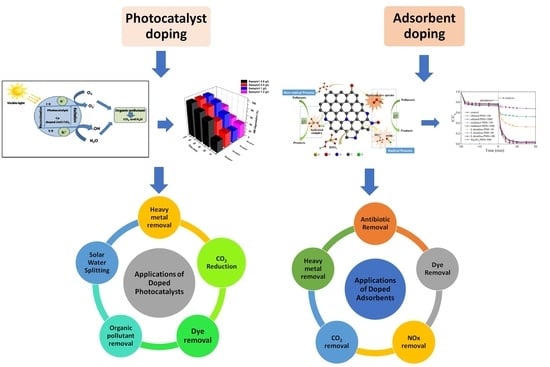
Advancements in Doping Strategies for Enhanced Photocatalysts and Adsorbents in Environmental Remediation
Abstract
:1. Introduction
- ➢
- To provide an extensive review of the methodologies employed in the synthesis of doped photocatalysts and adsorbents, encompassing the utilization of metals, metal oxides, and non-metals.
- ➢
- To examine the diverse applications of doped systems, shedding light on the substantial efficiency improvements realized in doped catalysts and adsorbents when compared to their undoped counterparts.
- ➢
- To scrutinize the influence of dopants on the performance of integrated adsorbent-photocatalytic units, uncovering the synergistic effects and benefits conferred by doping.
- ➢
- To identify and discuss the existing limitations inherent to doping processes and propose potential strategies to overcome these limitations.
2. Doping of Photocatalysts with Nanoparticles
2.1. Doping with Metal and Metal Oxides
2.1.1. Sol–Gel Method
2.1.2. Solvo/Hydrothermal Treatment
2.1.3. Pulsed Laser Ablation in Liquid (PLAL)
2.1.4. Flame Spray Pyrolysis
2.1.5. Chemical Precipitation and Co-Precipitation
2.1.6. Recent Progress in Metal-Doped Photocatalysts
2.2. Photocatalysts Doped with Non-Metals
2.3. Structural Modifications of Doped Photocatalysts
3. The Doping of Adsorbentswith Nanoparticles
3.1. Nitrogen Doping
Synthesis of Nitrogen-Doped Adsorbent
3.2. Metal Nanoparticle-Doped Adsorbents
The Synthesis of Metal-Doped Adsorbents
3.3. Recent Advancements in Doped Adsorbents
4. Applications of Doped Photocatalysts and Adsorbents
5. Integrated Photocatalyst-Adsorbent Systems with Doping
6. Conclusions and Future Perspective
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lin, C.; Song, Y.; Cao, L.; Chen, S. Effective Photocatalysis of Functional Nanocomposites Based on Carbon and TiO2 Nanoparticles. Nanoscale 2013, 5, 4986–4992. [Google Scholar] [CrossRef]
- Jagadale, T.C.; Takale, S.P.; Sonawane, R.S.; Joshi, H.M.; Patil, S.I.; Kale, B.B.; Ogale, S.B. N-Doped TiO2 Nanoparticle Based Visible Light Photocatalyst by Modified Peroxide Sol-Gel Method. J. Phys. Chem. C 2008, 112, 14595–14602. [Google Scholar] [CrossRef]
- Guan, B.; Yu, J.; Guo, S.; Yu, S.; Han, S. Porous Nickel Doped Titanium Dioxide Nanoparticles with Improved Visible Light Photocatalytic Activity. Nanoscale Adv. 2020, 2, 1352–1357. [Google Scholar] [CrossRef]
- Thambiliyagodage, C.; Mirihana, S. Photocatalytic Activity of Fe and Cu Co-Doped TiO2 Nanoparticles under Visible Light. J. Solgel Sci. Technol. 2021, 99, 109–121. [Google Scholar] [CrossRef]
- Negi, C.; Kandwal, P.; Rawat, J.; Sharma, M.; Sharma, H.; Dalapati, G.; Dwivedi, C. Carbon-Doped Titanium Dioxide Nanoparticles for Visible Light Driven Photocatalytic Activity. Appl. Surf. Sci. 2021, 554, 149553. [Google Scholar] [CrossRef]
- El-sayed, M.E.A. Nanoadsorbents for Water and Wastewater Remediation. Sci. Total Environ. 2020, 739, 139903. [Google Scholar] [CrossRef]
- Khajeh, M.; Laurent, S.; Dastafkan, K. Nanoadsorbents: Classification, Preparation, and Applications (with Emphasis on Aqueous Media). Chem. Rev. 2013, 113, 7728–7768. [Google Scholar] [CrossRef]
- Chakhtouna, H.; Benzeid, H.; Zari, N.; El Kacem Qaiss, A.; Bouhfid, R. Recent Progress on Ag/TiO2 Photocatalysts: Photocatalytic and Bactericidal Behaviors. Environ. Sci. Pollut. Res. 2021, 28, 44638–44666. [Google Scholar] [CrossRef]
- Medhi, R.; Marquez, M.D.; Lee, T.R. Visible-Light-Active Doped Metal Oxide Nanoparticles: Review of Their Synthesis, Properties, and Applications. ACS Appl. Nano Mater. 2020, 3, 6156–6185. [Google Scholar] [CrossRef]
- Melchionna, M.; Fornasiero, P. Updates on the Roadmap for Photocatalysis. ACS Catal. 2020, 10, 5493–5501. [Google Scholar] [CrossRef]
- Karuppasamy, P.; Ramzan Nilofar Nisha, N.; Pugazhendhi, A.; Kandasamy, S.; Pitchaimuthu, S. An Investigation of Transition Metal Doped TiO2 Photocatalysts for the Enhanced Photocatalytic Decoloration of Methylene Blue Dye under Visible Light Irradiation. J. Environ. Chem. Eng. 2021, 9, 105254. [Google Scholar] [CrossRef]
- Kanakaraju, D.; anakKutiang, F.D.; Lim, Y.C.; Goh, P.S. Recent Progress of Ag/TiO2 Photocatalyst for Wastewater Treatment: Doping, Co-Doping, and Green Materials Functionalization. Appl. Mater. Today 2022, 27, 101500. [Google Scholar]
- Akpan, U.G.; Hameed, B.H. The Advancements in Sol-Gel Method of Doped-TiO2 Photocatalysts. Appl. Catal. A Gen. 2010, 375, 1–11. [Google Scholar] [CrossRef]
- Bokov, D.; Turki Jalil, A.; Chupradit, S.; Suksatan, W.; Javed Ansari, M.; Shewael, I.H.; Valiev, G.H.; Kianfar, E. Nanomaterial by Sol-Gel Method: Synthesis and Application. Adv. Mater. Sci. Eng. 2021, 2021, 5102014. [Google Scholar] [CrossRef]
- Miditana, S.R.; Tirukkovalluri, S.R.; Raju, I.M.; Babu, A.B.; Babu, A.R. Review on the Synthesis of Doped TiO2 Nanomaterials by Sol-Gel Method and Description of Experimental Techniques. J. Water Environ. Nanotechnol. 2022, 7, 218–229. [Google Scholar]
- Thomas, J.; Radhika, S.; Yoon, M. Nd3+-Doped TiO2 Nanoparticles Incorporated with Heteropoly Phosphotungstic Acid: A Novel Solar Photocatalyst for Degradation of 4-Chlorophenol in Water. J. Mol. Catal. A Chem. 2015, 411, 146–156. [Google Scholar] [CrossRef]
- Sun, D.; Wang, K.; Xu, Z.; Li, R. Synthesis and Photocatalytic Activity of Sulfate Modified Nd-Doped TiO2 under Visible Light Irradiation. J. Rare Earths 2015, 33, 491–497. [Google Scholar] [CrossRef]
- Lal, M.; Sharma, P.; Ram, C. Synthesis and Photocatalytic Potential of Nd-Doped TiO2 under UV and Solar Light Irradiation Using a Sol-Gel Ultrasonication Method. Results Mater. 2022, 15, 100308. [Google Scholar] [CrossRef]
- Nithya, N.; Bhoopathi, G.; Magesh, G.; Kumar, C.D.N. Neodymium Doped TiO2 Nanoparticles by Sol-Gel Method for Antibacterial and Photocatalytic Activity. Mater. Sci. Semicond. Process. 2018, 83, 70–82. [Google Scholar] [CrossRef]
- Avram, D.; Patrascu, A.A.; Istrate, M.C.; Cojocaru, B.; Tiseanu, C. Lanthanide Doped TiO2: Coexistence of Discrete and Continuous Dopant Distribution in Anatase Phase. J. Alloys Compd. 2021, 851, 156849. [Google Scholar] [CrossRef]
- Caschera, D.; Federici, F.; de Caro, T.; Cortese, B.; Calandra, P.; Mezzi, A.; Lo Nigro, R.; Toro, R.G. Fabrication of Eu-TiO2 NCs Functionalized Cotton Textile as a Multifunctional Photocatalyst for Dye Pollutants Degradation. Appl. Surf. Sci. 2018, 427, 81–91. [Google Scholar] [CrossRef]
- Luo, Y.; Wang, K.; Qian, Q.; Zheng, W.; Xue, H.; Huang, B.; Xiao, L.; Chen, Q. Fabrication and Photocatalytic Properties of Gd-Doped ZnO Nanoparticle-Assembled Nanorods. Mater. Lett. 2015, 149, 70–73. [Google Scholar] [CrossRef]
- Mehtab, A.; Ahmed, J.; Alshehri, S.M.; Mao, Y.; Ahmad, T. Rare Earth Doped Metal Oxide Nanoparticles for Photocatalysis: A Perspective. Nanotechnology 2022, 33, 142001. [Google Scholar] [CrossRef] [PubMed]
- Dhandapani, C.; Narayanasamy, R.; Karthick, S.N.; Hemalatha, K.V.; Selvam, S.; Hemalatha, P.; Kumar, M.S.; Kirupha, S.D.; Kim, H.J. Drastic Photocatalytic Degradation of Methylene Blue Dye by Neodymium Doped Zirconium Oxide as Photocatalyst under Visible Light Irradiation. Optik 2016, 127, 10288–10296. [Google Scholar] [CrossRef]
- Zhang, J.; Deng, S.J.; Liu, S.Y.; Chen, J.M.; Han, B.Q.; Wang, Y.; Wang, Y.D. Preparation and Photocatalytic Activity of Nd Doped ZnO Nanoparticles. Mater. Technol. 2014, 29, 262–268. [Google Scholar] [CrossRef]
- Zaman, Y.; Ishaque, M.Z.; Waris, K.; Shahzad, M.; Siddique, A.B.; Arshad, M.I.; Zaman, H.; Ali, H.M.; Kanwal, F.; Aslam, M.; et al. Modified Physical Properties of Ni Doped ZnO NPs as Potential Photocatalyst and Antibacterial Agents. Arab. J. Chem. 2023, 16, 105230. [Google Scholar] [CrossRef]
- Choudhary, K.; Saini, R.; Purohit, L.P. Controllable Synthesis of Ce-Doped ZnO: TiO2 Nanospheres for Photocatalytic Degradation of MB Dye and Levofloxacin under Sunlight Light Irradiation. Opt. Mater. 2023, 143, 114167. [Google Scholar] [CrossRef]
- Palai, A.; Panda, N.R.; Sahu, D. Exploring the Photoluminescence Property, Photocatalytic Efficiency, and Antibacterial Activity of Eu-Doped ZnO/SnO2 Heterostructure. ECS J. Solid State Sci. Technol. 2023, 12, 076015. [Google Scholar] [CrossRef]
- Moradi, M.; Khorasheh, F.; Larimi, A. Pt Nanoparticles Decorated Bi-Doped TiO2 as an Efficient Photocatalyst for CO2 Photo-Reduction into CH4. Sol. Energy 2020, 211, 100–110. [Google Scholar] [CrossRef]
- Ochoa Rodríguez, P.A.; Pecchi, G.A.; Casuscelli, S.G.; Elías, V.R.; Eimer, G.A. A Simple Synthesis Way to Obtain Iron-Doped TiO2 Nanoparticles as Photocatalytic Surfaces. Chem. Phys. Lett. 2019, 732, 136643. [Google Scholar] [CrossRef]
- Sultana, R.; Liba, S.I.; Rahman, M.A.; Yeachin, N.; Syed, I.M.; Bhuiyan, M.A. Enhanced Photocatalytic Activity in RhB Dye Degradation by Mn and B Co-Doped Mixed Phase TiO2 Photocatalyst under Visible Light Irradiation. Surf. Interfaces 2023, 42, 103302. [Google Scholar] [CrossRef]
- Abbad, S.; Guergouri, K.; Gazaout, S.; Djebabra, S.; Zertal, A.; Barille, R.; Zaabat, M. Effect of Silver Doping on the Photocatalytic Activity of TiO2 Nanopowders Synthesized by the Sol-Gel Route. J. Environ. Chem. Eng. 2020, 8, 103718. [Google Scholar] [CrossRef]
- Pérez-González, M.; Tomás, S.A.; Santoyo-Salazar, J.; Gallardo-Hernández, S.; Tellez-Cruz, M.M.; Solorza-Feria, O. Sol-Gel Synthesis of Ag-Loaded TiO2-ZnO Thin Films with Enhanced Photocatalytic Activity. J. Alloys Compd. 2019, 779, 908–917. [Google Scholar] [CrossRef]
- Bibi, S.; Shah, S.S.; Muhammad, F.; Siddiq, M.; Kiran, L.; Aldossari, S.A.; Sheikh Saleh Mushab, M.; Sarwar, S. Cu-Doped Mesoporous TiO2 Photocatalyst for Efficient Degradation of Organic Dye via Visible Light Photocatalysis. Chemosphere 2023, 339, 139583. [Google Scholar] [CrossRef] [PubMed]
- Padmaja, B.; Dhanapandian, S.; Ashokkumar, K. Hydrothermally Derived Mg Doped Tin Oxide Nanostructures for Photocatalytic and Supercapacitor Applications. Mater. Sci. Eng. B 2023, 297, 116699. [Google Scholar] [CrossRef]
- Lertthanaphol, N.; Pienutsa, N.; Chusri, K.; Sornsuchat, T.; Chanthara, P.; Seeharaj, P.; Kim-Lohsoontorn, P.; Srinives, S. One-Step Hydrothermal Synthesis of Precious Metal-Doped Titanium Dioxide–Graphene Oxide Composites for Photocatalytic Conversion of CO2 to Ethanol. ACS Omega 2021, 6, 35769–35779. [Google Scholar] [CrossRef]
- Sunkhunthod, C.; Jiamprasertboon, A.; Waehayee, A.; Untarabut, P.; Phonsuksawang, P.; Butburee, T.; Suthirakun, S.; Siritanon, T. Enhanced Tetracycline Photocatalytic Degradation of FeOx/Fe-Bi2O2CO3 Synthesized by One-Step Hydrothermal Method. J. Alloys Compd. 2023, 960, 170632. [Google Scholar] [CrossRef]
- Tarasenka, N.; Kornev, V.; Ramanenka, A.; Li, R.; Tarasenko, N. Photoluminescent Neodymium-Doped ZnO Nanocrystals Prepared by Laser Ablation in Solution for NIR-II Fluorescence Bioimaging. Heliyon 2022, 8, e09554. [Google Scholar] [CrossRef]
- Chemin, A.; Lam, J.; Laurens, G.; Trichard, F.; Motto-Ros, V.; Ledoux, G.; Jarý, V.; Laguta, V.; Nikl, M.; Dujardin, C.; et al. Doping Nanoparticles Using Pulsed Laser Ablation in a Liquid Containing the Doping Agent. Nanoscale Adv. 2019, 1, 3963–3972. [Google Scholar] [CrossRef]
- Siriwong, C.; Wetchakun, N.; Inceesungvorn, B.; Channei, D.; Samerjai, T.; Phanichphant, S. Doped-Metal Oxide Nanoparticles for Use as Photocatalysts. Prog. Cryst. Growth Charact. Mater. 2012, 58, 145–163. [Google Scholar] [CrossRef]
- Psathas, P.; Zindrou, A.; Papachristodoulou, C.; Boukos, N.; Deligiannakis, Y. In Tandem Control of La-Doping and CuO-Heterojunction on SrTiO3 Perovskite by Double-Nozzle Flame Spray Pyrolysis: Selective H2 vs. CH4 Photocatalytic Production from H2O/CH3OH. Nanomaterials 2023, 13, 482. [Google Scholar] [CrossRef]
- Trung Tran, S.B.; Choi, H.S.; Oh, S.Y.; Moon, S.Y.; Park, J.Y. Iron-Doped ZnO as a Support for Pt-Based Catalysts to Improve Activity and Stability: Enhancement of Metal-Support Interaction by the Doping Effect. RSC Adv. 2018, 8, 21528–21533. [Google Scholar] [CrossRef] [PubMed]
- Radha, B.; Rathi, R.; Lalithambika, K.C.; Thayumanavan, A.; Ravichandran, K.; Sriram, S. Effect of Fe Doping on the Photocatalytic Activity of ZnO Nanoparticles: Experimental and Theoretical Investigations. J. Mater. Sci. Mater. Electron. 2018, 29, 13474–13482. [Google Scholar] [CrossRef]
- Rani, M.; Keshu; Pandey, S.; Rishabh; Sharma, S.; Shanker, U. Sunlight Assisted Highly Efficient Photocatalytic Remediation of Organic Pollutants by Green Biosynthesized ZnO@WO3 Nanocomposite. J. Photochem. Photobiol. A Chem. 2024, 446, 115160. [Google Scholar] [CrossRef]
- Zeid, E.F.F.A.; Obiedallah, F.M.M.; Abu-Sehly, A.H.; Mohamed, W.A.A.; El-Aal, M.A. A Comparative Study of Single and Bi-Doped Co3O4 Nanocatalysts for the Photodegradation of Methyl Orange Dye. J. Mol. Struct. 2023, 1293, 136203. [Google Scholar] [CrossRef]
- Ahmadi, N.; Nemati, A.; Bagherzadeh, M. Synthesis and Properties of Ce-Doped TiO2-Reduced Graphene Oxide Nanocomposite. J. Alloys Compd. 2018, 742, 986–995. [Google Scholar] [CrossRef]
- Velázquez-Martínez, S.; Silva-Martínez, S.; Pineda-Arellano, C.A.; Jiménez-González, A.; Salgado-Tránsito, I.; Morales-Pérez, A.A.; Peña-Cruz, M.I. Modified Sol-Gel/Hydrothermal Method for the Synthesis of Microsized TiO2 and Iron-Doped TiO2, Its Characterization and Solar Photocatalytic Activity for an Azo Dye Degradation. J. Photochem. Photobiol. A Chem. 2018, 359, 93–101. [Google Scholar] [CrossRef]
- Baig, F.; Butt, G.S. Impact of Copper Doping on Optical, UV Induced Wettability and Photo-Catalytic Properties of Sol-Gel Synthesized ZnO Thin Films. Optik 2023, 288, 171196. [Google Scholar] [CrossRef]
- Hameeda, B.; Mushtaq, A.; Saeed, M.; Munir, A.; Jabeen, U.; Waseem, A. Development of Cu-Doped NiO Nanoscale Material as Efficient Photocatalyst for Visible Light Dye Degradation. Toxin Rev. 2021, 40, 1396–1406. [Google Scholar] [CrossRef]
- Soleimani-Gorgani, A.; Al-Hazmi, H.E.; Esmaeili, A.; Habibzadeh, S. Screen-Printed Sn-Doped TiO2 Nanoparticles for Photocatalytic Dye Removal from Wastewater: A Technological Perspective. Environ. Res. 2023, 237, 117079. [Google Scholar] [CrossRef]
- Vishnu, G.; Singh, S.; Kaul, N.; Ramamurthy, P.C.; Naik, T.S.S.K.; Viswanath, R.; Kumar, V.; Bhojya Naik, H.S.; Prathap, A.; Anil Kumara, H.A.; et al. Green Synthesis of Nickel-Doped Magnesium Ferrite Nanoparticles via Combustion for Facile Microwave-Assisted Optical and Photocatalytic Applications. Environ. Res. 2023, 235, 116598. [Google Scholar] [CrossRef]
- Azimi-Fouladi, A.; Falak, P.; Hassanzadeh-Tabrizi, S.A. The Photodegradation of Antibiotics on Nano Cubic Spinel Ferrites Photocatalytic Systems: A Review. J. Alloys Compd. 2023, 961, 171075. [Google Scholar]
- Naik, M.M.; Naik, H.S.B.; Nagaraju, G.; Vinuth, M.; Vinu, K.; Rashmi, S.K. Effect of Aluminium Doping on Structural, Optical, Photocatalytic and Antibacterial Activity on Nickel Ferrite Nanoparticles by Sol–Gel Auto-Combustion Method. J. Mater. Sci. Mater. Electron. 2018, 29, 20395–20414. [Google Scholar] [CrossRef]
- Liu, D.; Sun, B.; Bai, S.; Gao, T.; Zhou, G. Dual Co-Catalysts Ag/Ti3C2/TiO2 Hierarchical Flower-like Microspheres with Enhanced Photocatalytic H2-Production Activity. Chin. J. Catal. 2023, 50, 273–283. [Google Scholar] [CrossRef]
- Dong, W.W.; Jia, J.; Wang, Y.; An, J.R.; Yang, O.Y.; Gao, X.J.; Liu, Y.L.; Zhao, J.; Li, D.S. Visible-Light-Driven Solvent-Free Photocatalytic CO2 Reduction to CO by Co-MOF/Cu2O Heterojunction with Superior Selectivity. Chem. Eng. J. 2022, 438, 135622. [Google Scholar] [CrossRef]
- An, J.R.; Wang, Y.; Dong, W.W.; Gao, X.J.; Yang, O.Y.; Liu, Y.L.; Zhao, J.; Li, D.S. Efficient Visible-Light Photoreduction of CO2 to CH4 over an Fe-Based Metal-Organic Framework (PCN-250-Fe3) in a Solid-Gas Mode. ACS Appl. Energy Mater. 2022, 5, 2384–2390. [Google Scholar] [CrossRef]
- Yang, O.Y.; Gao, X.J.; Qi, G.D.; Wang, Y.; Dong, W.W.; Tian, Z.F.; Zhao, J.; Li, D.S.; Zhang, Q. Dye-Anchoring Strategy with a Metal-Organic Framework for a Highly Efficient Visible-Light-Driven Photocatalytic CO2 Reduction through the Solid-Gas Mode. ACS Appl. Energy Mater. 2023, 6, 334–341. [Google Scholar] [CrossRef]
- Wang, W.; Sheng, Q.; Zhi, G.; Zhao, Y.; Qu, R.; Sun, L.; Zhang, S. A Strategy of Adjusting Band Alignment to Improve Photocatalytic Degradation and Photocatalytic Hydrogen Evolution of CuSbS2. Appl. Surf. Sci. 2023, 639, 158251. [Google Scholar] [CrossRef]
- Teng, Z.; Wang, B.; Hu, Y.; Zhang, W.; Wu, Z.; Xu, D. High Performance Ultrafiltration Membrane by Coupling Magnetic Migration and In-Situ Surface Modification. Polym. Test. 2021, 101, 107306. [Google Scholar] [CrossRef]
- Wang, L.; Sun, M.; Zhu, S.; Zhang, M.; Ma, Y.; Xie, D.; Li, S.; Mominou, N.; Jing, C. Cu-Ni Bimetallic Single Atoms Supported on TiO2@NG Core-Shell Material for the Removal of Dibenzothiophene under Visible Light. Sep. Purif. Technol. 2021, 279, 119646. [Google Scholar] [CrossRef]
- Zhang, Z.-R.; Guo, R.-T.; Xia, C.; Li, C.-F.; Pan, W.-G. B-TiO2/CuInS2 Photocatalyst Based on the Synergistic Effect of Oxygen Vacancy and Z-Scheme Heterojunction for Improving Photocatalyst CO2 Reduction. Sep. Purif. Technol. 2023, 323, 124461. [Google Scholar] [CrossRef]
- Liu, S.H.; Syu, H.R. High Visible-Light Photocatalytic Hydrogen Evolution of C,N-Codoped Mesoporous TiO2 Nanoparticles Prepared via an Ionic-Liquidtemplate Approach. Int. J. Hydrogen Energy 2013, 38, 13856–13865. [Google Scholar] [CrossRef]
- Ansari, S.A.; Khan, M.M.; Ansari, M.O.; Cho, M.H. Nitrogen-Doped Titanium Dioxide (N-Doped TiO2) for Visible Light Photocatalysis. New J. Chem. 2016, 40, 3000–3009. [Google Scholar] [CrossRef]
- Lin, L.-Y.; Nie, Y.; Kavadiya, S.; Soundappan, T.; Biswas, P. N-Doped Reduced Graphene Oxide Promoted Nano TiO2 as a Bifunctional Adsorbent/Photocatalyst for CO2 Photoreduction: Effect of N Species. Chem. Eng. J. 2017, 316, 449–460. [Google Scholar] [CrossRef]
- Mersal, M.; Zedan, A.F.; Mohamed, G.G.; Hassan, G.K. Fabrication of Nitrogen Doped TiO2/Fe2O3 Nanostructures for Photocatalytic Oxidation of Methanol Based Wastewater. Sci. Rep. 2023, 13, 4431. [Google Scholar] [CrossRef]
- Ellouzi, I.; El Hajjaji, S.; Harir, M.; Schmitt Koplin, P.; Robert, D.; Laânab, L. Synergistic Effects of C, N, S, Fe-Multidoped TiO2 for Photocatalytic Degradation of Methyl Orange Dye under UV and Visible Light Irradiations. SN Appl. Sci. 2019, 1, 930. [Google Scholar] [CrossRef]
- Shandilya, P.; Mittal, D.; Sudhaik, A.; Soni, M.; Raizada, P.; Saini, A.K.; Singh, P. GdVO4 Modified Fluorine Doped Graphene Nanosheets as Dispersed Photocatalyst for Mitigation of Phenolic Compounds in Aqueous Environment and Bacterial Disinfection. Sep. Purif. Technol. 2019, 210, 804–816. [Google Scholar] [CrossRef]
- Toe, E.D.; Kurniawan, W.; Mariquit, E.G.; Hinode, H. Synthesis of N-Doped Mesoporous TiO2 by Facile One-Step Solvothermal Process for Visible Light Photocatalytic Degradation of Organic Pollutant. J. Environ. Chem. Eng. 2018, 6, 5125–5134. [Google Scholar] [CrossRef]
- Tao, F.T.; Hu, C.; Wu, J.C.S.; Nguyen, V.H.; Tung, K.L. Influence of Nitrogen Sources on N-Doped Reduced TiO2 Prepared Using Atmospheric Plasma Spraying for Photocatalytic Tetracycline and Ciprofloxacin Degradation. Sep. Purif. Technol. 2023, 326, 124784. [Google Scholar] [CrossRef]
- Guan, J.; Li, D.; Feng, J.; Xu, P.; Li, Z.; Ge, S.; Chen, H.; Zhang, K. Enhanced Photocatalytic Ammonia Oxidation Activity and Nitrogen Selectivity over Ag/AgCl/N-TiO2 Photocatalyst. J. Environ. Sci. 2024, 138, 395–405. [Google Scholar] [CrossRef]
- Liu, X.; Wang, J.; Liao, T.; Li, Y.; Fan, X.; Zhang, F.; Peng, W. Electron Transfer Route Tuning Caused by Photo-Irradiation: Stability Enhancement of N-Doped Carbon Materials in Fenton-like Reactions. Appl. Catal. B 2024, 340, 123261. [Google Scholar] [CrossRef]
- Mustafa, G.; Bibi, I.; Majid, F.; Sultan, M.; Taj, B.; Nazeer, Z.; Muhammad Umair, H.; Elqahtani, Z.M.; Alwadai, N.; Khan, M.I.; et al. Zn and Ni Doping Effect on Barium Hexaferrite Ferroelectric, Optical Properties and Photocatalytic Activity under Visible Light Irradiation. Phys. B Condens. Matter 2023, 663, 415006. [Google Scholar] [CrossRef]
- Ordoñez, M.F.; Cerrato, G.; Giordana, A.; Di Michele, A.; Falletta, E.; Bianchi, C.L. One-Pot Synthesis of Ag-Modified SrTiO3: Synergistic Effect of Decoration and Doping for Highly Efficient Photocatalytic NOx Degradation under LED. J. Environ. Chem. Eng. 2023, 11, 110368. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, X.; Zhang, X.; Zhang, W.; Ma, J.; Wang, Q.; Su, S. Sulfur-Doped Sn3O4 Nanosheets for Improved Photocatalytic Performance. J. Alloys Compd. 2023, 961, 170904. [Google Scholar] [CrossRef]
- Meng, A.; Xing, J.; Li, Z.; Li, Q. Cr-Doped ZnO Nanoparticles: Synthesis, Characterization, Adsorption Property, and Recyclability. ACS Appl. Mater. Interfaces 2015, 7, 27449–27457. [Google Scholar] [CrossRef]
- Qumar, U.; Hassan, J.Z.; Bhatti, R.A.; Raza, A.; Nazir, G.; Nabgan, W.; Ikram, M. Photocatalysis vs Adsorption by Metal Oxide Nanoparticles. J. Mater. Sci. Technol. 2022, 131, 122–166. [Google Scholar] [CrossRef]
- Kumar, K.V.; Preuss, K.; Lu, L.; Guo, Z.X.; Titirici, M.M. Effect of Nitrogen Doping on the CO2 Adsorption Behavior in Nanoporous Carbon Structures: A Molecular Simulation Study. J. Phys. Chem. C 2015, 119, 22310–22321. [Google Scholar] [CrossRef]
- Al-Hajri, W.; De Luna, Y.; Bensalah, N. Review on Recent Applications of Nitrogen-Doped Carbon Materials in CO2 Capture and Energy Conversion and Storage. Energy Technol. 2022, 10, 2200498. [Google Scholar] [CrossRef]
- Ouyang, L.; Xiao, J.; Jiang, H.; Yuan, S. Nitrogen-Doped Porous Carbon Materials Derived from Graphene Oxide/Melamine Resin Composites for CO2 Adsorption. Molecules 2021, 26, 5293. [Google Scholar] [CrossRef]
- Wang, Q.; Han, L.; Wang, Y.; He, Z.; Meng, Q.; Wang, S.; Xiao, P.; Jia, X. Conversion of Coal into N-Doped Porous Carbon for High-Performance SO2 Adsorption. RSC Adv. 2022, 12, 20640–20648. [Google Scholar] [CrossRef]
- Han, W.; Wang, H.; Xia, K.; Chen, S.; Yan, P.; Deng, T.; Zhu, W. Superior Nitrogen-Doped Activated Carbon Materials for Water Cleaning and Energy Storing Prepared from Renewable Leather Wastes. Environ. Int. 2020, 142, 105846. [Google Scholar] [CrossRef] [PubMed]
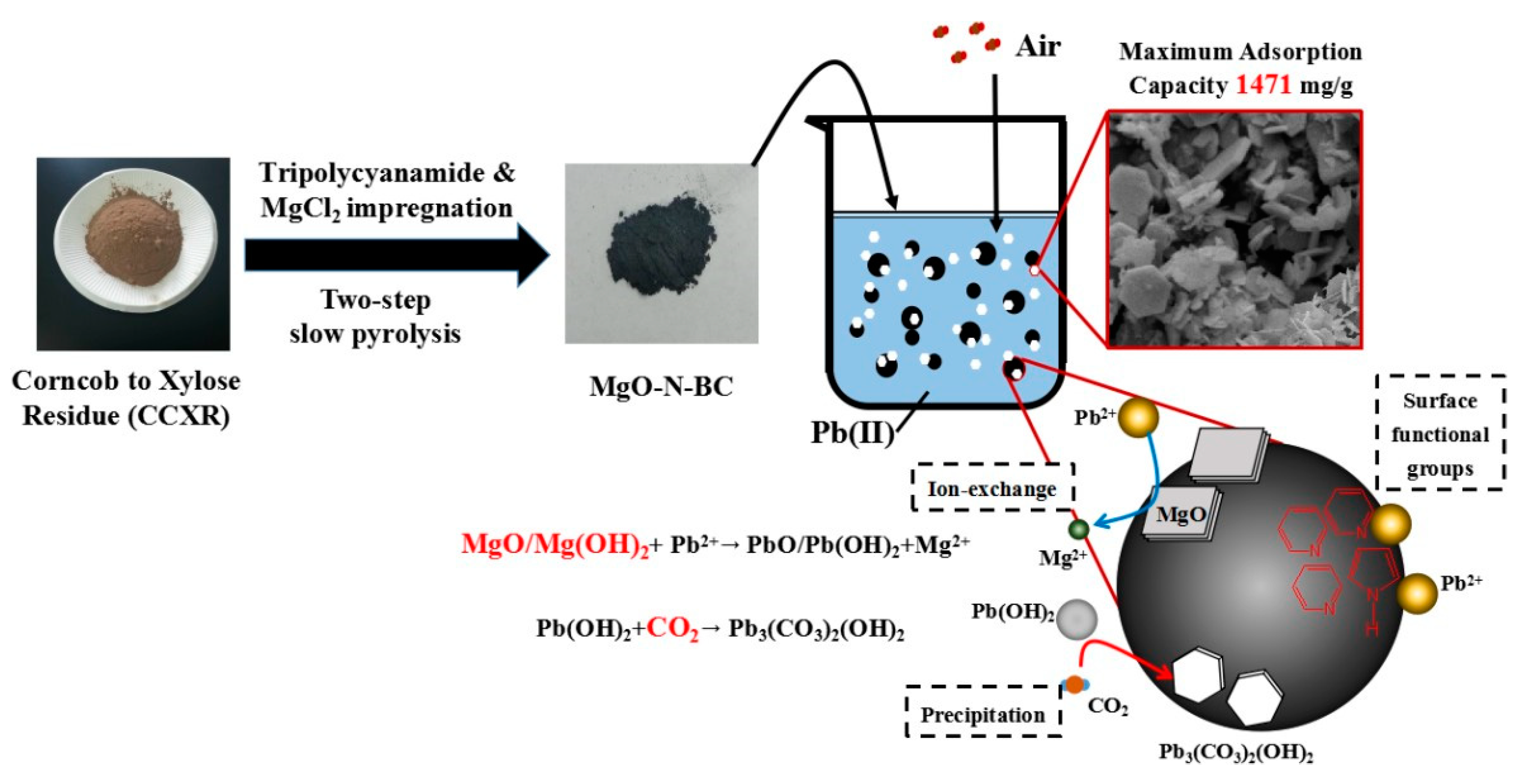
- Shang, H.; Li, Y.; Liu, J.; Wan, Y.; Feng, Y.; Yu, Y. Preparation of Nitrogen Doped Magnesium Oxide Modified Biochar and Its Sorption Efficiency of Lead Ions in Aqueous Solution. Bioresour Technol. 2020, 314, 123708. [Google Scholar] [CrossRef] [PubMed]
- Xiao, W.; Jiang, X.; Liu, X.; Zhou, W.; Garba, Z.N.; Lawan, I.; Wang, L.; Yuan, Z. Adsorption of Organic Dyes from Wastewater by Metal-Doped Porous Carbon Materials. J. Clean. Prod. 2021, 284, 124773. [Google Scholar] [CrossRef]
- Montazerolghaem, M.; Aghamiri, S.F.; Tangestaninejad, S.; Talaie, M.R. A Metal-Organic Framework MIL-101 Doped with Metal Nanoparticles (Ni & Cu) and Its Effect on CO2 Adsorption Properties. RSC Adv. 2015, 6, 632–640. [Google Scholar] [CrossRef]
- Qin, W.; Cao, W.; Liu, H.; Li, Z.; Li, Y. Metal-Organic Framework MIL-101 Doped with Palladium for Toluene Adsorption and Hydrogen Storage. RSC Adv. 2014, 4, 2414–2420. [Google Scholar] [CrossRef]
- Ebrahim, A.M.; Bandosz, T.J. Ce(III) Doped Zr-Based MOFs as Excellent NO2 Adsorbents at Ambient Conditions. ACS Appl. Mater. Interfaces 2013, 5, 10565–10573. [Google Scholar] [CrossRef]
- Kim, T.; Lee, S.K.; Lee, S.; Lee, J.S.; Kim, S.W. Development of Silver Nanoparticle–Doped Adsorbents for the Separation and Recovery of Radioactive Iodine from Alkaline Solutions. Appl. Radiat. Isot. 2017, 129, 215–221. [Google Scholar] [CrossRef]
- Cheng, Z.-L.; Sun, W. Preparation of Nano-CuO-Loaded Halloysite Nanotubes with High Catalytic Activity for Selective Oxidation of Cyclohexene. Chin. Chem. Lett. 2016, 27, 81–84. [Google Scholar] [CrossRef]
- Rahali, S.; Ben Aissa, M.A.; Khezami, L.; Elamin, N.; Seydou, M.; Modwi, A. Adsorption Behavior of Congo Red onto Barium-Doped ZnO Nanoparticles: Correlation between Experimental Results and DFT Calculations. Langmuir 2021, 37, 7285–7294. [Google Scholar] [CrossRef]
- Hu, P.; Wang, S.; Zhuo, Y. Research on CO2 Adsorption Performances of Metal-Doped (Ca, Fe and Al) MgO. Sep. Purif. Technol. 2021, 276, 119323. [Google Scholar] [CrossRef]
- Kim, T.S.; Song, H.J.; Dar, M.A.; Lee, H.J.; Kim, D.W. Fast Adsorption Kinetics of Highly Dispersed Ultrafine Nickel/Carbon Nanoparticles for Organic Dye Removal. Appl. Surf. Sci. 2018, 439, 364–370. [Google Scholar] [CrossRef]
- Fronczak, M.; Demby, K.; Strachowski, P.; Strawski, M.; Bystrzejewski, M. Graphitic Carbon Nitride Doped with the S-Block Metals: Adsorbent for the Removal of Methyl Blue and Copper(II) Ions. Langmuir 2018, 34, 7272–7283. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Cho, S.Y.; Son, K.; Attia, N.F.; Oh, H. A Metal-Doped Flexible Porous Carbon Cloth for Enhanced CO2/CH4 Separation. Sep. Purif. Technol. 2021, 277, 119511. [Google Scholar] [CrossRef]
- Lei, X.; Huang, L.; Liu, K.; Ouyang, L.; Shuai, Q.; Hu, S. Facile One-Pot Synthesis of Hierarchical N-Doped Porous Carbon for Efficient Ibuprofen Removal. J. Colloid. Interface Sci. 2021, 604, 823–831. [Google Scholar] [CrossRef]
- Lu, S.; Huang, X.; Tang, M.; Peng, Y.; Wang, S.; Makwarimba, C.P. Synthesis of N-Doped Hierarchical Porous Carbon with Excellent Toluene Adsorption Properties and Its Activation Mechanism. Environ. Pollut. 2021, 284, 117113. [Google Scholar] [CrossRef]
- Wang, R.; Wang, D.; Peng, W.; Zhang, J.; Liu, J.; Wang, Y.; Wang, X. Removal of F− from Water by Magnetic Floriform Magnesium Zirconium Hydrotalcite-like Material Doped with Fe2O3 and ZrO2. Desalination 2022, 544, 116142. [Google Scholar] [CrossRef]
- Bakry, A.M.; Awad, F.S.; Bobb, J.A.; El-Shall, M.S. Multifunctional Binding Sites on Nitrogen-Doped Carboxylated Porous Carbon for Highly Efficient Adsorption of Pb(II), Hg(II), and Cr(VI) Ions. ACS Omega 2020, 5, 33090–33100. [Google Scholar] [CrossRef]
- Kamran, U.; Park, S.J. Microwave-Assisted Acid Functionalized Carbon Nanofibers Decorated with Mn Doped TNTs Nanocomposites: Efficient Contenders for Lithium Adsorption and Recovery from Aqueous Media. J. Ind. Eng. Chem. 2020, 92, 263–277. [Google Scholar] [CrossRef]
- Mukhtar, A.; Saqib, S.; Mellon, N.B.; Rafiq, S.; Babar, M.; Ullah, S.; Muhammad, N.; Khan, A.L.; Ayoub, M.; Ibrahim, M.; et al. A Review on CO2 Capture via Nitrogen-Doped Porous Polymers and Catalytic Conversion as a Feedstock for Fuels. J. Clean. Prod. 2020, 277, 123999. [Google Scholar] [CrossRef]
- Sanjabi, A.; Azizian, S.; Torabi, M.; Zolfigol, M.A.; Yarie, M. On the Applicability of Triazine-Based Covalent Organic Polymer as Adsorbent for Dye Removal from Aqueous Solution. Microporous Mesoporous Mater. 2023, 348, 112367. [Google Scholar] [CrossRef]
- Aliabadi, H.M.; Zargoosh, K. Synthesis of 3-Amino-1,2,4-Triazole-5-Thiol Functionalized p-Phenylenediamine Covalent Organic Polymer as a Highly Selective Adsorbent for Hg2+ Ions. React. Funct. Polym. 2023, 186, 105575. [Google Scholar] [CrossRef]
- Zolfaghari, S.; Sharafdini, R.; Ghaedi, M.; Javadian, H.; Shokrollahi, A.; Shahvandi, S.K.; Rodrigues, V.H.N.; Razmjoue, D. Synthesis of a Coordination Polymer Based on Zn(DMF)(Tp) as a Novel Adsorbent for the Simultaneous Removal of Quinoline Yellow and Azure B. J. Mol. Struct. 2023, 1294, 136572. [Google Scholar] [CrossRef]
- Abdelmoaty, Y.H.; Tessema, T.D.; Choudhury, F.A.; El-Kadri, O.M.; El-Kaderi, H.M. Nitrogen-Rich Porous Polymers for Carbon Dioxide and Iodine Sequestration for Environmental Remediation. ACS Appl. Mater. Interfaces 2018, 10, 16049–16058. [Google Scholar] [CrossRef]
- Qu, J.G.; Li, N.N.; Liu, B.J.; He, J.X. Preparation of BiVO4/Bentonite Catalysts and Their Photocatalytic Properties under Simulated Solar Irradiation. Mater. Sci. Semicond. Process. 2013, 16, 99–105. [Google Scholar] [CrossRef]
- Vaiano, V.; Iervolino, G.; Rizzo, L. Cu-Doped ZnO as Efficient Photocatalyst for the Oxidation of Arsenite to Arsenate under Visible Light. Appl. Catal. B 2018, 238, 471–479. [Google Scholar] [CrossRef]
- Rosa, D.; D’Agostino, F.; Bavasso, I.; Bracciale, M.P.; Di Palma, L. Easy Way to Produce Iron-Doped Titania Nanoparticles via the Solid-State Method and Investigation Their Photocatalytic Activity. J. Mater. Res. 2023, 38, 1282–1292. [Google Scholar] [CrossRef]
- Xing, M.; Fang, W.; Nasir, M.; Ma, Y.; Zhang, J.; Anpo, M. Self-Doped Ti3+-Enhanced TiO2 Nanoparticles with a High-Performance Photocatalysis. J. Catal. 2013, 297, 236–243. [Google Scholar] [CrossRef]
- Chen, Z.; Li, Y.; Guo, M.; Xu, F.; Wang, P.; Du, Y.; Na, P. One-Pot Synthesis of Mn-Doped TiO2 Grown on Graphene and the Mechanism for Removal of Cr(VI) and Cr(III). J. Hazard. Mater. 2016, 310, 188–198. [Google Scholar] [CrossRef]
- Jiang, Y.; Chowdhury, S.; Balasubramanian, R. Nitrogen-Doped Graphene Hydrogels as Potential Adsorbents and Photocatalysts for Environmental Remediation. Chem. Eng. J. 2017, 327, 751–763. [Google Scholar] [CrossRef]
- Hong, S.P.; Kim, S.; Kim, N.; Yoon, J.; Kim, C. A Short Review on Electrochemically Self-Doped TiO2 Nanotube Arrays: Synthesis and Applications. Korean J. Chem. Eng. 2019, 36, 1753–1766. [Google Scholar] [CrossRef]
- Zhang, Z.; Hedhili, M.N.; Zhu, H.; Wang, P. Electrochemical Reduction Induced Self-Doping of Ti3+ for Efficient Water Splitting Performance on TiO2 Based Photoelectrodes. Phys. Chem. Chem. Phys. 2013, 15, 15637–15644. [Google Scholar] [CrossRef]
- Cao, J.; Yang, Z.-H.; Xiong, W.-P.; Zhou, Y.-Y.; Peng, Y.-R.; Li, X.; Zhou, C.-Y.; Xu, R.; Zhang, Y.-R. One-Step Synthesis of Co-Doped UiO-66 Nanoparticle with Enhanced Removal Efficiency of Tetracycline: Simultaneous Adsorption and Photocatalysis. Chem. Eng. J. 2018, 353, 126–137. [Google Scholar] [CrossRef]
- Adam, F.A.; Ghoniem, M.G.; Diawara, M.; Rahali, S.; Abdulkhair, B.Y.; Elamin, M.R.; Ben Aissa, M.A.; Seydou, M. Enhanced Adsorptive Removal of Indigo Carmine Dye by Bismuth Oxide Doped MgO Based Adsorbents from Aqueous Solution: Equilibrium, Kinetic and Computational Studies. RSC Adv. 2022, 12, 24786–24803. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.; Zhang, L.; Xia, H.; Peng, J.; Shu, J.; Li, C. Ultrasound and Microwave-Assisted Preparation of Fe-Activated Carbon as an Effective Low-Cost Adsorbent for Dyes Wastewater Treatment. RSC Adv. 2016, 6, 78936–78946. [Google Scholar] [CrossRef]
- Wang, Y.; Xiao, J.; Wang, H.; Zhang, T.C.; Yuan, S. Binary Doping of Nitrogen and Phosphorus into Porous Carbon: A Novel Di-Functional Material for Enhancing CO2 Capture and Super-Capacitance. J. Mater. Sci. Technol. 2022, 99, 73–81. [Google Scholar] [CrossRef]
- Fouda-Mbanga, B.G.; Prabakaran, E.; Pillay, K. Cd2+ Ion Adsorption and Re-Use of Spent Adsorbent with N-Doped Carbon Nanoparticles Coated on Cerium Oxide Nanorods Nanocomposite for Fingerprint Detection. Chem. Phys. Impact 2022, 5, 100083. [Google Scholar] [CrossRef]
- Bhat, S.A.; Zafar, F.; Mirza, A.U.; Mondal, A.H.; Kareem, A.; Haq, Q.M.R.; Nishat, N. NiO Nanoparticle Doped-PVA-MF Polymer Nanocomposites: Preparation, Congo Red Dye Adsorption and Antibacterial Activity. Arab. J. Chem. 2020, 13, 5724–5739. [Google Scholar] [CrossRef]
- Lather, R.; Jeevanandam, P. Synthesis of Zn2+ Doped SnS2 Nanoparticles Using a Novel Thermal Decomposition Approach and Their Application as Adsorbent. J. Alloys Compd. 2022, 891, 161989. [Google Scholar] [CrossRef]
- Dadfarnia, S.; Haji Shabani, A.M.; Moradi, S.E.; Emami, S. Methyl Red Removal from Water by Iron Based Metal-Organic Frameworks Loaded onto Iron Oxide Nanoparticle Adsorbent. Appl. Surf. Sci. 2015, 330, 85–93. [Google Scholar] [CrossRef]
- Li, H.; Tang, M.; Huang, X.; Wang, L.; Liu, Q.; Lu, S. An Efficient Biochar Adsorbent for CO2 Capture: Combined Experimental and Theoretical Study on the Promotion Mechanism of N-Doping. Chem. Eng. J. 2023, 466, 143095. [Google Scholar] [CrossRef]
- Guaya, D.; Jiménez, R.; Sarango, J.; Valderrama, C.; Cortina, J.L. Iron-Doped Natural Clays: Low-Cost Inorganic Adsorbents for Phosphate Recovering from Simulated Urban Treated Wastewater. J. Water Process Eng. 2021, 43, 102274. [Google Scholar] [CrossRef]
- Naskar, A.; Khan, H.; Jana, S. One Pot Low Temperature Synthesis of Graphene Coupled Gd-Doped ZnFe2O4 Nanocomposite for Effective Removal of Antibiotic Levofloxacin Drug. J. Solgel Sci. Technol. 2018, 86, 599–609. [Google Scholar] [CrossRef]
- Ma, J.; Zhang, M.; Ji, M.; Zhang, L.; Qin, Z.; Zhang, Y.; Gao, L.; Jiao, T. Magnetic Graphene Oxide-Containing Chitosan-sodium Alginate Hydrogel Beads for Highly Efficient and Sustainable Removal of Cationic Dyes. Int. J. Biol. Macromol. 2021, 193, 2221–2231. [Google Scholar] [CrossRef] [PubMed]
- Kong, L.; Yan, Q.; Wang, Y.; Wang, Q.; Andrews, C.B.; Zheng, C. Self-Supported Trimetallic NiZnLa Nanosheets on Hierarchical Porous Graphene Oxide-Polymer Composite Fibers for Enhanced Phosphate Removal from Water. J. Colloid. Interface Sci. 2022, 628, 807–818. [Google Scholar] [CrossRef] [PubMed]
- Fang, Z.B.; Liu, T.T.; Liu, J.; Jin, S.; Wu, X.P.; Gong, X.Q.; Wang, K.; Yin, Q.; Liu, T.F.; Cao, R.; et al. Boosting Interfacial Charge-Transfer Kinetics for Efficient Overall CO2 Photoreduction via Rational Design of Coordination Spheres on Metal-Organic Frameworks. J. Am. Chem. Soc. 2020, 142, 12515–12523. [Google Scholar] [CrossRef] [PubMed]
- Cao, Z.; Qin, M.; Gu, Y.; Jia, B.; Chen, P.; Qu, X. Synthesis and Characterization of Sn-Doped Hematite as Visible Light Photocatalyst. Mater. Res. Bull. 2016, 77, 41–47. [Google Scholar] [CrossRef]
- Albukhari, S.M.; Ismail, A.A. Highly Dispersed Pt Nanoparticle-Doped Mesoporous ZnO Photocatalysts for Promoting Photoconversion of CO2 to Methanol. ACS Omega 2021, 6, 23378–23388. [Google Scholar] [CrossRef]
- Singh, R.; Ladol, J.; Khajuria, H.; Nawaz Sheikh, H. Nitrogen Doped Graphene Nickel Ferrite Magnetic Photocatalyst for the Visible Light Degradation of Methylene Blue. Acta Chim. Slov. 2017, 64, 170–178. [Google Scholar] [CrossRef]
- Lavorato, C.; Primo, A.; Molinari, R.; Garcia, H. N-Doped Graphene Derived from Biomass as a Visible-Light Photocatalyst for Hydrogen Generation from Water/Methanol Mixtures. Chem.-A Eur. J. 2014, 20, 187–194. [Google Scholar] [CrossRef]
- Velu, M.; Balasubramanian, B.; Velmurugan, P.; Kamyab, H.; Ravi, A.V.; Chelliapan, S.; Lee, C.T.; Palaniyappan, J. Fabrication of Nanocomposites Mediated from Aluminium Nanoparticles/Moringa Oleifera Gum Activated Carbon for Effective Photocatalytic Removal of Nitrate and Phosphate in Aqueous Solution. J. Clean. Prod. 2021, 281, 124553. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, Y.; Hei, Y.; Wang, S.; Shi, Y.; Jiang, F.; Luo, L. Design and Controllable Synthesis of C-Doped Bi2O3 Nanowires with Superior Performance for Removal of Bisphenol A. Mater. Sci. Semicond. Process. 2021, 132, 105875. [Google Scholar] [CrossRef]
- Nguyen, L.T.T.; Nguyen, H.T.T.; Nguyen, L.T.H.; Duong, A.T.T.; Nguyen, H.Q.; Ngo, V.T.M.; Vu, N.V.; Nguyen, D.T.C.; Tran, T. Van Efficient and Recyclable Nd3+-Doped CoFe2O4 for Boosted Visible Light-Driven Photocatalytic Degradation of Rhodamine B Dye. RSC Adv. 2023, 13, 10650–10656. [Google Scholar] [CrossRef] [PubMed]
- Yahya, N.; Aziz, F.; Jamaludin, N.; Mutalib, M.A.; Ismail, A.; Salleh, W.W.; Jaafar, J.; Yusof, N.; Ludin, N.A. A Review of Integrated Photocatalyst Adsorbents for Wastewater Treatment. J. Environ. Chem. Eng. 2018, 6, 7411–7425. [Google Scholar] [CrossRef]
- Xu, J.; Ao, Y.; Fu, D.; Yuan, C. Synthesis of Fluorine-Doped Titania-Coated Activated Carbon under Low Temperature with High Photocatalytic Activity under Visible Light. J. Phys. Chem. Solids 2008, 69, 2366–2370. [Google Scholar] [CrossRef]
- Belver, C.; Bedia, J.; Rodriguez, J.J. Zr-Doped TiO2 Supported on Delaminated Clay Materials for Solar Photocatalytic Treatment of Emerging Pollutants. J. Hazard. Mater. 2017, 322, 233–242. [Google Scholar] [CrossRef]
- Bian, Z.; Feng, Y.; Li, H.; Yu, H.; Wu, H. Adsorption-Photocatalytic Degradation and Kinetic of Sodium Isobutyl Xanthate Using the Nitrogen and Cerium Co-Doping TiO2−Coated Activated Carbon. Chemosphere 2021, 263, 128254. [Google Scholar] [CrossRef]
- Wang, T.; Dissanayake, P.D.; Sun, M.; Tao, Z.; Han, W.; An, N.; Gu, Q.; Xia, D.; Tian, B.; Ok, Y.S.; et al. Adsorption and Visible-Light Photocatalytic Degradation of Organic Pollutants by Functionalized Biochar: Role of Iodine Doping and Reactive Species. Environ. Res. 2021, 197, 111026. [Google Scholar] [CrossRef]
- Henych, J.; Št’astný, M.; Němečková, Z.; Kormunda, M.; Šanderová, Z.; Žmudová, Z.; Ryšánek, P.; Stehlík, Š.; Ederer, J.; Liegertová, M.; et al. Cerium-Bismuth Oxides/Oxynitrates with Low Toxicity for the Removal and Degradation of Organophosphates and Bisphenols. ACS Appl. Nano Mater. 2022, 5, 17956–17968. [Google Scholar] [CrossRef]
- Primo, A.; Corma, A.; García, H. Titania Supported Gold Nanoparticles as Photocatalyst. Phys. Chem. Chem. Phys. 2011, 13, 886–910. [Google Scholar] [CrossRef]
- Oladoye, P.O.; Adegboyega, S.A.; Giwa, A.R.A. Remediation Potentials of Composite Metal-Organic Frameworks (MOFs) for Dyes as Water Contaminants: A Comprehensive Review of Recent Literatures. Environ. Nanotechnol. Monit. Manag. 2021, 16, 100568. [Google Scholar] [CrossRef]
- Dong, H.; Zeng, G.; Tang, L.; Fan, C.; Zhang, C.; He, X.; He, Y. An Overview on Limitations of TiO2-Based Particles for Photocatalytic Degradation of Organic Pollutants and the Corresponding Countermeasures. Water Res. 2015, 79, 128–146. [Google Scholar] [CrossRef] [PubMed]
- Cheng, R.; Xia, J.; Wen, J.; Xu, P.; Zheng, X. Nano Metal-Containing Photocatalysts for the Removal of Volatile Organic Compounds: Doping, Performance, and Mechanisms. Nanomaterials 2022, 12, 1335. [Google Scholar] [CrossRef] [PubMed]
- Barua, A.; Mehra, P.; Paul, A. Role of Nitrogen Doping and Pore Volume for CO2 Capture in Metal-Organic Framework Derived Ultramicroporous Carbon Material. Results Chem. 2023, 5, 100807. [Google Scholar] [CrossRef]






| Material | Method of Synthesis | Key Findings | Performance Improvement by Doping | References |
|---|---|---|---|---|
| Iron-based metal–organic framework (MOF) with azobenzene tetracarboxylic acid organic linkers | Facile hydrothermal method | CH4 product yield as high as 16.32 µmol g−1 catalyst and 77.57% selectivity during visible light-driven CO2 photoreduction | Product yield improved fourfold compared to the metal cluster Fe3(µ3-O)-(CH3COO)6 | [56] |
| Nanocomposites of TiO2 and nitrogen-doped reduced graphene oxide | Urea-assisted hydrothermal method | CO yield 356.5 µmole g−1 in CO2 photoreduction reaction | 4.4-fold enhancement inthe yield of carbon monoxide compared to undoped TiO2 | [64] |
| Fluorine-doped graphene nanosheets (FG)-based photocatalyst modified with GdVO4 | Ultrasonication-assisted hydrothermal method (Exfoliation method) | 97% removal of phenol from water | 120% enhancement in phenol degradation using GdVO4 modified FG catalyst compared to pristine FG | [67] |
| TiO2 with manganese doping grown on reduced graphene oxide | One-pot hydrothermal method | 99% chromium removal efficiency in 60 min under sunlight | 12.85% enhancement in photocatalytic activity in the doped catalyst | [108] |
| Nitrogen-doped graphene hydrogels | Facile one plot hydrothermal method | Excellent adsorption and photocatalytic activities for organic pollutant removal | Increase in degradation percentage from 60 to 70 using doped catalyst | [109] |
| Self-doped reduced TiO2 nanotube array (r-TiO2 NTA) | Electrochemical self-doping | Photocurrent density achieved was 2.8 mA/cm2 at 1.23 V vs. reversible hydrogen electrode (RHE) and photoconversion efficiency 1.27% in solar-assisted water-splitting reactions. | Photoconversion efficiency increases from 0.97% in TiO2 NTAs to 1.27% in electrochemically self-doped TiO2. | [110,111] |
| Pyrazolyl porphyrinic nickel-based MOF | Hydrothermal method | CH4 product yield as high as 101 µmol g−1 catalyst and 62.73% CH4 selectivity during visible light driven CO2 photoreduction | Product yield increased threefold compared to other porphyrinic MOF | [125] |
| Sn-doped haematite | Solution combustion synthesis | 5% Sn-doped haematite shows 100% photocatalytic activity in dye degradation | Photocatalytic activity was intact after three cycles compared to the pristine haematite | [126] |
| Platinum nanoparticle-doped mesoporous/ZnO photocatalyst | Sol–gel synthesis | Methanol yield of 668 µmol g−1 during CO2 photoreduction | 18.5-fold higher CH3OH yield than pristine ZnO | [127] |
| Magnetic NiFe2O4 catalyst supported on nitrogen-doped graphene | One-step hydrothermal method | 100% Methylene blue degradation in 3 h | - | [128] |
| Chitosan-based nitrogen-doped graphene | Pyrolysis | 90 µmol hydrogen generation in 180 min from a water/methanol mixture | Increase from 20 µmol in undoped photocatalyst to 90 µmol in doped photocatalyst | [129] |
| Doped aluminium nanoparticles/Moringa oleifera gum-activated carbon | Sol–gel method | ~95% removal of nitrate and phosphate under LED | Increase in removal efficiency from ~30% using undoped catalyst to 95% in doped catalyst | [130] |
| C-doped Bi2O3 nanowires | Solvothermal method using bismuth-based adsorbent as a precursor | 98.9% photocatalytic removal of bisphenol | Increase in photocatalytic activity from 30% degradation of the pollutant with commercial Bi2O3 to 98.9% in doped catalyst. | [131] |
| Neodymium (Nd3+)-doped CoFe2O4 (cobalt ferrite) | Solvothermal treatment | 94.7% dye degradation | Increase dye degradation percentage from 29.4% in undoped cobalt ferrite to 94.7% in doped catalyst. | [132] |
| Material | Synthesis Method | Applications and Key Findings | Performance Improvement by Doping | References |
|---|---|---|---|---|
| Bismuth oxide-doped MgO adsorbent. | Co-precipitation method | Indigo carmine dye adsorption with a maximum dye removal capacity of 126 mg g−1 was shown by 5% Bi2O3 doped MgO. | The adsorption capacity of the material was higher than other magnesium-based adsorbents. | [113] |
| Fe(NO3)3-impregnated activated carbon | Ultrasound–microwave combined method | Dye removal from wastewater. Maximum adsorption capacity 259.74 mg/g | The removal capacity of iron-activated carbon was enhanced by 17.12% compared to carbon without activation | [114] |
| Nitrogen- and phosphorus-co-doped porous carbon. | Solvothermal method | The CO2 adsorption capacity of 5.68 mmol g−1 at 5 bar | Adsorption of CO2 increased 2.63 times compared to the undoped adsorbent | [115] |
| Cerium oxide nanorod nanocomposite coated with nitrogen-doped carbon nanoparticles | Hydrothermal method | Cadmium ion (Cd2+) adsorption with 99.9% removal at 10 mg adsorbent dosage and pH 8 at 10 ppm Cd2+ concentration | − | [116] |
| Nickel oxide nanoparticle-doped PVA MF polymer nanocomposites | Co-precipitation and condensation | Congo red dye adsorption, dye removal efficiency of 80% was achieved | Increased in dye adsorption capacity from ~45% in undoped composites to ~80% in doped composites | [117] |
| Zinc-doped SnS2 nanoparticles | Thermal decomposition method | Rhodamine B adsorption up to ~90% | Dye adsorption increased from ~30% in undoped nanoparticles to ~90% in doped adsorbent. | [118] |
| Metal–organic framework with iron base loaded on iron oxide nanoparticles | Solvothermal method | Methyl red dye removal with an adsorption capacity of 600 mg/g | - | [119] |
| Nitrogen-doped biochar | Solvent-free heating at 800 °C | CO2 adsorption with maximum adsorption capacity 250 mg g−1 at 1 bar and 273 K and selectivity 38.24 | Adsorption capacity increased from 121 mg g−1 to 250 mg g−1 | [120] |
| Iron-doped natural clay | Mixing pretreated clay with iron sulphate solution | Maximum recovery of 38 mg g−1 phosphate from urban wastewater | Increase in phosphate removal efficiency from 21 mg g−1 in undoped clay to 38 mg g−1 in doped clay | [121] |
| Reduced graphene oxide/gadolinium-doped ZnFe2O4. | One pot Solvothermal method | Maximum levofloxacin adsorption capacity of 70% | Enhanced adsorption capacity from 30% in undoped to 70% in doped composites | [122] |
| UiO-66 nanoparticle doped with cobalt | Single-step solvothermal method | 94% removal of tetracycline | 7.6 times greater adsorption capacity compared to pristine UiO-66 adsorbents | [112] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sen, P.; Bhattacharya, P.; Mukherjee, G.; Ganguly, J.; Marik, B.; Thapliyal, D.; Verma, S.; Verros, G.D.; Chauhan, M.S.; Arya, R.K. Advancements in Doping Strategies for Enhanced Photocatalysts and Adsorbents in Environmental Remediation. Technologies 2023, 11, 144. https://doi.org/10.3390/technologies11050144
Sen P, Bhattacharya P, Mukherjee G, Ganguly J, Marik B, Thapliyal D, Verma S, Verros GD, Chauhan MS, Arya RK. Advancements in Doping Strategies for Enhanced Photocatalysts and Adsorbents in Environmental Remediation. Technologies. 2023; 11(5):144. https://doi.org/10.3390/technologies11050144
Chicago/Turabian StyleSen, Pramita, Praneel Bhattacharya, Gargi Mukherjee, Jumasri Ganguly, Berochan Marik, Devyani Thapliyal, Sarojini Verma, George D. Verros, Manvendra Singh Chauhan, and Raj Kumar Arya. 2023. "Advancements in Doping Strategies for Enhanced Photocatalysts and Adsorbents in Environmental Remediation" Technologies 11, no. 5: 144. https://doi.org/10.3390/technologies11050144