Green Electrospun Nanofibers for Biomedicine and Biotechnology
Abstract
:1. Introduction
1.1. Overview of the Topic
1.2. Importance of Green Electrospun Nanofiber Materials in Biomedicine and Biotechnology
1.3. Purpose of the Review
2. Fundamentals
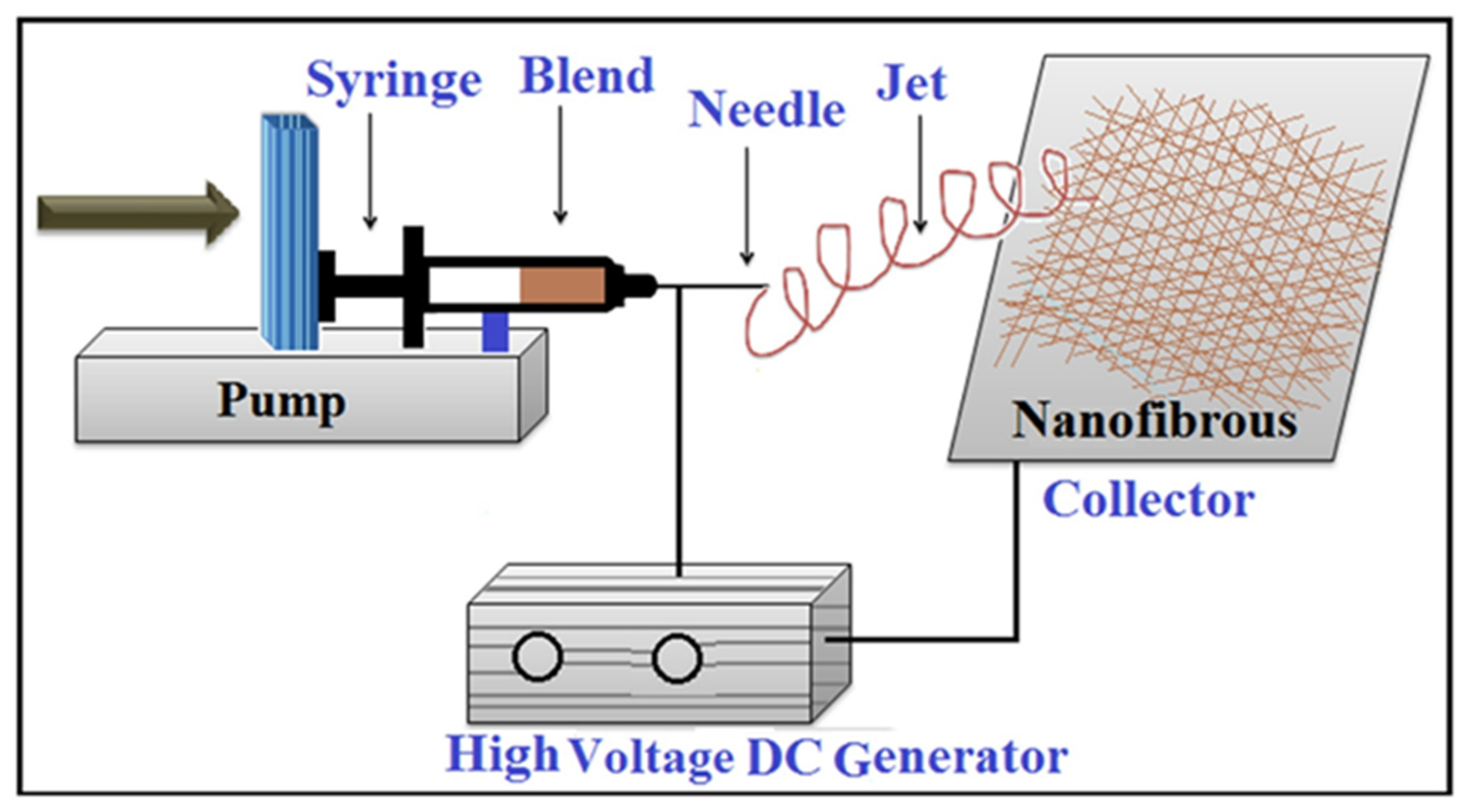
2.1. Basics of Electrospun Nanofiber Materials
2.2. Green Electrospinning Methods
3. Applications in Biomedicine and Biotechnology
3.1. Tissue Engineering and Regeneration
3.1.1. Tissue Engineering Scaffolds
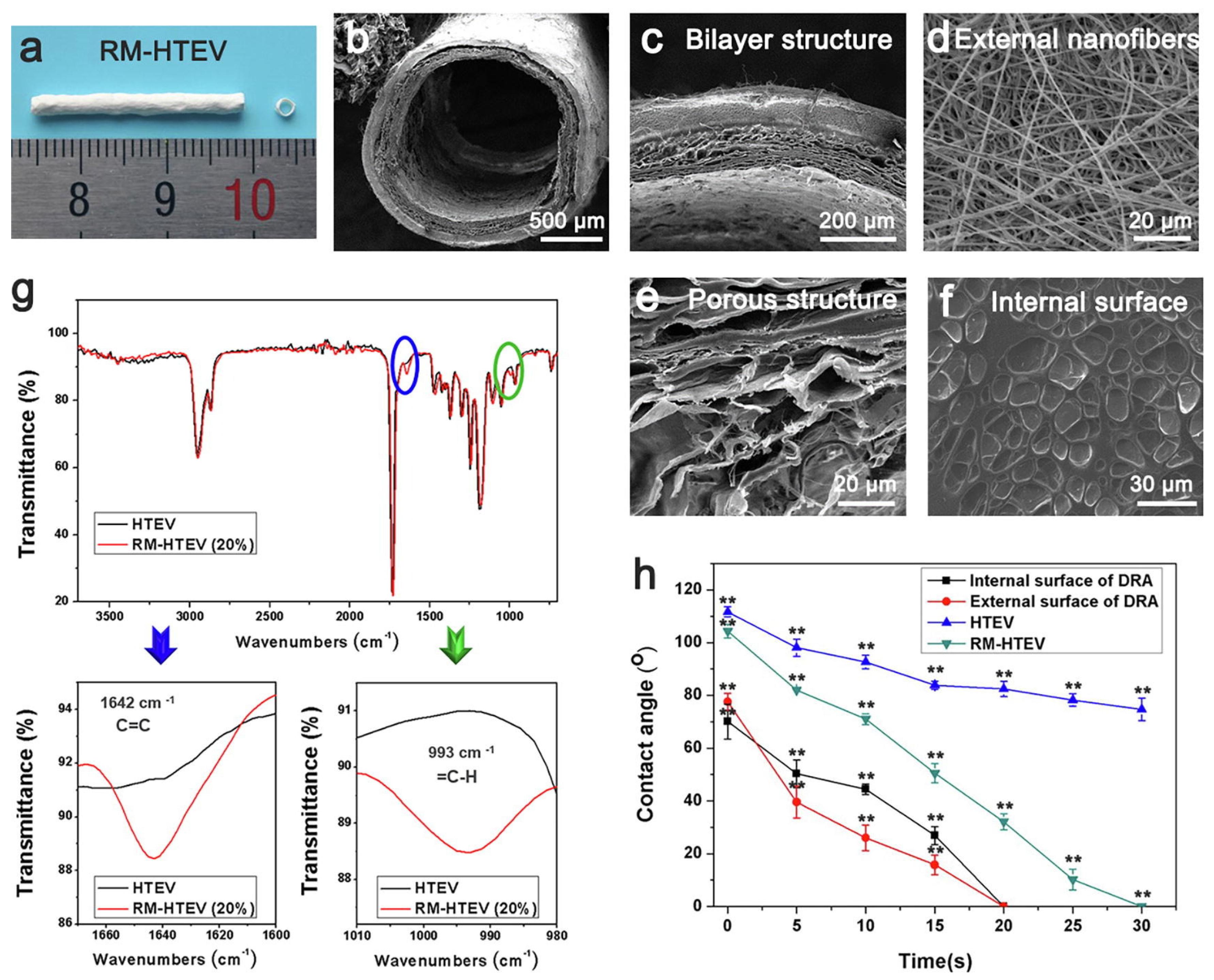
3.1.2. Vascular Grafts
3.2. Controlled Drug Delivery
3.2.1. Antibiotic and Anticancer Delivery
3.2.2. Gene Delivery
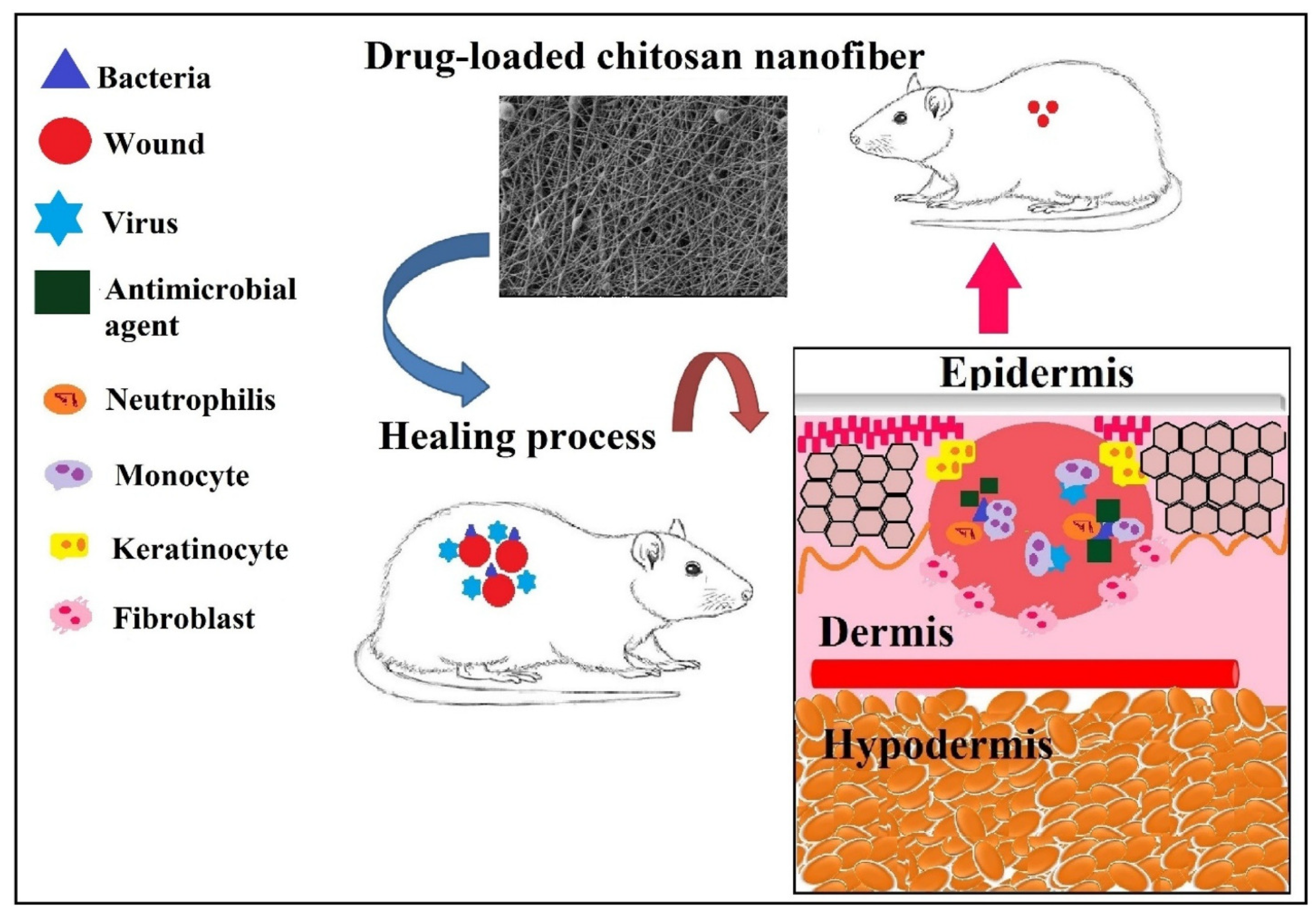
3.2.3. Hemostatic Dressing
3.2.4. Burn Wound Dressing
3.2.5. Sutures/Medical Textiles
3.2.6. Implants
3.3. Applications in Biotechnology
3.3.1. Biosensors
Enzyme Immobilization
Immunosensors
Cell Growth Substrates
Tissue Modelling
3.4. Environmental Remediation
3.4.1. Water Purification
3.4.2. Air Filters
3.4.3. Controlled Release Fertilizers/Pesticides
3.4.4. Food Packaging
3.4.5. Cell Encapsulation
3.4.6. Prodrug Activation
3.4.7. Fiber Diameter in Electrospinning
4. Environmental Impact and Sustainability
Discussion on the Environmental Footprint of Green Electrospun Nanofiber Materials
5. Future Perspectives and Challenges
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Naz, M.; Jabeen, S.; Gull, N.; Ghaffar, A.; Islam, A.; Rizwan, M.; Abdullah, H.; Rasool, A.; Khan, S.; Khan, R. novel silane crosslinked chitosan based electrospun nanofiber for controlled release of benzocaine. Front. Mater. 2022, 9, 826251. [Google Scholar] [CrossRef]
- Adam, A.A.; Dennis, J.O.; Al-Hadeethi, Y.; Mkawi, E.M.; Abdulkadir, B.A.; Usman, F.; Hassan, Y.M.; Wadi, I.A.; Sani, M. state of the art and new directions on electrospun lignin/cellulose nanofibers for supercapacitor application: A systematic literature review. Polymers 2020, 12, 2884. [Google Scholar] [CrossRef] [PubMed]
- Wsoo, M.A.; Shahir, S.; Bohari, S.P.M.; Nayan, N.H.M.; Razak, S.I.A. A review on the properties of electrospun cellulose acetate and its application in drug delivery systems: A new perspective. Carbohydr. Res. 2020, 491, 107978. [Google Scholar] [CrossRef] [PubMed]
- Kerwald, J.; Junior, C.F.d.M.; Freitas, E.D.; Segundo, J.d.D.P.d.M.; Vieira, R.S.; Beppu, M.M. Cellulose-based electrospun nanofibers: A review. Cellulose 2022, 29, 25–54. [Google Scholar] [CrossRef]
- Khoshnevisan, K.; Maleki, H.; Samadian, H.; Shahsavari, S.; Sarrafzadeh, M.H.; Larijani, B.; Dorkoosh, F.A.; Haghpanah, V.; Khorramizadeh, M.R. cellulose acetate electrospun nanofibers for drug delivery systems: Applications and recent advances. Carbohydr. Polym. 2018, 198, 131–141. [Google Scholar] [CrossRef]
- Yang, Y.; Du, Y.; Zhang, J.; Zhang, H.; Guo, B. structural and functional design of electrospun nanofibers for hemostasis and wound healing. Adv. Fiber Mater. 2022, 4, 1027–1057. [Google Scholar] [CrossRef]
- Feng, Y.; Shi, Y.; Tian, Y.; Yang, Y.; Wang, J.; Guo, H.; Banitaba, S.N.; Khademolqorani, S.; Li, J. Collagen-Based Scaffolds for Bone Regeneration: A Journey through Electrospun Composites Integrated with Organic and Inorganic Additives. Processes 2023, 11, 2105. [Google Scholar] [CrossRef]
- Bazrafshan, Z.; Stylios, G.K. Spinnability of collagen as a biomimetic material: A review. Int. J. Biol. Macromol. 2019, 129, 693–705. [Google Scholar] [CrossRef]
- Mbese, Z.; Alven, S.; Aderibigbe, B.A. collagen-based nanofibers for skin regeneration and wound dressing applications. Polymers 2021, 13, 4368. [Google Scholar] [CrossRef] [PubMed]
- Han, S.; Nie, K.; Li, J.; Sun, Q.; Wang, X.; Li, X.; Li, Q. 3D electrospun nanofiber-based scaffolds: From preparations and properties to tissue regeneration applications. Stem Cells Int. 2021, 2021, 8790143. [Google Scholar] [CrossRef]
- Hernández-Rangel, A.; Martin-Martinez, E.S. Collagen based electrospun materials for skin wounds treatment. J. Biomed. Mater. Res. Part A 2021, 109, 1751–1764. [Google Scholar] [CrossRef] [PubMed]
- Campiglio, C.E.; Contessi Negrini, N.; Farè, S.; Draghi, L.J.M. Cross-linking strategies for electrospun gelatin scaffolds. Materials 2019, 12, 2476. [Google Scholar] [CrossRef]
- Nazrin, A.; Sapuan, S.M.; Zuhri, M.Y.M.; Ilyas, R.A.; Syafiq, R.; Sherwani, S.F.K. Nanocellulose reinforced thermoplastic starch (TPS), polylactic acid (PLA), and polybutylene succinate (PBS) for food pack-aging applications. Front. Chem. 2020, 8, 213. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Tian, H.; Mao, J.; Ma, F.; Zhang, M.; Chen, F.; Yang, P. Preparation and application of chitosan-based medical electrospun nanofibers. Int. J. Biol. Macromol. 2023, 226, 410–422. [Google Scholar] [CrossRef] [PubMed]
- Kalantari, K.; Afifi, A.M.; Jahangirian, H.; Webster, T.J. Biomedical applications of chitosan electrospun nanofibers as a green polymer—Review. Carbohydr. Polym. 2019, 207, 588–600. [Google Scholar] [CrossRef] [PubMed]
- Castro-Muñoz, R.; Kharazmi, M.S.; Jafari, S.M. Chitosan-based electrospun nanofibers for encapsulating food bioactive ingredients: A review. Int. J. Biol. Macromol. 2023, 245, 125424. [Google Scholar] [CrossRef] [PubMed]
- Kour, K.; Kumar, R.; Singh, G.; Singh, G.; Singh, S.; Sandhu, K. Additive manufacturing of polylactic acid-based nanofibers composites for innovative scaffolding applications. Int. J. Interact. Des. Manuf. 2023, 13, 1–25. [Google Scholar] [CrossRef]
- More, N.; Avhad, M.; Utekar, S.; More, A. Polylactic acid (PLA) membrane—Significance, synthesis, and applications: A review. Polym. Bull. 2023, 80, 1117–1153. [Google Scholar] [CrossRef]
- Kajdič, S.; Planinšek, O.; Gašperlin, M.; Kocbek, P. Electrospun nanofibers for customized drug-delivery systems. J. Drug Deliv. Sci. Technol. 2019, 51, 672–681. [Google Scholar] [CrossRef]
- Wang, Z.; Kang, K.; Wu, J.; Hu, Q.; Harper, D.P.; Du, G.; Wang, S.; Xu, K. Comparative effects of electrospinning ways for fabricating green, sustainable, flexible, porous, nanofibrous cellulose/chitosan carbon mats as anode materials for lithium-ion batteries. J. Mater. Res. Technol. 2021, 11, 50–61. [Google Scholar] [CrossRef]
- Strnad, S.; Zemljič, L.F. Cellulose–chitosan functional biocomposites. Polymers 2023, 15, 425. [Google Scholar] [CrossRef] [PubMed]
- Nouri, A.; Faraji Dizaji, B.; Kianinejad, N.; Jafari Rad, A.; Rahimi, S.; Irani, M.; Sharifian Jazi, F. Simultaneous linear release of folic acid and doxorubicin from ethyl cellulose/chitosan/g-C3N4/MoS2 core-shell nanofibers and its anticancer properties. J. Biomed. Mater. Res. Part A 2021, 109, 903–914. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, C.; Wang, Y. Recent progress in cellulose-based electrospun nanofibers as multifunctional materials. Nanoscale Adv. 2021, 3, 6040–6047. [Google Scholar] [CrossRef] [PubMed]
- Ranganathan, S.; Balagangadharan, K.; Selvamurugan, N. Chitosan and gelatin-based electrospun fibers for bone tissue engineering. Int. J. Biol. Macromol. 2019, 133, 354–364. [Google Scholar] [CrossRef] [PubMed]
- Arun, A.; Malrautu, P.; Laha, A.; Ramakrishna, S. Gelatin nanofibers in drug delivery systems and tissue engineering. Eng. Sci. 2021, 16, 71–81. [Google Scholar]
- Ehrmann, A. Non-toxic crosslinking of electrospun gelatin nanofibers for tissue engineering and biomedicine—A review. Polymers 2021, 13, 1973. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Sun, M.; Wu, S. State-of-the-art review of electrospun gelatin-based nanofiber dressings for wound healing applications. Nanomaterials 2022, 12, 784. [Google Scholar] [CrossRef] [PubMed]
- Cui, C.; Sun, S.; Wu, S.; Chen, S.; Ma, J.; Zhou, F. Electrospun chitosan nanofibers for wound healing application. Eng. Regen. 2021, 2, 82–90. [Google Scholar] [CrossRef]
- Mirbagheri, M.S.; Akhavan-Mahdavi, S.; Hasan, A.; Kharazmi, M.S.; Jafari, S.M. Chitosan-based electrospun nanofibers for diabetic foot ulcer management; recent advances. Carbohydr. Polym. 2023, 313, 120512. [Google Scholar] [CrossRef]
- Sakib, M.N.; Mallik, A.K.; Rahman, M.M. Update on chitosan-based electrospun nanofibers for wastewater treatment: A review. Carbohydr. Polym. Technol. Appl. 2021, 2, 100064. [Google Scholar] [CrossRef]
- Wu, J.-H.; Hu, T.-G.; Wang, H.; Zong, M.-H.; Wu, H.; Wen, P. Electrospinning of PLA nanofibers: Recent advances and its potential application for food packaging. J. Agric. Food Chem. 2022, 70, 8207–8221. [Google Scholar] [CrossRef]
- Fattahi, F.; Khoddami, A.; Avinc, O. Poly (Lactic Acid) nanofibers as drug delivery systems: Opportunities and challenges. Nanomed. Res. J. 2019, 4, 130–140. [Google Scholar]
- Wu, J.; Liu, S.; Zhang, M.; Wu, G.; Yu, H.; Li, H.; Li, F.; Jia, L. Coaxial electrospinning preparation and antibacterial property of polylactic acid/tea polyphenol nanofiber membrane. J. Ind. Text. 2022, 51, 1778S–1792S. [Google Scholar] [CrossRef]
- Vatanpour, V.; Dehqan, A.; Paziresh, S.; Zinadini, S.; Zinatizadeh, A.A.; Koyuncu, I. Polylactic acid in the fabrication of separation membranes: A review. Sep. Purif. Technol. 2022, 296, 121433. [Google Scholar] [CrossRef]
- Maleki, H.; Azimi, B.; Ismaeilimoghadam, S.; Danti, S. Poly(lactic acid)-based electrospun fibrous structures for biomedical applications. Appl. Sci. 2022, 12, 3192. [Google Scholar] [CrossRef]
- Mtibe, A.; Muniyasamy, S.; Mokhena, T.C.; Ofosu, O.; Ojijo, V.; John, M. Recent insight into the biomedical applications of polybutylene succinate and polybutylene succinate-based materials. Express Polym. Lett. 2023, 17, 2–28. [Google Scholar] [CrossRef]
- Zhao, X.; Zhang, D.; Yu, S.; Zhou, H.; Peng, S. Recent advances in compatibility and toughness of poly (lactic acid)/poly (butylene succinate) blends. e-Polymers 2021, 21, 793–810. [Google Scholar] [CrossRef]
- Platnieks, O.; Gaidukovs, S.; Thakur, V.K.; Barkane, A.; Beluns, S. Bio-based poly (butylene succinate): Recent progress, challenges and future opportunities. Eur. Polym. J. 2021, 161, 110855. [Google Scholar] [CrossRef]
- Dodero, A.; Schlatter, G.; Hébraud, A.; Vicini, S.; Castellano, M. Polymer-free cyclodextrin and natural polymer-cyclodextrin electrospun nanofibers: A comprehensive review on current applications and future perspectives. Carbohydr. Polym. 2021, 264, 118042. [Google Scholar] [CrossRef]
- Wu, T.; Ding, M.; Shi, C.; Qiao, Y.; Wang, P.; Qiao, R.; Wang, X.; Zhong, J. Resorbable polymer electrospun nanofibers: History, shapes and application for tissue engineering. Chin. Chem. Lett. 2020, 31, 617–625. [Google Scholar] [CrossRef]
- Fadil, F.; Affandi, N.D.N.; Misnon, M.I.; Bonnia, N.N.; Harun, A.M.; Alam, M.K. Review on electrospun nanofiber-applied products. Polymers 2021, 13, 2087. [Google Scholar] [CrossRef]
- Keshvardoostchokami, M.; Majidi, S.S.; Huo, P.; Ramachandran, R.; Chen, M.; Liu, B. electrospun nanofibers of natural and synthetic polymers as artificial extracellular matrix for tissue engineering. Nanomaterials 2020, 11, 21. [Google Scholar] [CrossRef]
- Lin, C.-M.; Chang, Y.-C.; Cheng, L.-C.; Liu, C.-H.; Chang, S.C.; Hsien, T.-Y.; Wang, D.-M.; Hsieh, H.-J. Preparation of graphene-embedded hydroxypropyl cellulose/chitosan/polyethylene oxide nanofiber membranes as wound dressings with enhanced antibacterial properties. Cellulose 2020, 27, 2651–2667. [Google Scholar] [CrossRef]
- Dieterle, M.P.; Steinberg, T.; Tomakidi, P.; Nohava, J.; Vach, K.; Schulz, S.D.; Hellwig, E.; Proksch, S. Novel In Situ-Cross-Linked Electrospun Gelatin/Hydroxyapatite Nonwoven Scaffolds Prove Suitable for Periodontal Tissue Engineering. Pharmaceutics 2022, 14, 1286. [Google Scholar] [CrossRef]
- Shahi, S.; Sharifi, S.; Khalilov, R.; Dizaj, S.M.; Abdolahinia, E.D. Gelatin-hydroxyapatite Fibrous Nanocomposite for Regenerative Dentistry and bone Tissue Engineering. Open Dent. J. 2022, 16, e187421062208200. [Google Scholar] [CrossRef]
- Sadeghi, E.; Zebarjad, S.M.; Khademi, F.; Bagherzadeh, E. Enhancing structural strength and improving cell survival through Polycaprolactone/(gelatin/hydroxyapatite) Core-Shell nanofibers for tissue engineering. Polym. Compos. 2022, 43, 7379–7389. [Google Scholar] [CrossRef]
- Sun, C.-K.; Weng, P.-W.; Chang, J.Z.-C.; Lin, Y.-W.; Tsuang, F.-Y.; Lin, F.-H.; Tsai, T.-H.; Sun, J.-S. Metformin-incorporated gelatin/hydroxyapatite nanofiber scaffold for bone regeneration. Tissue Eng. Part A 2022, 28, 1–12. [Google Scholar] [CrossRef]
- Liu, J.; Yao, R.; Guo, J.; Gao, T.; He, J.; Meng, G.; Wu, F. The regulating effect of trace elements Si, Zn and Sr on mineralization of gelatin-hydroxyapatite electrospun fiber. Colloids Surf. B Biointerfaces 2021, 204, 111822. [Google Scholar] [CrossRef]
- Liu, J.; Wu, S.; Ma, J.; Liu, C.; Dai, T.; Wu, X.; Zhao, H.; Zhou, D. Polycaprolactone/Gelatin/Hydroxyapatite Electrospun Nanomembrane Materials Incorporated with Different Proportions of Attapulgite Synergistically Promote Bone Formation. Int. J. Nanomed. 2022, 17, 4087–4103. [Google Scholar] [CrossRef]
- Londhe, P.V.; Chavan, S.S.; Pawar, A.M.; Shendokar, S.M. Optimization of Parameters for diameter of Nanofibers and FTIR, XRD Characterization for Synthesized Biofunctionalized Nanofibers (Curcumin, Gelatin and Formic Acid) using Electrospinning Process. Int. J. Recent Technol. Mech. Electr. Eng. 2019, 6, 2349–7947. [Google Scholar]
- Sharma, A.; Mittal, A.; Puri, V.; Kumar, P.; Singh, I. Curcumin-loaded, alginate–gelatin composite fibers for wound healing applications. 3 Biotech 2020, 10, 464. [Google Scholar] [CrossRef]
- Sharifi, S.; Khosroshahi, A.Z.; Dizaj, S.M.; Rezaei, Y. Preparation, physicochemical assessment and the antimicrobial action of hydroxyapatite–gelatin/curcumin nanofibrous composites as a dental biomaterial. Biomimetics 2021, 7, 4. [Google Scholar] [CrossRef]
- Huang, S.-M.; Liu, S.-M.; Tseng, H.-Y.; Chen, W.-C. Development and In Vitro Analysis of Layer-by-Layer Assembled Membranes for Potential Wound Dressing: Electrospun Curcumin/Gelatin as Middle Layer and Gentamicin/Polyvinyl Alcohol as Outer Layers. Membranes 2023, 13, 564. [Google Scholar] [CrossRef]
- Gulsun, T.; Inal, M.; Akdag, Y.; Izat, N.; Oner, L.; Sahin, S. The development and characterization of electrospun gelatin nanofibers containing indomethacin and curcumin for accelerated wound healing. J. Drug Deliv. Sci. Technol. 2022, 67, 103000. [Google Scholar] [CrossRef]
- Kanu, N.J.; Gupta, E.; Sutar, V.; Singh, G.K.; Vates, U.K. An Insight into Biofunctional Curcumin/Gelatin Nanofibers. In Nanofibers-Synthesis, Properties and Applications; IntechOpen: London, UK, 2021. [Google Scholar]
- Kanu, N.J.; Gupta, E.; Vates, U.K.; Singh, G.K. Electrospinning process parameters optimization for biofunctional curcumin/gelatin nanofibers. Mater. Res. Express 2020, 7, 035022. [Google Scholar] [CrossRef]
- Chakraborty, P.K.; Adhikari, J.; Saha, P. Facile fabrication of electrospun regenerated cellulose nanofiber scaffold for potential bone-tissue engineering application. Int. J. Biol. Macromol. 2019, 122, 644–652. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, T.; Hua, W.; Li, P.; Wang, X. 3D Porous poly (lactic acid)/regenerated cellulose composite scaffolds based on electrospun nanofibers for biomineralization. Colloids Surf. A Physicochem. Eng. Asp. 2020, 585, 124048. [Google Scholar] [CrossRef]
- Moon, J.Y.; Lee, J.; Hwang, T.I.; Park, C.H.; Kim, C.S. A multifunctional, one-step gas foaming strategy for antimicrobial silver nanoparticle-decorated 3D cellulose nanofiber scaffolds. Carbohydr. Polym. 2021, 273, 118603. [Google Scholar] [CrossRef]
- Tan, H.-L.; Kai, D.; Pasbakhsh, P.; Teow, S.-Y.; Lim, Y.-Y.; Pushpamalar, J. Electrospun cellulose acetate butyrate/polyethylene glycol (CAB/PEG) composite nanofibers: A potential scaffold for tissue engineering. Colloids Surf. B Biointerfaces 2020, 188, 110713. [Google Scholar] [CrossRef]
- Zhijiang, C.; Ping, X.; Shiqi, H.; Cong, Z. Soy protein nanoparticles modified bacterial cellulose electrospun nanofiber membrane scaffold by ultrasound-induced self-assembly technique: Characterization and cytocompatibility. Cellulose 2019, 26, 6133–6150. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, T.; Li, J.; Cui, X.; Jiang, M.; Zhang, M.; Wang, X.; Zhang, W.; Liu, Z. Bilayer membrane composed of mineralized collagen and chitosan cast film coated with berberine-loaded PCL/PVP electrospun nanofiber promotes bone regeneration. Front. Bioeng. Biotechnol. 2021, 9, 684335. [Google Scholar] [CrossRef]
- Huo, P.; Han, X.; Zhang, W.; Zhang, J.; Kumar, P.; Liu, B. Electrospun nanofibers of polycaprolactone/collagen as a sustained-release drug delivery system for artemisinin. Pharmaceutics 2021, 13, 1228. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Xu, W.; Shafiq, M.; Tang, J.; Hao, J.; Xie, X.; Yuan, Z.; Xiao, X.; Liu, Y.; Mo, X. Three-dimensional porous gas-foamed electrospun nanofiber scaffold for cartilage regeneration. J. Colloid Interface Sci. 2021, 603, 94–109. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Dong, X.; Shafiq, M.; Myles, G.; Radacsi, N.; Mo, X. Recent advancements on three-dimensional electrospun nanofiber scaffolds for tissue engineering. Adv. Fiber Mater. 2022, 4, 959–986. [Google Scholar] [CrossRef]
- Xie, X.; Chen, Y.; Wang, X.; Xu, X.; Shen, Y.; Khan, A.U.R.; Aldalbahi, A.; Fetz, A.E.; Bowlin, G.L.; El-Newehy, M.; et al. Electrospinning nanofiber scaffolds for soft and hard tissue regeneration. J. Mater. Sci. Technol. 2020, 59, 243–261. [Google Scholar] [CrossRef]
- Zarei, M.; Samimi, A.; Khorram, M.; Abdi, M.M.; Golestaneh, S.I. Fabrication and characterization of conductive polypyrrole/chitosan/collagen electrospun nanofiber scaffold for tissue engineering application. Int. J. Biol. Macromol. 2021, 168, 175–186. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.G.; Mousa, H.M.; Blaudez, F.; El-Sadek, M.A.; Mohamed, M.; Abdel-Jaber, G.; Abdal-Hay, A.; Ivanovski, S. Dual nanofiber scaffolds composed of polyurethane- gelatin/nylon 6- gelatin for bone tissue engineering. Colloids Surf. A Physicochem. Eng. Asp. 2020, 597, 124817. [Google Scholar] [CrossRef]
- Xu, F.; Wang, H.; Zhang, J.; Jiang, L.; Zhang, W.; Hu, Y. A facile design of EGF conjugated PLA/gelatin electrospun nanofibers for nursing care of in vivo wound healing applications. J. Ind. Text. 2022, 51, 420S–440S. [Google Scholar] [CrossRef]
- Niu, Y.; Stadler, F.J.; Fu, M. Biomimetic electrospun tubular PLLA/gelatin nanofiber scaffold promoting regeneration of sciatic nerve transection in SD rat. Mater. Sci. Eng. C 2021, 121, 111858. [Google Scholar] [CrossRef]
- Ajmal, G.; Bonde, G.V.; Mittal, P.; Pandey, V.K.; Yadav, N.; Mishra, B. PLGA/Gelatin-based electrospun nanofiber scaffold encapsulating antibacterial and antioxidant molecules for accelerated tissue regeneration. Mater. Today Commun. 2023, 35, 105633. [Google Scholar] [CrossRef]
- Preeth, D.R.; Saravanan, S.; Shairam, M.; Selvakumar, N.; Raja, I.S.; Dhanasekaran, A.; Vimalraj, S.; Rajalakshmi, S. Bioactive Zinc(II) complex incorporated PCL/gelatin electrospun nanofiber enhanced bone tissue regeneration. Eur. J. Pharm. Sci. 2021, 160, 105768. [Google Scholar] [CrossRef]
- Fathi-Karkan, S.; Banimohamad-Shotorbani, B.; Saghati, S.; Rahbarghazi, R.; Davaran, S. A critical review of fibrous polyurethane-based vascular tissue engineering scaffolds. J. Biol. Eng. 2022, 16, 6. [Google Scholar] [CrossRef]
- Ibrahim, H.M.; Klingner, A. A review on electrospun polymeric nanofibers: Production parameters and potential applications. Polym. Test. 2020, 90, 106647. [Google Scholar] [CrossRef]
- Mishra, P.; Srivastava, A.K.; Yadav, T.C.; Pruthi, V.; Prasad, R. Advances in Natural Polymer-Based Electrospun Nanomaterials for Soft Tissue Engineering. In Engineered Nanomaterials for Innovative Therapies and Biomedicine; Springer: Berlin/Heidelberg, Germany, 2022; pp. 29–52. [Google Scholar]
- Hayat, U.; Raza, A.; Bilal, M.; Iqbal, H.M.; Wang, J.-Y. Biodegradable polymeric conduits: Platform materials for guided nerve regeneration and vascular tissue engineering. J. Drug Deliv. Sci. Technol. 2022, 67, 103014. [Google Scholar] [CrossRef]
- Wu, J.; Wang, S.; Zheng, Z.; Li, J. Fabrication of Biologically Inspired Electrospun Collagen/Silk fibroin/bioactive glass composited nanofibrous scaffold to accelerate the treatment efficiency of bone repair. Regen. Ther. 2022, 21, 122–138. [Google Scholar] [CrossRef]
- Do, T.M.; Yang, Y.; Deng, A. Porous bilayer vascular grafts fabricated from electrospinning of the recombinant human collagen (RHC) peptide-based blend. Polymers 2021, 13, 4042. [Google Scholar] [CrossRef]
- Mozaffari, A.; Gashti, M.P.; Mirjalili, M.; Parsania, M. Argon and argon–oxygen plasma surface modification of gelatin nanofibers for tissue engineering applications. Membranes 2021, 11, 31. [Google Scholar] [CrossRef]
- Li, G.; Yang, T.; Liu, Y.; Su, H.; Liu, W.; Fang, D.; Jin, L.; Jin, F.; Xu, T.; Duan, C. The proteins derived from platelet-rich plasma improve the endothelialization and vascularization of small diameter vascular grafts. Int. J. Biol. Macromol. 2023, 225, 574–587. [Google Scholar] [CrossRef]
- Chen, Y.; Shafiq, M.; Liu, M.; Morsi, Y.; Mo, X. Advanced fabrication for electrospun three-dimensional nanofiber aerogels and scaffolds. Bioact. Mater. 2020, 5, 963–979. [Google Scholar] [CrossRef]
- Capuana, E.; Lopresti, F.; Ceraulo, M.; La Carrubba, V. Poly-L-lactic acid (PLLA)-based biomaterials for regenerative medicine: A review on processing and applications. Polymers 2022, 14, 1153. [Google Scholar] [CrossRef]
- Zhang, Q.; He, S.; Zhu, X.; Luo, H.; Gama, M.; Peng, M.; Deng, X.; Wan, Y. Heparinization and hybridization of electrospun tubular graft for improved endothelialization and anticoagulation. Mater. Sci. Eng. C 2021, 122, 111861. [Google Scholar] [CrossRef]
- Fooladi, S.; Nematollahi, M.H.; Rabiee, N.; Iravani, S. Bacterial Cellulose-Based Materials: A Perspective on Cardiovascular Tissue Engineering Applications. ACS Biomater. Sci. Eng. 2023, 9, 2949–2969. [Google Scholar] [CrossRef]
- Wang, H.; Xia, H.; Xu, Z.; Natsuki, T.; Ni, Q.-Q. Effect of surface structure on the antithrombogenicity performance of poly(-caprolactone)-cellulose acetate small-diameter tubular scaffolds. Int. J. Biol. Macromol. 2023, 226, 132–142. [Google Scholar] [CrossRef]
- Iravani, S.; Varma, R.S. Varma, Cellulose-Based Composites as Scaffolds for Tissue Engineering: Recent Advances. Molecules 2022, 27, 8830. [Google Scholar] [CrossRef]
- Yoo, H.S.; Kim, T.G.; Park, T.G. Surface-functionalized electrospun nanofibers for tissue engineering and drug delivery. Adv. Drug Deliv. Rev. 2009, 61, 1033–1042. [Google Scholar] [CrossRef]
- Contreras-Cáceres, R.; Cabeza, L.; Perazzoli, G.; Díaz, A.; López-Romero, J.M.; Melguizo, C.; Prados, J. Electrospun nanofibers: Recent applications in drug delivery and cancer therapy. Nanomaterials 2019, 9, 656. [Google Scholar] [CrossRef]
- Pillay, V.; Dott, C.; Choonara, Y.E.; Tyagi, C.; Tomar, L.; Kumar, P.; du Toit, L.C.; Ndesendo, V.M.K. A review of the effect of processing variables on the fabrication of electrospun nanofibers for drug delivery applications. J. Nanomater. 2013, 2013, 789289. [Google Scholar] [CrossRef]
- Shahriar, S.M.S.; Mondal, J.; Hasan, M.N.; Revuri, V.; Lee, D.Y.; Lee, Y.-K. Electrospinning nanofibers for therapeutics delivery. Nanomaterials 2019, 9, 532. [Google Scholar] [CrossRef]
- Aytac, Z.; Uyar, T. Electrospun Nanofibers for Drug Delivery Applications. In Applications of Polymer Nanofibers; John Wiley & Sons: Hoboken, NJ, USA, 2022; pp. 202–254. [Google Scholar]
- Torres-Martinez, E.J.; Pérez-González, G.L.; Serrano-Medina, A.; Grande, D.; Vera-Graziano, R.; Cornejo-Bravo, J.M.; Villarreal-Gómez, L.J. Drugs loaded into electrospun polymeric nanofibers for delivery. J. Pharm. Pharm. Sci. 2019, 22, 313–331. [Google Scholar] [CrossRef]
- Sill, T.J.; von Recum, H.A. Electrospinning: Applications in drug delivery and tissue engineering. Biomaterials 2008, 29, 1989–2006. [Google Scholar] [CrossRef]
- Hadjiargyrou, M.; Chiu, J.B. Enhanced composite electrospun nanofiber scaffolds for use in drug delivery. Expert Opin. Drug Deliv. 2008, 5, 1093–1106. [Google Scholar] [CrossRef]
- Bhattarai, R.S.; Bachu, R.D.; Boddu, S.H.S.; Bhaduri, S. Biomedical applications of electrospun nanofibers: Drug and nanoparticle delivery. Pharmaceutics 2018, 11, 5. [Google Scholar] [CrossRef]
- Hu, X.; Liu, S.; Zhou, G.; Huang, Y.; Xie, Z.; Jing, X. Electrospinning of polymeric nanofibers for drug delivery applications. J. Control. Release 2014, 185, 12–21. [Google Scholar] [CrossRef]
- Torres-Martinez, E.J.; Cornejo-Bravo, J.M.; Serrano-Medina, A.; Perez-González, G.L.; Gómez, L.J.V. A summary of electrospun nanofibers as drug delivery system: Drugs loaded and biopolymers used as matrices. Curr. Drug Deliv. 2018, 15, 1360–1374. [Google Scholar] [CrossRef]
- Jiang, J.; Ceylan, M.; Zheng, Y.; Yao, L.; Asmatulu, R.; Yang, S.-Y. Poly-ε-caprolactone electrospun nanofiber mesh as a gene delivery tool. AIMS Bioeng. 2016, 3, 528–537. [Google Scholar] [CrossRef]
- Nguyen, L.H.; Gao, M.; Lin, J.; Wu, W.; Wang, J.; Chew, S.Y. Three-dimensional aligned nanofibers-hydrogel scaffold for controlled non-viral drug/gene delivery to direct axon regeneration in spinal cord injury treatment. Sci. Rep. 2017, 7, srep42212. [Google Scholar] [CrossRef]
- Che, H.-L.; Lee, H.J.; Uto, K.; Ebara, M.; Kim, W.J.; Aoyagi, T.; Park, I.-K. Simultaneous drug and gene delivery from the biodegradable poly(ε-caprolactone) nanofibers for the treatment of liver cancer. J. Nanosci. Nanotechnol. 2015, 15, 7971–7975. [Google Scholar] [CrossRef]
- Rao, G.K.; Kurakula, M.; Yadav, K.S. Application of Electrospun Materials in Gene Delivery. In Electrospun Materials and Their Allied Applications; Scrivener Publishing: Austin, TX, USA, 2020; pp. 265–306. [Google Scholar]
- Zhang, J.; Duan, Y.; Wei, D.; Wang, L.; Wang, H.; Gu, Z.; Kong, D. Co-electrospun fibrous scaffold-adsorbed DNA for substrate-mediated gene delivery. J. Biomed. Mater. Res. Part A 2011, 96, 212–220. [Google Scholar] [CrossRef]
- Ghaderpour, A.; Hoseinkhani, Z.; Yarani, R.; Mohammadiani, S.; Amiri, F.; Mansouri, K. Altering the characterization of nanofibers by changing the electrospinning parameters and their application in tissue engineering, drug delivery, and gene delivery systems. Polym. Adv. Technol. 2021, 32, 1924–1950. [Google Scholar] [CrossRef]
- Lee, S.; Jin, G.; Jang, J.-H. Electrospun nanofibers as versatile interfaces for efficient gene delivery. J. Biol. Eng. 2014, 8, 30. [Google Scholar] [CrossRef]
- Choi, J.S.; Leong, K.W.; Yoo, H.S. In vivo wound healing of diabetic ulcers using electrospun nanofibers immobilized with human epidermal growth factor (EGF). Biomaterials 2008, 29, 587–596. [Google Scholar] [CrossRef]
- Ranjbar-Mohammadi, M.; Rabbani, S.; Bahrami, S.H.; Joghataei, M.; Moayer, F. Antibacterial performance and in vivo diabetic wound healing of curcumin loaded gum tragacanth/poly(ε-caprolactone) electrospun nanofibers. Mater. Sci. Eng. C 2016, 69, 1183–1191. [Google Scholar] [CrossRef]
- Abrigo, M.; McArthur, S.L.; Kingshott, P. Electrospun nanofibers as dressings for chronic wound care: Advances, challenges, and future prospects. Macromol. Biosci. 2014, 14, 772–792. [Google Scholar] [CrossRef]
- Rieger, K.A.; Birch, N.P.; Schiffman, J.D. Designing electrospun nanofiber mats to promote wound healing—A review. J. Mater. Chem. B 2013, 1, 4531–4541. [Google Scholar] [CrossRef]
- Gao, C.; Zhang, L.; Wang, J.; Jin, M.; Tang, Q.; Chen, Z.; Cheng, Y.; Yang, R.; Zhao, G. Electrospun nanofibers promote wound healing: Theories, techniques, and perspectives. J. Mater. Chem. B 2021, 9, 3106–3130. [Google Scholar] [CrossRef]
- Chen, S.; Liu, B.; Carlson, M.A.; Gombart, A.F.; Reilly, D.A.; Xie, J. Recent advances in electrospun nanofibers for wound healing. Nanomedicine 2017, 12, 1335–1352. [Google Scholar] [CrossRef]
- Memic, A.; Abudula, T.; Mohammed, H.S.; Joshi Navare, K.; Colombani, T.; Bencherif, S.A. Latest progress in electrospun nanofibers for wound healing applications. ACS Appl. Bio Mater. 2019, 2, 952–969. [Google Scholar] [CrossRef]
- Liu, M.; Duan, X.P.; Li, Y.M.; Yang, D.P.; Long, Y.Z. Electrospun nanofibers for wound healing. Mater. Sci. Eng. C 2017, 76, 1413–1423. [Google Scholar] [CrossRef]
- Özen, İ.; Wang, X. Biomedicine: Electrospun nanofibrous hormonal therapies through skin/tissue—A review. Int. J. Polym. Mater. Polym. Biomater. 2023, 72, 21–39. [Google Scholar] [CrossRef]
- Dalavi, P.A.; Murugan, S.S.; Anil, S.; Venkatesan, J. Biological macromolecules in tissue engineering. In Biological Macromolecules; Elsevier: Amsterdam, The Netherlands, 2021; pp. 381–392. [Google Scholar]
- Xiao, Z.; Liu, H.; Zhao, Q.; Niu, Y.; Chen, Z.; Zhao, D. Application of microencapsulation technology in silk fibers. J. Appl. Polym. Sci. 2022, 139, 52351. [Google Scholar] [CrossRef]
- Yılmaz, S.S.; Aytac, A. The highly absorbent polyurethane/polylactic acid blend electrospun tissue scaffold for dermal wound dressing. Polym. Bull. 2023, 80, 12787–12813. [Google Scholar] [CrossRef]
- Wang, J.; Zhan, L.; Zhang, X.; Wu, R.; Liao, L.; Wei, J. Silver nanoparticles coated poly (L-lactide) electrospun membrane for implant associated infections prevention. Front. Pharmacol. 2020, 11, 431. [Google Scholar] [CrossRef]
- Chen, Z.; Benecke, L.; Kempert, P.; Stoppe, T.; Bornitz, M.; Neudert, M.; Aibibu, D.; Cherif, C.; Zahnert, T. Simulation and Development of Biomimetic Electrospun PCL Nanofibrous Tympanic Membrane Implants. PAMM 2021, 20, e202000100. [Google Scholar] [CrossRef]
- Bakhsheshi-Rad, H.R.; Ismail, A.F.; Aziz, M.; Hadisi, Z.; Omidi, M.; Chen, X. Antibacterial activity and corrosion resistance of Ta2O5 thin film and electrospun PCL/MgO-Ag nanofiber coatings on biodegradable Mg alloy implants. Ceram. Int. 2019, 45, 11883–11892. [Google Scholar] [CrossRef]
- Jia, W.; Cui, D.; Liu, Y.; Ji, X.; Sun, M.; Cheng, Z.; Luo, Y.; Liu, G. Polyether-ether-ketone/poly (methyl methacrylate)/carbon fiber ternary composites prepared by electrospinning and hot pressing for bone implant applications. Mater. Des. 2021, 209, 109893. [Google Scholar] [CrossRef]
- Eldurini, S.; El-Hady, B.M.A.; Shafaa, M.W.; Gad, A.A.M.; Tolba, E. A multicompartment vascular implant of electrospun wintergreen oil/ polycaprolactone fibers coated with poly(ethylene oxide). Biomed. J. 2021, 44, 589–597. [Google Scholar] [CrossRef]
- Pouponneau, P.; Perrey, O.; Brunon, C.; Grossiord, C.; Courtois, N.; Salles, V.; Alves, A. Electrospun Bioresorbable Membrane Eluting Chlorhexidine for Dental Implants. Polymers 2020, 12, 66. [Google Scholar] [CrossRef]
- Hernandez, J.L.; Park, J.; Yao, S.; Blakney, A.K.; Nguyen, H.V.; Katz, B.H.; Jensen, J.T.; Woodrow, K.A. Effect of tissue microenvironment on fibrous capsule formation to biomaterial-coated implants. Biomaterials 2021, 273, 120806. [Google Scholar] [CrossRef]
- Morais, M.; Coimbra, P.; Pina, M.E. Comparative analysis of morphological and release profiles in ocular implants of acetazolamide prepared by electrospinning. Pharmaceutics 2021, 13, 260. [Google Scholar] [CrossRef]
- Ji, H.; Wang, Y.; Liu, H.; Liu, Y.; Zhang, X.; Xu, J.; Li, Z.; Luo, E. Programmed core-shell electrospun nanofibers to sequentially regulate osteogenesis-osteoclastogenesis balance for promoting immediate implant osseointegration. Acta Biomater. 2021, 135, 274–288. [Google Scholar] [CrossRef]
- Ribeiro, E.S.; de Farias, B.S.; Junior, T.R.S.C.; Pinto, L.A.d.A.; Diaz, P.S. Chitosan–based nanofibers for enzyme immobilization. Int. J. Biol. Macromol. 2021, 183, 1959–1970. [Google Scholar] [CrossRef] [PubMed]
- Zdarta, J.; Jankowska, K.; Wyszowska, M.; Kijeńska-Gawrońska, E.; Zgoła-Grześkowiak, A.; Pinelo, M.; Meyer, A.S.; Moszyński, D.; Jesionowski, T. Robust biodegradation of naproxen and diclofenac by laccase immobilized using electrospun nanofibers with enhanced stability and reusability. Mater. Sci. Eng. C 2019, 103, 109789. [Google Scholar] [CrossRef] [PubMed]
- Jun, S.-H.; Yang, J.; Jeon, H.; Kim, H.S.; Pack, S.P.; Jin, E.; Kim, J. Stabilized and immobilized carbonic anhydrase on electrospun nanofibers for enzymatic CO2 conversion and utilization in expedited microalgal growth. Environ. Sci. Technol. 2020, 54, 1223–1231. [Google Scholar] [CrossRef] [PubMed]
- Kamaci, U.D.; Peksel, A. Enhanced catalytic activity of immobilized phytase into polyvinyl alcohol-sodium alginate based electrospun nanofibers. Catal. Lett. 2020, 151, 821–831. [Google Scholar] [CrossRef]
- Chen, H.; Cheng, K.; Hsu, R.; Hsieh, C.; Wang, H.; Ting, Y. Enzymatic degradation of ginkgolic acid by laccase immobilized on novel electrospun nanofiber mat. J. Sci. Food Agric. 2020, 100, 2705–2712. [Google Scholar] [CrossRef] [PubMed]
- Syukri, M.S.M.; Rahman, R.A.; Mohamad, Z.; Illias, R.M.; Mahmood, N.A.N.; Jaafar, N.R. Optimization strategy for laccase immobilization on polyethylene terephthalate grafted with maleic anhydride electrospun nanofiber mat. Int. J. Biol. Macromol. 2021, 166, 876–883. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Wang, Q.; Huang, F.; Wei, Q. Electrospun nanofibers for enzyme immobilization. In Electrospinning: Nanofabrication and Applications; Elsevier: Amsterdam, The Netherlands, 2019; pp. 765–781. [Google Scholar]
- Smith, S.; Goodge, K.; Delaney, M.; Struzyk, A.; Tansey, N.; Frey, M. A Comprehensive review of the covalent immobilization of biomolecules onto electrospun nanofibers. Nanomaterials 2020, 10, 2142. [Google Scholar] [CrossRef] [PubMed]
- Rather, A.H.; Khan, R.S.; Wani, T.U.; Beigh, M.A.; Sheikh, F.A. Overview on immobilization of enzymes on synthetic polymeric nanofibers fabricated by electrospinning. Biotechnol. Bioeng. 2022, 119, 9–33. [Google Scholar] [CrossRef]
- Sun, Y.; Cheng, S.; Lu, W.; Wang, Y.; Zhang, P.; Yao, Q. Electrospun fibers and their application in drug controlled release, biological dressings, tissue repair, and enzyme immobilization. RSC Adv. 2019, 9, 25712–25729. [Google Scholar] [CrossRef]
- Myndrul, V.; Coy, E.; Bechelany, M.; Iatsunskyi, I. Photoluminescence label-free immunosensor for the detection of Aflatoxin B1 using polyacrylonitrile/zinc oxide nanofibers. Mater. Sci. Eng. C 2021, 118, 111401. [Google Scholar] [CrossRef]
- Maleki, F.; Rashidi, M.R.; Razmi, H.; Ghorbani, M. Label-free electrochemical immunosensor for detection of insulin-like growth factor-1 (IGF-1) using a specific monoclonal receptor on electrospun Zein-based nanofibers/rGO-modified electrode. Talanta 2023, 265, 124844. [Google Scholar] [CrossRef]
- Dos Santos, D.M.; Migliorini, F.L.; Soares, A.C.; Mattoso, L.H.C.; Oliveira, O.N.; Correa, D.S. Electrochemical Immunosensor Made with Zein-based Nanofibers for On-site Detection of Aflatoxin B1. Electroanalysis 2023, 35, 131–138. [Google Scholar] [CrossRef]
- Li, Z.; Liu, Y.; Chen, X.; Cao, H.; Shen, H.; Mou, L.; Deng, X.; Jiang, X.; Cong, Y. Surface-modified mesoporous nanofibers for microfluidic immunosensor with an ultra-sensitivity and high signal-to-noise ratio. Biosens. Bioelectron. 2020, 166, 112444. [Google Scholar] [CrossRef]
- Adabi, M.; Esnaashari, S.S.; Adabi, M. An electrochemical immunosensor based on electrospun carbon nanofiber mat decorated with gold nanoparticles and carbon nanotubes for the detection of breast cancer. J. Porous Mater. 2021, 28, 415–421. [Google Scholar] [CrossRef]
- Yin, W.-J.; Zhang, J.-X.; Wang, H.; Wang, Y.; Zeng, X.; Xu, Z.-L.; Yang, J.-Y.; Xiao, Z.-L.; Hammock, B.D.; Wen, P. A highly sensitive electrochemical immunosensor based on electrospun nanocomposite for the detection of parathion. Food Chem. 2023, 404, 134371. [Google Scholar] [CrossRef]
- Zeybekler, S.E.; Odaci, D. Carbon Nanotube-Incorporated Nanofibers for Immunosensor Preparation against CD36. ACS Omega 2023, 8, 5776–5786. [Google Scholar] [CrossRef]
- Evren, G.; Er, E.; Yalcinkaya, E.E.; Horzum, N.; Odaci, D. Electrospun Nanofibers including Organic/Inorganic Nanohybrids: Polystyrene- and Clay-Based Architectures in Immunosensor Preparation for Serum Amyloid A. Biosensors 2023, 13, 673. [Google Scholar] [CrossRef]
- Paimard, G.; Shahlaei, M.; Moradipour, P.; Akbari, H.; Jafari, M.; Arkan, E. An Impedimetric Immunosensor modified with electrospun core-shell nanofibers for determination of the carcinoma embryonic antigen. Sens. Actuators B Chem. 2020, 311, 127928. [Google Scholar] [CrossRef]
- Horne, J.; McLoughlin, L.; Bridgers, B.; Wujcik, E.K. Recent developments in nanofiber-based sensors for disease detection, immunosensing, and monitoring. Sensors Actuators Rep. 2020, 2, 100005. [Google Scholar]
- Ketmen, S.; Zeybekler, S.E.; Gelen, S.S.; Odaci, D. Graphene Oxide-Magnetic Nanoparticles Loaded Polystyrene-Polydopamine Electrospun Nanofibers Based Nanocomposites for Immunosensing Application of C-Reactive Protein. Biosensors 2022, 12, 1175. [Google Scholar] [CrossRef]
- Wen, P.; Yang, Y.-Y.; Yin, W.-J.; Hu, J.-C.; Shen, Y.-D.; Wang, Y.; Xu, Z.-L.; Xiao, Z.-L.; Lei, H.-T.; Yang, J.-Y.; et al. Preparation of an ultrasensitive electrochemical immunosensor for the rapid detection of 19-nortestosterone based on polyvinyl alcohol/polyacrylic acid electrospun nanofiber mat. Sens. Actuators B Chem. 2022, 370, 132450. [Google Scholar] [CrossRef]
- Asiri, A.; Saidin, S.; Sani, M.H.; Al-Ashwal, R.H. Epidermal and fibroblast growth factors incorporated polyvinyl alcohol electrospun nanofibers as biological dressing scaffold. Sci. Rep. 2021, 11, 5634. [Google Scholar] [CrossRef]
- Stojanov, S.; Berlec, A. Electrospun nanofibers as carriers of microorganisms, stem cells, proteins, and nucleic acids in therapeutic and other applications. Front. Bioeng. Biotechnol. 2020, 8, 130. [Google Scholar] [CrossRef]
- Behere, I.; Ingavle, G. In vitro and in vivo advancement of multifunctional electrospun nanofiber scaffolds in wound healing applications: Innovative nanofiber designs, stem cell approaches, and future perspectives. J. Biomed. Mater. Res. Part A 2022, 110, 443–461. [Google Scholar] [CrossRef]
- Mayoral, I.; Bevilacqua, E.; Gómez, G.; Hmadcha, A.; González-Loscertales, I.; Reina, E.; Sotelo, J.; Domínguez, A.; Pérez-Alcántara, P.; Smani, Y.; et al. Tissue engineered in-vitro vascular patch fabrication using hybrid 3D printing and electrospinning. Mater. Today Bio 2022, 14, 100252. [Google Scholar] [CrossRef]
- Carangelo, F. Reproducing Cardiac Fibrosis: From State of the Art Analysis to the Design of Bioartificial Electrospun Fibers for In Vitro Pathological Cardiac Tissue Modelling. Ph.D. Thesis, Politecnico di Torino, Torino, Italy, 2020. [Google Scholar]
- Dorati, R.; Chiesa, E.; Pisani, S.; Genta, I.; Modena, T.; Bruni, G.; Brambilla, C.R.; Benazzo, M.; Conti, B. The effect of process parameters on alignment of tubular electrospun nanofibers for tissue regeneration purposes. J. Drug Deliv. Sci. Technol. 2020, 58, 101781. [Google Scholar] [CrossRef]
- Lotfi, Z.; Khakbiz, M.; Davari, N.; Bonakdar, S.; Mohammadi, J.; Shokrgozar, M.A.; Derhambakhsh, S. Fabrication and multiscale modeling of polycaprolactone/amniotic membrane electrospun nanofiber scaffolds for wound healing. Artif. Organs 2023, 47, 1267–1284. [Google Scholar] [CrossRef]
- Shukla, R.; Mishra, P.; Ujjwal, R.R.; Kesharwani, P. Electrospun nanofibers for wound healing. In Theory and Applications of Nonparenteral Nanomedicines; Elsevier: Amsterdam, The Netherlands, 2021; pp. 289–318. [Google Scholar]
- Cavo, M.; Serio, F.; Kale, N.; D’Amone, E.; Gigli, G.; del Mercato, L.L. Electrospun nanofibers in cancer research: From engineering of in vitro 3D cancer models to therapy. Biomater. Sci. 2020, 8, 4887–4905. [Google Scholar] [CrossRef]
- Cui, J.; Li, F.; Wang, Y.; Zhang, Q.; Ma, W.; Huang, C. Electrospun nanofiber membranes for wastewater treatment applications. Sep. Purif. Technol. 2020, 250, 117116. [Google Scholar] [CrossRef]
- Blanco, M.; Monteserín, C.; Angulo, A.; Pérez-Márquez, A.; Maudes, J.; Murillo, N.; Aranzabe, E.; Ruiz-Rubio, L.; Vilas, J.L. TiO2-doped electrospun nanofibrous membrane for photocatalytic water treatment. Polymers 2019, 11, 747. [Google Scholar] [CrossRef]
- Fahimirad, S.; Fahimirad, Z.; Sillanpää, M. Efficient removal of water bacteria and viruses using electrospun nanofibers. Sci. Total. Environ. 2021, 751, 141673. [Google Scholar] [CrossRef]
- Bates, I.I.C.; Loranger, É.; Mathew, A.P.; Chabot, B. Cellulose reinforced electrospun chitosan nanofibers bio-based composite sorbent for water treatment applications. Cellulose 2021, 28, 4865–4885. [Google Scholar] [CrossRef]
- Subrahmanya, T.M.; Arshad, A.B.; Lin, P.T.; Widakdo, J.; Makari, H.K.; Austria, H.F.M.; Hu, C.-C.; Lai, J.-Y.; Hung, W.-S. A review of recent progress in polymeric electrospun nanofiber membranes in addressing safe water global issues. RSC Adv. 2021, 11, 9638–9663. [Google Scholar]
- Yanar, N.; Liang, Y.; Yang, E.; Kim, M.; Kim, H.; Byun, J.; Son, M.; Choi, H. Robust and fouling-resistant ultrathin membranes for water purification tailored via semi-dissolved electrospun nanofibers. J. Clean. Prod. 2023, 418, 138056. [Google Scholar] [CrossRef]
- Abd Halim, N.S.; Wirzal, M.D.H.; Hizam, S.M.; Bilad, M.R.; Nordin, N.A.H.M.; Sambudi, N.S.; Putra, Z.A.; Yusoff, A.R.M. Recent development on electrospun nanofiber membrane for produced water treatment: A review. J. Environ. Chem. Eng. 2021, 9, 104613. [Google Scholar] [CrossRef]
- Kugarajah, V.; Ojha, A.K.; Ranjan, S.; Dasgupta, N.; Ganesapillai, M.; Dharmalingam, S.; Elmoll, A.; Hosseini, S.A.; Muthulakshmi, L.; Vijayakumar, S.; et al. Future applications of electrospun nanofibers in pressure driven water treatment: A brief review and research update. J. Environ. Chem. Eng. 2021, 9, 105107. [Google Scholar] [CrossRef]
- Chen, H.; Huang, M.; Liu, Y.; Meng, L.; Ma, M. Functionalized electrospun nanofiber membranes for water treatment: A review. Sci. Total. Environ. 2020, 739, 139944. [Google Scholar] [CrossRef]
- Orlando, R.; Polat, M.; Afshari, A.; Johnson, M.S.; Fojan, P. Electrospun Nanofibre air Filters for Particles and Gaseous Pollutants. Sustainability 2021, 13, 6553. [Google Scholar] [CrossRef]
- Shin, J.; Jeong, S.; Kim, J.; Choi, Y.Y.; Choi, J.; Lee, J.G.; Kim, S.; Kim, M.; Rho, Y.; Hong, S.; et al. Dynamic pore modulation of stretchable electrospun nanofiber filter for adaptive machine learned respiratory protection. ACS Nano 2021, 15, 15730–15740. [Google Scholar] [CrossRef]
- Demirel, O.; Kolgesiz, S.; Yuce, S.; Soytaş, S.H.; Koseoglu-Imer, D.Y.; Unal, H. Photothermal electrospun nanofibers containing polydopamine-coated halloysite nanotubes as antibacterial air filters. ACS Appl. Nano Mater. 2022, 5, 18127–18137. [Google Scholar] [CrossRef]
- He, W.; Guo, Y.; Zhao, Y.B.; Jiang, F.; Schmitt, J.; Yue, Y.; Liu, J.; Cao, J.; Wang, J. Self-supporting smart air filters based on PZT/PVDF electrospun nanofiber composite membrane. Chem. Eng. J. 2021, 423, 130247. [Google Scholar] [CrossRef]
- Mamun, A.; Blachowicz, T.; Sabantina, L. Electrospun nanofiber mats for filtering applications—Technology, structure and materials. Polymers 2021, 13, 1368. [Google Scholar] [CrossRef]
- Deng, Y.; Lu, T.; Cui, J.; Samal, S.K.; Xiong, R.; Huang, C. Bio-based electrospun nanofiber as building blocks for a novel eco-friendly air filtration membrane: A review. Sep. Purif. Technol. 2021, 277, 1196230. [Google Scholar] [CrossRef]
- Zhou, Y.; Liu, Y.; Zhang, M.; Feng, Z.; Yu, D.-G.; Wang, K. Electrospun nanofiber membranes for air filtration: A review. Nanomaterials 2022, 12, 1077. [Google Scholar] [CrossRef]
- Niu, Z.; Bian, Y.; Xia, T.; Zhang, L.; Chen, C. An optimization approach for fabricating electrospun nanofiber air filters with minimized pressure drop for indoor PM2.5 control. J. Affect. Disord. 2021, 188, 107449. [Google Scholar] [CrossRef]
- Bian, Y.; Wang, S.; Zhang, L.; Chen, C. Influence of fiber diameter, filter thickness, and packing density on PM2.5 removal efficiency of electrospun nanofiber air filters for indoor applications. J. Affect. Disord. 2020, 170, 106628. [Google Scholar] [CrossRef]
- Rajak, A.; Hapidin, D.A.; Iskandar, F.; Munir, M.M.; Khairurrijal, K. Electrospun nanofiber from various source of expanded polystyrene (EPS) waste and their characterization as potential air filter media. Waste Manag. 2020, 103, 76–86. [Google Scholar] [CrossRef]
- Chadha, S. Recent advances in nano-encapsulation technologies for controlled release of biostimulants and antimicrobial agents. In Advances in Nano-Fertilizers and Nano-Pesticides in Agriculture; Woodhead Publishing: Sawston, UK, 2021; pp. 29–55. [Google Scholar]
- Zaim, N.S.H.B.H.; Tan, H.L.; Rahman, S.M.A.; Abu Bakar, N.F.; Osman, M.S.; Thakur, V.K.; Radacsi, N. Recent Advances in Seed Coating Treatment Using Nanoparticles and Nanofibers for Enhanced Seed Germination and Protection. J. Plant Growth Regul. 2023, 1–29. [Google Scholar] [CrossRef]
- Martins, D.; Scagion, V.P.; Schneider, R.; Lemos, A.C.C.; Oliveira, J.; Correa, D.S. Biodegradable polymer nanofibers applied in slow release systems for agri-food applications. In Polymers for Agri-Food Applications; Springer: Cham, Switzerland, 2019; pp. 291–316. [Google Scholar]
- Das, K.P.; Sharma, D.; Satapathy, B.K. Electrospun fibrous constructs towards clean and sustainable agricultural prospects: SWOT analysis and TOWS based strategy assessment. J. Clean. Prod. 2022, 368, 133137. [Google Scholar] [CrossRef]
- Rashidi, M.; Mansour, S.S.; Mostashari, P.; Ramezani, S.; Mohammadi, M.; Ghorbani, M. Electrospun nanofiber based on Ethyl cellulose/Soy protein isolated integrated with bitter orange peel extract for antimicrobial and antioxidant active food packaging. Int. J. Biol. Macromol. 2021, 193, 1313–1323. [Google Scholar] [CrossRef]
- Bodbodak, S.; Shahabi, N.; Mohammadi, M.; Ghorbani, M.; Pezeshki, A. Development of a novel antimicrobial electrospun nanofiber based on polylactic acid/hydroxypropyl methylcellulose containing pomegranate peel extract for active food packaging. Food Bioprocess Technol. 2021, 14, 2260–2272. [Google Scholar] [CrossRef]
- Zhang, C.; Li, Y.; Wang, P.; Zhang, H. Electrospinning of nanofibers: Potentials and perspectives for active food packaging. Compr. Rev. Food Sci. Food Saf. 2020, 19, 479–502. [Google Scholar] [CrossRef]
- Forghani, S.; Almasi, H.; Moradi, M. Electrospun nanofibers as food freshness and time-temperature indicators: A new approach in food intelligent packaging. Innov. Food Sci. Emerg. Technol. 2021, 73, 102804. [Google Scholar] [CrossRef]
- Kowsalya, E.; Mosa Christas, K.; Balashanmugam, P.; Rani, J.C. Biocompatible silver nanoparticles/poly(vinyl alcohol) electrospun nanofibers for potential antimicrobial food packaging applications. Food Packag. Shelf Life 2019, 21, 100379. [Google Scholar]
- Shi, C.; Zhou, A.; Fang, D.; Lu, T.; Wang, J.; Song, Y.; Lyu, L.; Wu, W.; Huang, C.; Li, W. Oregano essential oil/β-cyclodextrin inclusion compound polylactic acid/polycaprolactone electrospun nanofibers for active food packaging. Chem. Eng. J. 2022, 445, 136746. [Google Scholar] [CrossRef]
- Duan, M.; Yu, S.; Sun, J.; Jiang, H.; Zhao, J.; Tong, C.; Hu, Y.; Pang, J.; Wu, C. Development and characterization of electrospun nanofibers based on pullulan/chitin nanofibers containing curcumin and anthocyanins for active-intelligent food packaging. Int. J. Biol. Macromol. 2021, 187, 332–340. [Google Scholar] [CrossRef]
- Mohammadi, M.A.; Dakhili, S.; Alizadeh, A.M.; Kooki, S.; Hassanzadazar, H.; Alizadeh-Sani, M.; McClements, D.J. New perspectives on electrospun nanofiber applications in smart and active food packaging materials. Crit. Rev. Food Sci. Nutr. 2022, 1–17. [Google Scholar] [CrossRef]
- Topuz, F.; Uyar, T. Antioxidant, antibacterial and antifungal electrospun nanofibers for food packaging applications. Food Res. Int. 2020, 130, 108927. [Google Scholar] [CrossRef]
- Zhu, C.; Cao, R.; Zhang, Y.; Chen, R. Metallic ions encapsulated in electrospun nanofiber for antibacterial and angiogenesis function to promote wound repair. Front. Cell Dev. Biol. 2021, 9, 660571. [Google Scholar] [CrossRef]
- Miller, B.; Hansrisuk, A.; Highley, C.B.; Caliari, S.R. Guest–host supramolecular assembly of injectable hydrogel nanofibers for cell encapsulation. ACS Biomater. Sci. Eng. 2021, 7, 4164–4174. [Google Scholar] [CrossRef]
- Diep, E.; Schiffman, J.D. Encapsulating bacteria in alginate-based electrospun nanofibers. Biomater. Sci. 2021, 9, 4364–4373. [Google Scholar] [CrossRef]
- Eom, S.; Park, S.M.; Hong, H.; Kwon, J.; Oh, S.-R.; Kim, J.; Kim, D.S. Hydrogel-assisted electrospinning for fabrication of a 3D complex tailored nanofiber macrostructure. ACS Appl. Mater. Interfaces 2020, 12, 51212–51224. [Google Scholar] [CrossRef]
- Duan, F.; Sun, T.; Zhang, J.; Wang, K.; Wen, Y.; Lu, L. Recent innovations in immobilization of β-galactosidases for industrial and therapeutic applications. Biotechnol. Adv. 2022, 61, 108053. [Google Scholar] [CrossRef]
- Luo, H.; Jie, T.; Zheng, L.; Huang, C.; Chen, G.; Cui, W. Electrospun nanofibers for cancer therapy. Adv. Exp. Med. Biol. 2021, 1295, 163–190. [Google Scholar]
- Li, H.; Sanchez-Vazquez, B.; Trindade, R.P.; Zou, Q.; Mai, Y.; Dou, L.; Zhu, L.-M.; Williams, G.R. Electrospun oral formulations for combined photo-chemotherapy of colon cancer. Colloids Surf. B Biointerfaces 2019, 183, 110411. [Google Scholar] [CrossRef]
- Law, K.C.L.; Mahmoudi, N.; Zadeh, Z.E.; Williams, R.J.; Hunt, C.P.J.; Nagy, A.; Thompson, L.H.; Nisbet, D.R.; Parish, C.L. A Selective, Hydrogel-Based Prodrug Delivery System Efficiently Activates a Suicide Gene to Remove Undifferentiated Human Stem Cells Within Neural Grafts. Adv. Funct. Mater. 2023, 33, 2305771. [Google Scholar] [CrossRef]
- Ye, J.; Gong, M.; Song, J.; Chen, S.; Meng, Q.; Shi, R.; Zhang, L.; Xue, J. Integrating Inflammation-Responsive Prodrug with Electrospun Nanofibers for Anti-Inflammation Application. Pharmaceutics 2022, 14, 1273. [Google Scholar] [CrossRef]
- Dart, A.; Roy, D.; Vlaskin, V.; Limqueco, E.; Lowe, N.M.; Srinivasan, S.; Ratner, D.M.; Bhave, M.; Stayton, P.; Kingshott, P. A nanofiber based antiviral (TAF) prodrug delivery system. Mater. Sci. Eng. C 2022, 133, 112626. [Google Scholar] [CrossRef]


| Nanofiber Type | Basic Properties | Biomedical Properties | Biotechnological Properties | Ref. |
|---|---|---|---|---|
| Cellulose | Renewable from plants/wood, Biocompatible, biodegradable, High strength, flexibility | Tissue scaffolds, Wound dressings, Vascular grafts | Biosensors, Biocatalyst, immobilization | [1,2,3,4,5] |
| Collagen | Obtained from animal tissues, Contains bioactive peptides, Supports cell adhesion | Skin/bone regeneration, Hernia repair meshes, Nerve conduits | N/A | [11,12,13,14,15,16] |
| Gelatin | Collagen derivative, Tunable degradation, Low immunogenicity | Drug delivery, Wound healing, Soft tissue scaffolds | Enzyme immobilization, Affinity membranes | [6,7,8,9,10] |
| Chitosan | From shrimp shells, Antimicrobial activity, Biocompatible | Wound dressings, Tissue engineering, Bone regeneration | Biosensing, Bioremediation | [17,18,19,20,21,22] |
| Polymer Nanofiber Type | Source | Basic Properties | Biomedical Properties | Biotechnological Properties | Ref. |
|---|---|---|---|---|---|
| Poly(lactic acid) (PLA) | Plant starch/sugar fermentation | Thermoplastic, Tunable degradation rate, High strength/elasticity | Implants, Sutures/meshes, Tissue engineering scaffolds | N/A | [23,24,25,26,27,28,29] |
| Poly(butylene succinate) (PBS) | Succinic acid from plants/microbes | Flexibility, Impact resistance, Biodegradation in soil/compost | Implants, Medical devices, Drug delivery carriers | N/A | [30,31,32,33] |
| Composite | Characteristics | Biomedical Properties | Biotechnological Properties | Ref. |
|---|---|---|---|---|
| Cellulose/Chitosan | Biocompatible, renewable polymers | Tissue engineering scaffolds, Wound dressings | Biosensors, Affinity membranes | [16,39,40,41,42] |
| Gelatin/Hydroxyapatite | Mimics bone composition | Bone regeneration, Oral implants | N/A | [43,44,45,46,47,48] |
| Curcumin/Gelatin | Natural anti-inflammatory drug | Controlled drug delivery, Wound healing | N/A | [49,50,51,52,53,54,55] |
| Technique | Description | Benefits |
|---|---|---|
| Aqueous Electrospinning | Uses water as a solvent instead of organic chemicals. Suitable for water-soluble polymers like collagen, gelatin, chitosan. | Eliminates the use of toxic organic solvents. |
| Emulsion Electrospinning | Involves water-in-oil or oil-in-water emulsions for insoluble polymers. | Provides benefits of aqueous systems while maintaining material compatibility. |
| Melt Electrospinning | Feeds polymers in melt/semisolid form directly through the nozzle without solvents. | Applicable to thermoresponsive polymers like PLA, PCL. Solvent-free process. |
| Near-Field Electrospinning | Performs electrospinning at short, 1–5 mm tip-collector distances and low voltages (<5 kV). | Requires lower electric field strengths. |
| Centrifugal Spinning | Uses centrifugal rather than electrostatic force to form fibers. | No chemical exposure, high voltages or expensive equipment needed. |
| Bacterial Nanocellulose Spinning | Facilitates in situ growth of nanocellulose hydrogels on a rotating surface via bacterial culture. | Completely biomass-derived and renewable fiber production. |
| Solar Electrospinning | Replaces high voltage supply with photovoltaic cells powered by sunlight. | Highly sustainable process with no electrical energy requirement. |
| Material | Basic Details | Main Responsible | Greenness | Future Suggestions | Ref. |
|---|---|---|---|---|---|
| Cellulose | Nanostructure, >90% porosity, degradation over months | Skin, bone regeneration | Renewable source, biodegradable | Functionalization with growth factors, mechanical strengthening | [56,57,58,59,60] |
| Collagen | Diameter 50–500 nm, 80–90% porosity, degradation over months | Skin, cartilage regeneration | Biomimicking ECM, biodegradable | Angiogenic/osteogenic functionalization, controlled degradation | [61,62,63,64,65,66] |
| Gelatin | Diameter 100–300 nm, 70–85% porosity, degradation over weeks | Skin, nerve regeneration | Biodegradable, processed from collagen | Crosslinking for strength–porosity control, drug release studies | [67,68,69,70,71] |
| Material | Basic Details | Main Responsible | Greenness | Future Suggestions | Ref. |
|---|---|---|---|---|---|
| Collagen/Silk | Diameter 50–500 nm, resembles veins/arteries | Arterial grafts | Biomimicking ECM, biodegradable | Angiogenesis cues, mechanical properties | [74,75,76,77,78] |
| Gelatin/PLLA | Tunable strength-degradation, moderate compliance | Venous grafts | Biodegradable polymers | Strengthening, nonthrombogenic surface | [79,80,81,82,83] |
| Cellulose/Gelatin | High porosity, nonthrombogenic, remodelling | Arterial/venous grafts | Renewable materials | Mechanical properties, controlled degradation | [84,85,86,87] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Berdimurodov, E.; Dagdag, O.; Berdimuradov, K.; Wan Nik, W.M.N.; Eliboev, I.; Ashirov, M.; Niyozkulov, S.; Demir, M.; Yodgorov, C.; Aliev, N. Green Electrospun Nanofibers for Biomedicine and Biotechnology. Technologies 2023, 11, 150. https://doi.org/10.3390/technologies11050150
Berdimurodov E, Dagdag O, Berdimuradov K, Wan Nik WMN, Eliboev I, Ashirov M, Niyozkulov S, Demir M, Yodgorov C, Aliev N. Green Electrospun Nanofibers for Biomedicine and Biotechnology. Technologies. 2023; 11(5):150. https://doi.org/10.3390/technologies11050150
Chicago/Turabian StyleBerdimurodov, Elyor, Omar Dagdag, Khasan Berdimuradov, Wan Mohd Norsani Wan Nik, Ilyos Eliboev, Mansur Ashirov, Sherzod Niyozkulov, Muslum Demir, Chinmurot Yodgorov, and Nizomiddin Aliev. 2023. "Green Electrospun Nanofibers for Biomedicine and Biotechnology" Technologies 11, no. 5: 150. https://doi.org/10.3390/technologies11050150







