tissue Eng. 2006, 12, 1197–1211. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Still, T.J.; von Recum, H.A. Electrospinning: Applications in drug delivery and tissue engineering. Biomaterials 2008, 29, 1989–2006. [Google Scholar] [CrossRef]
- Lowery, J.L.; Datta, N.G.C. Rutledge, Effect of fiber diameter, pore size and seeding method on growth of human dermal fibroblasts in electrosoun poly(e-caprolactone) fibrous mats. Biomaterials 2010, 31, 491–504. [Google Scholar] [CrossRef] [PubMed]
- Amini, F.; Semnani, D.; Karbasi, S.; Banitaba, S.N. A novel bilayer drug-loaded wound dressing of PVDF and PHB/Chitosan nanofibers applicable for post-surgical ulcers. Int. J. Polym. Mater. 2018, 68, 772–777. [Google Scholar] [CrossRef]
- Kundrat, V.; Cernekova, N.; Kovalcik, A.; Enev, V.; Marova, I. Drug Release Kinetics of Electrospun PHB Meshes. Materials 2019, 12, 1924. [Google Scholar] [CrossRef] [Green Version]
- Naveen, N.; Kumar, R.; Balaji, S.; Uma, T.; Natrajan, T.; Sehgal, P. Synthesis of Nonwoven Nanofibers by Electrospinnin—A Promising Biomaterial for Tissue Engineering and Drug Delivery. Adv. Eng. Mater. 2010, 12, B380–B387. [Google Scholar] [CrossRef]
- Mukheem, A.; Muthoosamy, K.; Manickam, S.; Sudesh, K.; Shahabuddin, S.; Saidur, R.; Akbar, N.; Sridewi, N. Fabrication and Characterization of an Electrospun PHA/Graphene Silver Nanocomposite Scaffold for Antibacterial Applications. Materials 2018, 11, 1673. [Google Scholar] [CrossRef] [Green Version]
- Mukheem, A.; Shahabuddin, S.; Akbar, N.; Miskon, A.; Sarih, N.M.; Sudesh, K.; Khan, N.A.; Saidur, R.; Sridewi, N. Boron Nitride Doped Polyhydroxyalkanoate/Chitosan Nanocomposite for Antibacterial and Biological Applications. Nanomaterials 2019, 9, 645. [Google Scholar] [CrossRef] [Green Version]
- Xing, Z.C.; Chae, W.P.; Baek, J.Y.; Choi, M.J.; Jung, Y.; Kang, I.K. In Vitro Assessment of Antibacterial Activity and Cytocompatibility of Silver-Containing PHBV Nanofibrous Scaffolds for Tissue Engineering. Biomacromolecules 2010, 11, 1248–1253. [Google Scholar] [CrossRef]
- Douglass, M.; Hopkins, S.; Pandey, R.; Singha, P.; Norman, M.; Handa, H. S-Nitrosoglutathione-Based Nitric Oxide-Releasing Nanofibers Exhibit Dual Antimicrobial and Antithrombotic Activity for Biomedical Applications. Macromol. Biosci. 2021, 21, 2000248. [Google Scholar] [CrossRef]
- Shiny, P.J.; Devi, M.V.; Grace Felciya, S.J.; Ramanathan, G.; Fardim, P.; Sivagnanam, U.T. In vitro and in vivo evaluation of poly-3-hydroxybutyric acid-sodium alginate as a core-shell nanofibrous matrix with arginine and bacitracin-nanoclay complex for dermal reconstruction of excision wound. Int. Biol. Macromol. 2021, 168, 46–58. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Cicek, N.; Levin, D.B.; Logsetty, S.; Liu, S. Bacteria-triggered release of a potent biocide from core-shell polyhydroxyalkanoate (PHA)-based nanofibers for wound dressing applications. J. Biomater. Sci. Polym. Ed. 2020, 31, 394–406. [Google Scholar] [CrossRef]
- Volova, T.; Shishatskaya, E. Bacterial Strain VKPM B-10646—A Producer of Polyhydroxyalkanoates and a Method of Their Production). RF Patent No. 2439143, 10 January 2012. [Google Scholar]
- Volova, T.; Kiselev, E.; Nemtsev, I.; Lukyanenko, A.; Sukovatyi, A.; Kuzmin, A.; Ryltseva, G.; Shishatskaya, E. Properties of Degradable Polyhydroxyalkanoates with Different Monomer Compositions. Int. J. Biol. Macromol. 2021, 182, 98–114. [Google Scholar] [CrossRef] [PubMed]
- Kaelble, D.H. Dispersion-Polar Surface Tension Properties of Organic Solids. J. Adhes. 1970, 2, 66–81. [Google Scholar] [CrossRef]
- Owens, D.K.; Wendt, R.C. Estimation of the Surface Free Energy of Polymers. J. Appl. Polym. Sci. 1969, 13, 1741–1747. [Google Scholar] [CrossRef]
- Dash, S.; Murthy, P.N.; Nath, L.; Chowdhury, P. Kinetic Modeling on Drug Release from Controlled Drug Delivery Systems. Acta Pol. Pharm. 2010, 67, 217–223. [Google Scholar]
- Fu, Y.; Kao, W.J. Drug Release Kinetics and Transport Mechanisms of Non-Degradable and Degradable Polymeric Delivery Systems. Expert Opin. Drug Deliv. 2010, 7, 429–444. [Google Scholar] [CrossRef]
- Cavalieri, S.J. Manual of Antimicrobial Susceptibility Testing; American Society for Microbiology: Washington, DC, USA, 2009; p. 241. [Google Scholar]
- Martin, D.P.; Williams, S.F. Medical applications of poly-4-hydroxybutyrate: A strong flexible absorbable biomaterial. Biochem. Eng. J. 2003, 16, 97–105. [Google Scholar] [CrossRef]
- de Macedo, M.A.; Oliveira Filho, E.R.; Taciro, M.K.; Piccoli, R.A.M.; Gomez, J.G.C.; Silva, L.F. Poly(3 hydroxybutyrate co 4 hydroxybutyrate) [P(3HB co 4HB)] biotechnological production: Challenges and opportunities. Biomass Convers. Biorefinery 2022. [Google Scholar] [CrossRef]
- Nelson, T.; Kaufman, E.; Kline, J.; Sokoloff, L. The extraneural distribution of g-hydroxybutyrate. J. Neurochem. 1981, 37, 1345–1348. [Google Scholar] [CrossRef] [PubMed]
- Sendelbeck, S.L.; Girdis, C.L. Disposition of a 14C-labeled bioerodiblepolyorthoester and its hydrolysis products, 4-hydroxybutyrate and cis, trans1,4-bis(hydroxymethyl) cyclohexane, in rats. Drug Metab. Dispos. 1985, 13, 291–295. [Google Scholar]
- Lide, D.R. Handbook of Chemistry and Physics, 86th ed.; CRC; Taylor & Francis: Boca Raton, FL, USA, 2005. [Google Scholar]
- Serjeant, E.P.; Dempsey, B. Ionisation Constants of Organic acids in Aqueous Solution; Pergamon Press: New York, NY, USA, 1979. [Google Scholar]
- O’Neil, M.J. The Merck Index: An Encyclopedia of Chemicals, Drugs, and Biologicals; RSC Publishing: Cambridge, UK, 2013. [Google Scholar]
- Volova, T.G.; Prudnikova, S.V.; Vinogradova, O.N.; Syrvacheva, D.A.; Shishatskaya, E.I. A study of microorganisms degrading PHAs with different chemical compositions and PHA biodegradation behavior. Microb. Ecol. 2017, 73, 353–367. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Prabhakaran, M.P.; Tian, L.L.; Ding, X.; Ramakrishna, S. Drug-loaded emulsion electrospun nanofibers: Characterization, drug release and in vitro biocompatibility. RSC Adv. 2015, 5, 100256–100267. [Google Scholar] [CrossRef]
- Wang, W.; Cao, J.D.; Lan, P.; Wu, W. Drug release from electrospun fibers of poly (3-hydroxybutyrate-co-3-hydroxyvalerate) grafted with poly (N-vinylpyrrolidone). J. Appl. Polym. Sci. 2012, 124, 1919–1928. [Google Scholar] [CrossRef]
- Kaniuk, L.; Stachewicz, U. Development and Advantages of Biodegradable PHA Polymers Based on Electrospun PHBV Fibers for Tissue Engineering and Other Biomedical Applications. ACS Biomater. Sci. Eng. 2021, 7, 5339–5362. [Google Scholar] [CrossRef]
- Sanhueza, C.; Acevedo, F.; Rocha, S.; Villegas, P.; Seeger, M.; Navia, R. Polyhydroxyalkanoates as Biomaterial for Electrospun Scaffolds. Int. J. Biol. Macromol. 2019, 124, 102–110. [Google Scholar] [CrossRef]
- Ding, J.; Zhang, J.; Li, J.; Li, D.; Xiao, C.; Xiao, H.; Yang, H.; Zhuang, X.; Chen, X. Electrospun Polymer Biomaterials. Prog. Polym. Sci. 2019, 90, 1–34. [Google Scholar] [CrossRef]
- Chen, Y.; Abdalkarima, S.Y.; Yu, H.Y.; Li, Y.; Xu, J.; Marek, J.; Yao, J.; Tam, K. Double stimuli-responsive cellulose nanocrystals reinforced electrospun PHBV composites membrane for intelligent drug release. Inter. J. Biol. Macromol. 2020, 155, 330–339. [Google Scholar] [CrossRef]
- Bhattacharjee, A.; Kumar, K.; Arora, A.; Katti, D.S. Fabrication and characterization of Pluronic modified poly(hydroxybutyrate) fibers for potential wound dressing applications. Mater. Sci. Engin. C 2016, 63, 266–273. [Google Scholar] [CrossRef] [PubMed]
- Ang, S.L.; Shaharuddin, B.; Chuah, J.A.; Sudesh, K. Electrospun poly(3-hydroxybutyrate-co-3-hydroxyhexanoate)/silk fibroin film is a promising scaffold for bone tissue engineering. Inter. J. Biol. Macromol. 2020, 145, 173–188. [Google Scholar] [CrossRef] [PubMed]
- Safdari, M.; Shakiba, E.; Kiaie, S.; Fattahi, A. Preparation and characterization of ceftazidime loaded electrospun silk fibroin/gelatin mat for wound dressing. Fibers Polym. 2016, 17, 744–750. [Google Scholar] [CrossRef]
- Mendez, A.; Mantovani, L.; Barbosa, F.; Sayago, C.T.M.; Garcia, C.V.; Paula, F.R.; Denardin, F. Characterization of the antibiotic doripinem using physicochemical methods—chromatography, spectrophotometry, spectroscopy and thermal analysis. Quim. Nova 2011, 34, 1634–1638. [Google Scholar] [CrossRef] [Green Version]
- Lee, Y.F.; Sridewi, N.; Ramanathan, S.; Sudesh, K. The Influence of Electrospinning Parameters and Drug Loading on Polyhydroxyalkanoate (PHA) Nanofibers for Drug Delivery. Inter. J. Biotech. Wellness Ind. 2015, 4, 103–113. [Google Scholar] [CrossRef]
- Jiffrin, R.; Razak, S.I.; Jamaludin, M.I.; Hamzah, A.S.; Mazian, M.A.; Jaya, M.A.; Nasrullah, M.Z.; Majrashi, M.; Theyab, A.; Aldarmahi, A.A.; et al. Electrospun Nanofiber Composites for Drug Delivery: A Review on Current Progresses. Polymers 2022, 14, 3725. [Google Scholar] [CrossRef] [PubMed]
- Anderson, A.J.; Dawes, E.A. Occurrence, metabolism, metabolic role, and industrial uses of bacterial polyhydroxyalkanoates. Microbiol. Rev. 1990, 54, 450–472. [Google Scholar] [CrossRef] [PubMed]
- Ong, S.; Chee, J.Y.; Sudesh, K. Degradation of polyhydroxyalkanoate (PHA): A review. J. Sib. Fed. Univ. Biol. 2017, 10, 211–225. [Google Scholar] [CrossRef]
- Cheng, G.; Cai, Z.; Wang, L. Biocompatibility and biodegradation of poly(hydroxybutyrate)/ poly(ethylene glycol) blend films. J. Mater. Sci. Mater. Med. 2003, 14, 1073–1078. [Google Scholar] [CrossRef]
- Freier, T.; Kunze, C.; Nischan, C.; Kramer, S.; Sternberg, K.; Hoptu, T.; Schmitz, K.P. In vitro and in vivo degradation studies for development of a biodegradable patch based on poly(3-hydroxybutyrate). Biomaterials 2002, 23, 2649–2657. [Google Scholar] [CrossRef]
- Machicao, F.; Muresanu, D.F.; Hundsberger, H.; Pflüger, M.; Guekht, A. Pleiotropic neuroprotective and metabolic effects of actovegin. Neuromuscul. Dis. 2012, 4, 28–35. (In Russia) [Google Scholar]
- Drug Database Online. Available online: https://go.drugbank.com/ (accessed on 20 January 2023).
- Peppas, N.A.; Narasimhan, B. Mathematical models in drug delivery: How modeling has shaped the way we design new drug delivery systems. J. Control. Release 2014, 190, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Ladhari, S.; Vu, N.N.; Boisvert, C.; Saidi, A.; Nguyen-Tri, P. Recent Development of Polyhydroxyalkanoates (PHA)-Based Materials for Antibacterial Applications: A Review. ACS Appl. Bio Mater. 2023, 6, 1398–1430. [Google Scholar] [CrossRef] [PubMed]
- Arkoun, M.; Daigle, F.; Heuzey, M.C.; Ajji, A. Mechanism of Action of Electrospun Chitosan-Based Nanofibers against Meat Spoilage and Pathogenic Bacteria. Molecules 2017, 22, 585. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abadi, M.S.S.; Mirzaei, E.; Bazargani, A.; Gholipour, A.; Heidari, H.; Hadi, N. Antibacterial activity and mechanism of action of chitosan nanofibers against toxigenic Clostridioides (Clostridium) difficile Isolates. Ann. Ig. 2020, 32, 72–80. [Google Scholar] [CrossRef]
- Hamdan, N.; Yamin, A.; Hamid, S.A.; Khodir, W.K.; Guarino, V. Functionalized Antimicrobial Nanofibers: Design Criteria and Recent Advances. J. Funct. Biomater. 2021, 12, 59. [Google Scholar] [CrossRef]
- Kehail, A.; Brigham, C.J. Anti-biofilm Activity of Solvent-Cast and ElectrospunPolyhydroxyalkanoate Membranes Treated with Lysozyme. J. Polym. Environ. 2018, 26, 66–72. [Google Scholar] [CrossRef]
- Murueva, A.V.; Shershneva, A.M.; Abanina, K.V.; Prudnikova, S.V.; Shishatskaya, E.I. Development and characterization of ceftriaxone-loaded P3HB-based microparticles for drug delivery. J. Drying Tech. 2019, 37, 1131–1142. [Google Scholar] [CrossRef] [Green Version]
- Giacometti, A.; Cirioni, O.; Schimizzi, A.M.; Del Prete, M.S.; Barchiesi, F.; D’Errico, M.M.; Petrelli, E.; Scalise, G. Epidemiology and microbiology of surgical wound infections. J. Clin. Microbiol. 2000, 38, 918–922. [Google Scholar] [CrossRef]
- Soares, G.M.S.; Figueiredo, L.C.; Faveri, M.; Cortelli, S.C.; Duarte, P.M.; Feres, M. Mechanisms of action of systemic antibiotics used in periodontal treatment and mechanisms of bacterial resistance to these drugs. J. Appl. Oral Sci. 2012, 20, 295–309. [Google Scholar] [CrossRef] [Green Version]
- Kapoor, G.; Saigal, S.; Elongavan, A. Action and resistance mechanisms of antibiotics: A guide for clinicians. J. Anaesthesiol. Clin. Pharmacol. 2017, 33, 300–305. [Google Scholar] [CrossRef] [PubMed]
- Hutmacher, D.W. Scaffold design and fabrication technologies for engineering tissues–state of the art and future perspectives. J. Biomater. Sci. Polym. Ed. 2001, 12, 107–124. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.H.; Chi, C.Y.; Chen, Y.-S.; Chen, K.-Y.; Chen, P.-L.; Yao, C.-H. Evaluation of proanthocyanidin-crosslinked electrospun gelatin nanofibers for drug delivering system. Mater. Sci. Engin. C 2012, 32, 2476–2483. [Google Scholar] [CrossRef]
- Thakur, R.A.; Florek, C.A.; Kohn, J.; Michniak, B.B. Electrospun nanofibrous polymeric scaffold with targeted drug release profiles for potential application as wound dressing. Inter. J. of Pharm. 2008, 364, 87–93. [Google Scholar] [CrossRef] [PubMed]








| Drug | Structural Formula | Mechanism of Action |
|---|---|---|
| Ceftazidime |  | Ceftazidime is a third-generation cephalosporin antibiotic. It has a bactericidal effect, disrupting the synthesis of the bacterial cell wall. It has a high natural activity against gram-negative bacteria and is not inactivated by many β-lactamases. Degraded by extended spectrum β-lactamases and class C β-lactamases. |
| Doripinem |  | Antibiotic of the beta-lactam group. It has a wide spectrum of antimicrobial activity, including many Gram-positive and Gram-negative aerobes and anaerobes. Resistant to penicillinases and cephalosporinases. The mechanism of action is based on the binding of specific beta-lactamotropic proteins of the cell wall and inhibition of peptidoglycan synthesis, leading to the lysis of sensitive bacteria. |
| Actovegin | * It is a deproteinized hemoderivative of calf blood, which includes amino acids, oligopeptides, nucleosides, intermediate products of carbohydrate and fat metabolism, antioxidant enzymes, electrolytes, microelements. | Antihypoxant has three types of effects: metabolic, neuroprotective and microcirculatory; activates the metabolism in tissues, improves trophism, and stimulates the regeneration processes; is widely used in Russia in clinical practice in the form of intravenous injections and as part of gels and ointments for wound healing. |
| Matrix Thickness, mm | Fiber Diameter, µm | Average Fibers Diameter, µm | Fiber Bulk Density, g/cm3 |
|---|---|---|---|
| P(3HB-co-4HB) | |||
| 0.185 | 1.4–3.7 | 2.7 ± 0.5 | 0.4 |
| P(3HB-co-4HB)/doripinem | |||
| 0.120 | 4.3–9.2 | 6.8 ± 0.9 | 0.9 |
| P(3HB-co-4HB)/ceftazidime | |||
| 0.155 | 0.1–2.7 | 1.2 ± 0.6 | 0.4 |
| P(3HB-co-4HB)/actovegin | |||
| 0.160 | 0.2–2.6 | 1.1 ± 0.5 | 0.6 ± 0.1 |
| Contact Angle of Wetting with Water [°] | Free Surface Energy, mN/m | Dispersion Component, mN/m | Polar Component, mN/m |
|---|---|---|---|
| P(3HB-co-4HB) | |||
| 123 ± 5 | 47.4 ± 0.4 | 44.1 ± 0.2 | 3.3 ± 0.2 |
| P(3HB-co-4HB)/doripinem | |||
| 72.3 ± 0.9 | 38.1 ± 0.4 | 27.4 ± 0.3 | 10.7 ± 0.1 |
| P(3HB-co-4HB)/ceftazidime | |||
| 98.4 ± 2.2 | 31.8 ± 0.3 | 31.1 ± 0.3 | 0.7 ± 0.1 |
| P(3HB-co-4HB)/actovegin | |||
| 77.3 ± 3.9 | 36.7 ± 1.1 | 29.2 ± 0.7 | 7.6 ± 0.3 |
| Drug | Zero-Order | First-Order | Higuchi | Hixon-Crowell | Korsmeyer-Peppas | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| R2 | K0 × h−1 | R2 | K1 × h−1 | R2 | KH × h−0.5 | R2 | KSC(h−1/3) | R2 | n | |
| DOR | 0.66 | 0.14 | 0.70 | −0.0009 | 0.93 | 2.56 | 0.82 | −0.007 | 0.99 | 0.25 |
| CEF | 0.63 | 0.11 | 0.85 | −0.0005 | 0.84 | 2.26 | 0.67 | −0.005 | 0.98 | 0.24 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Volova, T.G.; Demidenko, A.V.; Murueva, A.V.; Dudaev, A.E.; Nemtsev, I.; Shishatskaya, E.I. Biodegradable Polyhydroxyalkanoates Formed by 3- and 4-Hydroxybutyrate Monomers to Produce Nanomembranes Suitable for Drug Delivery and Cell Culture. Technologies 2023, 11, 106. https://doi.org/10.3390/technologies11040106
Volova TG, Demidenko AV, Murueva AV, Dudaev AE, Nemtsev I, Shishatskaya EI. Biodegradable Polyhydroxyalkanoates Formed by 3- and 4-Hydroxybutyrate Monomers to Produce Nanomembranes Suitable for Drug Delivery and Cell Culture. Technologies. 2023; 11(4):106. https://doi.org/10.3390/technologies11040106
Chicago/Turabian StyleVolova, Tatiana G., Aleksey V. Demidenko, Anastasiya V. Murueva, Alexey E. Dudaev, Ivan Nemtsev, and Ekaterina I. Shishatskaya. 2023. "Biodegradable Polyhydroxyalkanoates Formed by 3- and 4-Hydroxybutyrate Monomers to Produce Nanomembranes Suitable for Drug Delivery and Cell Culture" Technologies 11, no. 4: 106. https://doi.org/10.3390/technologies11040106