Cost-Effective 3D Printing of Silicone Structures Using an Advanced Intra-Layer Curing Approach
Abstract
:1. Introduction
2. Experiments
2.1. Materials
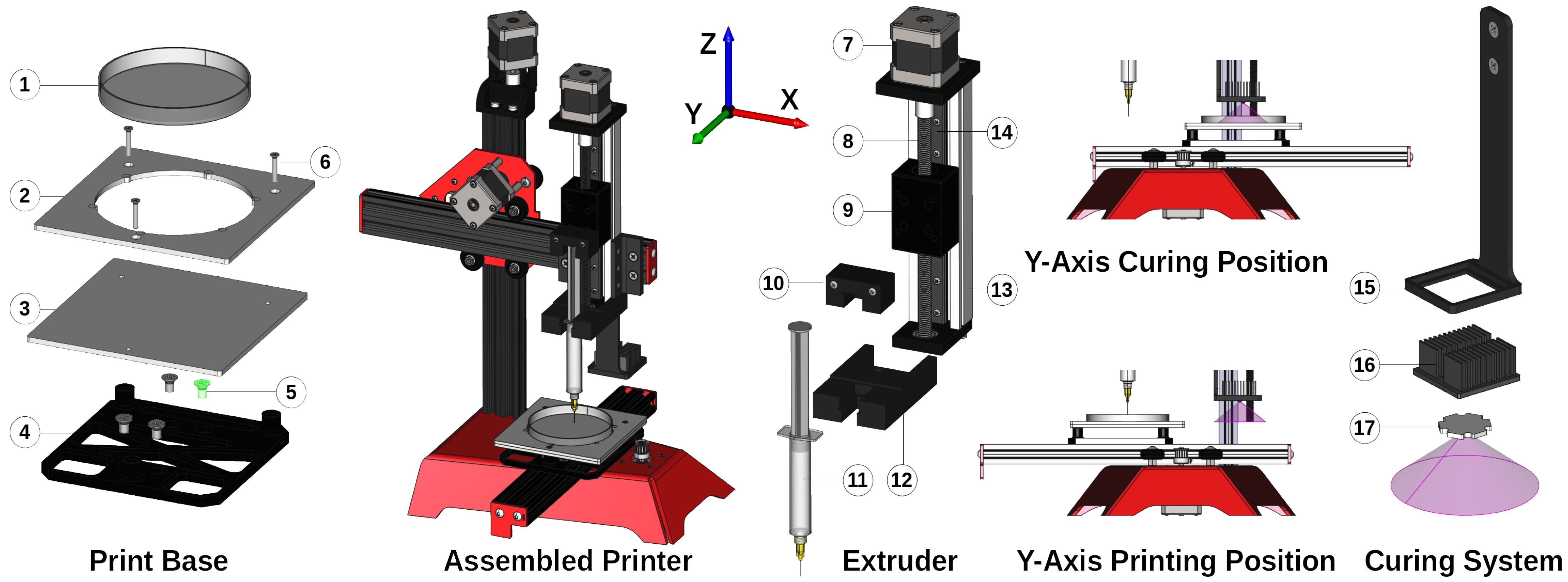
2.2. Fabrication System Design
2.2.1. 3D Printer Design
2.2.2. Control System
2.3. PDMS Structures
2.3.1. Preparation/Setup
2.3.2. Printing Process
- Print one layer using a rectilinear pattern [49];
- Stop XYZ movement and retract the extruder to stop the material extrusion;
- Increase Z so that the tip is above the height of the petri dish;
- Move the print base (via the y-axis) to beneath the LED for curing;
- Lower Z so that the distance between the layer and the LED is 5 cm;
- Turn on the LED and irradiate the layer (3 min);
- Turn off the LED and increase Z;
- Move the print base (via the y-axis) back to the printing position;
- Move Z to the next layer height.
3. Results and Discussion
3.1. Extrusion System and Calibration
3.2. Developed Printer
3.3. Fabricated Structures
3.4. Structural Analysis
3.5. Comparative Platforms
3.6. Applicable Areas and Future Directions
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| PDMS | Polydimethylsiloxane |
| FDM | Fused deposition modelling |
| FFF | Fused filament fabrication |
| DP | Direct printing |
| IM | Injection moulding |
| LSR | Liquid silicone rubber |
| CAD | Computer-aided design |
| UV | Ultraviolet |
| LED | Light-emitting diode |
References
- Berman, B. 3D printing: The new industrial revolution. IEEE Eng. Manag. Rev. 2013, 41, 72–80. [Google Scholar] [CrossRef]
- Barnatt, C. 3D Printing: The Next Industrial Revolution. 2013. Available online: https://www.explainingthefuture.com/ (accessed on 7 December 2023).
- Delvenne, P.; Vigneron, L. On the disruptive potential of 3D printing. In Embedding New Technologies into Society; Jenny Stanford Publishing: Singapore, 2017; pp. 335–355. [Google Scholar]
- Niranjan, Y.C.; Channabasavanna, S.G.; Krishnapillai, S.; Velmurugan, R.; Kannan, A.R.; Mohan, D.G.; Karganroudi, S.S. The Unprecedented Role of 3D Printing Technology in Fighting the COVID-19 Pandemic: A Comprehensive Review. Materials 2022, 15, 6827. [Google Scholar] [CrossRef]
- Dogan, E.; Bhusal, A.; Cecen, B.; Miri, A.K. 3D Printing metamaterials towards tissue engineering. Appl. Mater. Today 2020, 20, 100752. [Google Scholar] [CrossRef]
- Poomathi, N.; Singh, S.; Prakash, C.; Subramanian, A.; Sahay, R.; Cinappan, A.; Ramakrishna, S. 3D printing in tissue engineering: A state of the art review of technologies and biomaterials. Rapid Prototyp. J. 2020, 26, 1313–1334. [Google Scholar] [CrossRef]
- Etxebarria-Elezgarai, J.; Garcia-Hernando, M.; Basabe-Desmonts, L.; Benito-Lopez, F. Precise Integration of Polymeric Sensing Functional Materials within 3D Printed Microfluidic Devices. Chemosensors 2023, 11, 253. [Google Scholar] [CrossRef]
- Bhattacharjee, N.; Urrios, A.; Kang, S.; Folch, A. The upcoming 3D-printing revolution in microfluidics. Lab Chip 2016, 16, 1720–1742. [Google Scholar] [CrossRef]
- Deepika Raj, K.D.M. Disruptive Potential of 3D Printing for Clothing and Textile Sector. In International Textile and Apparel Association Annual Conference Proceedings; Iowa State University Digital Press: Ames, IA, USA, 2016; Volume 73. [Google Scholar]
- Xiao, Y.Q.; Kan, C.W. Review on Development and Application of 3D-Printing Technology in Textile and Fashion Design. Coatings 2022, 12, 267. [Google Scholar] [CrossRef]
- Van Overwalle, G.; Leys, R. 3D Printing and Patent Law: A Disruptive Technology Disrupting Patent Law? Iic Int. Rev. Intellect. Prop. Compet. Law 2017, 48, 504–537. [Google Scholar] [CrossRef]
- Trimmer, B.; Lewis, J.A.; Shepherd, R.F.; Lipson, H. 3D Printing Soft Materials: What Is Possible? Soft Robot. 2015, 2, 3–6. [Google Scholar] [CrossRef]
- Wolf, M.P.; Salieb-Beugelaar, G.B.; Hunziker, P. PDMS with designer functionalities—Properties, modifications strategies, and applications. Prog. Polym. Sci. 2018, 83, 97–134. [Google Scholar] [CrossRef]
- Bhattacharjee, N.; Parra-Cabrera, C.; Kim, Y.T.; Kuo, A.P.; Folch, A. Desktop-Stereolithography 3D-Printing of a Poly(dimethylsiloxane)-Based Material with Sylgard-184 Properties. Adv. Mater. 2018, 30, 1800001. [Google Scholar] [CrossRef]
- Moretto, H.H.; Schulze, M.; Wagner, G. Silicones, Ullmann’s Encyclopedia of Industrial Chemistry; Wiley: Weinheim, Germany, 2005; Volume 10, p. a24057. [Google Scholar]
- Kim, S.M.; Cho, Y.J.; Eo, M.Y.; Kim, J.S.; Lee, S.K. Silicone Facial Prosthesis: A Preliminary Report on Silicone Adhesion to Magnet. J. Craniofacial Surg. 2018, 29, e6–e8. [Google Scholar] [CrossRef]
- Liravi, F.; Toyserkani, E. Additive manufacturing of silicone structures: A review and prospective. Addit. Manuf. 2018, 24, 232–242. [Google Scholar] [CrossRef]
- Jiménez, M.; Romero, L.; Domínguez, I.A.; Espinosa, M.d.M.; Domínguez, M. Additive Manufacturing Technologies: An Overview about 3D Printing Methods and Future Prospects. Complexity 2019, 2019, 9656938. [Google Scholar] [CrossRef]
- Li, J.; Wu, S.; Zhang, W.; Ma, K.; Jin, G. 3D Printing of Silicone Elastomers for Soft Actuators. Actuators 2022, 11, 200. [Google Scholar] [CrossRef]
- Gutierrez, D.B.; Caldona, E.B.; Yang, Z.; Suo, X.; Cheng, X.; Dai, S.; Espiritu, R.D.; Advincula, R.C. PDMS-silica composite gas separation membranes by direct ink writing. J. Appl. Polym. Sci. 2023, 140, e54277. [Google Scholar] [CrossRef]
- Shrestha, M.; Depari, L.; Shakerzadeh, M.; Shivakumar, R.; Teo, E.H. Ink-based transparent compliant electrode for direct coating on untreated hydrophobic PDMS surface. Sensors Actuators Rep. 2023, 5, 100162. [Google Scholar] [CrossRef]
- Fuenmayor, E.; Forde, M.; Healy, A.V.; Devine, D.M.; Lyons, J.G.; McConville, C.; Major, I. Comparison of fused-filament fabrication to direct compression and injection molding in the manufacture of oral tablets. Int. J. Pharm. 2019, 558, 328–340. [Google Scholar] [CrossRef] [PubMed]
- Yirmibesoglu, O.D.; Morrow, J.; Walker, S.; Gosrich, W.; Cañizares, R.; Kim, H.; Daalkhaijav, U.; Fleming, C.; Branyan, C.; Menguc, Y. Direct 3D Printing of Silicone Elastomer Soft Robots and TheirPerformance Comparison with Molded Counterparts. In Proceedings of the IEEE International Conference on Soft Robotics (RoboSoft), Livorno, Italy, 24–28 April 2018; pp. 295–302. [Google Scholar] [CrossRef]
- Riedle, H.; Seitz, V.; Schraudolf, L.; Franke, J. Generation of 3D Silicone Models of Anatomic Soft Tissue Structures—A Comparison of Direct 3D Printing and Molding Techniques. In Proceedings of the 2018 IEEE-EMBS Conference on Biomedical Engineering and Sciences (IECBES), Sarawak, Malaysia, 3–6 December 2018; pp. 539–543. [Google Scholar] [CrossRef]
- Duoss, E.B.; Weisgraber, T.H.; Hearon, K.; Zhu, C.; Small IV, W.; Metz, T.R.; Vericella, J.J.; Barth, H.D.; Kuntz, J.D.; Maxwell, R.S.; et al. Three-Dimensional Printing of Elastomeric, Cellular Architectures with Negative Stiffness. Adv. Funct. Mater. 2014, 24, 4905–4913. [Google Scholar] [CrossRef]
- Kolesky, D.B.; Truby, R.L.; Gladman, A.S.; Busbee, T.A.; Homan, K.A.; Lewis, J.A. 3D Bioprinting of Vascularized, Heterogeneous Cell-Laden Tissue Constructs. Adv. Mater. 2014, 26, 3124–3130. [Google Scholar] [CrossRef]
- Coulter, F.B.; Schaffner, M.; Faber, J.A.; Rafsanjani, A.; Smith, R.; Appa, H.; Zilla, P.; Bezuidenhout, D.; Studart, A.R. Bioinspired Heart Valve Prosthesis Made by Silicone Additive Manufacturing. Matter 2019, 1, 266–279. [Google Scholar] [CrossRef]
- Holländer, J.; Hakala, R.; Suominen, J.; Moritz, N.; Yliruusi, J.; Sandler, N. 3D printed UV light cured polydimethylsiloxane devices for drug delivery. Int. J. Pharm. 2018, 544, 433–442. [Google Scholar] [CrossRef]
- Robinson, S.S.; O’Brien, K.W.; Zhao, H.; Peele, B.N.; Larson, C.M.; Mac Murray, B.C.; Van Meerbeek, I.M.; Dunham, S.N.; Shepherd, R.F. Integrated soft sensors and elastomeric actuators for tactile machines with kinesthetic sense. Extrem. Mech. Lett. 2015, 5, 47–53. [Google Scholar] [CrossRef]
- Zhu, G.; Dai, H.; Yao, Y.; Tang, W.; Shi, J.; Yang, J.; Zhu, L. 3D Printed Skin-Inspired Flexible Pressure Sensor with Gradient Porous Structure for Tunable High Sensitivity and Wide Linearity Range. Adv. Mater. Technol. 2022, 7, 2101239. [Google Scholar] [CrossRef]
- Plott, J.; Shih, A. The extrusion-based additive manufacturing of moisture-cured silicone elastomer with minimal void for pneumatic actuators. Addit. Manuf. 2017, 17, 1–14. [Google Scholar] [CrossRef]
- Tian, K.; Bae, J.; Bakarich, S.E.; Yang, C.; Gately, R.D.; Spinks, G.M.; Suo, Z.; Vlassak, J.J. 3D Printing of Transparent and Conductive Heterogeneous Hydrogel–Elastomer Systems. Adv. Mater. 2017, 29, 1604827. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.; Plott, J.; Wang, H.; Zhu, B.; Shih, A.J. Silicone Foam Additive Manufacturing by Liquid Rope Coiling. Procedia CIRP 2017, 65, 196–201. [Google Scholar] [CrossRef]
- Ozbolat, V.; Dey, M.; Ayan, B.; Povilianskas, A.; Demirel, M.C.; Ozbolat, I.T. 3D Printing of PDMS Improves Its Mechanical and Cell Adhesion Properties. ACS Biomater. Sci. Eng. 2018, 4, 682–693. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Xue, G.h.; Fu, J.z. Fabrication of low cost soft tissue prostheses with the desktop 3D printer. Sci. Rep. 2014, 4, 6973. [Google Scholar] [CrossRef]
- Petersen, E.E.; Pearce, J. Emergence of Home Manufacturing in the Developed World: Return on Investment for Open-Source 3-D Printers. Technologies 2017, 5, 7. [Google Scholar] [CrossRef]
- Wohlers, T.; Campbell, R.I.; Diegel, O.; Huff, R.; Kowen, J. Wohlers Report 2020 3D Printing and Additive Manufacturing State of the Industry; Wohlers Associates: Fort Collins, CO, USA, 2020. [Google Scholar]
- Jo, B.W.; Song, C.S. Thermoplastics and Photopolymer Desktop 3D Printing System Selection Criteria Based on Technical Specifications and Performances for Instructional Applications. Technologies 2021, 9, 91. [Google Scholar] [CrossRef]
- Araújo, N.; Pacheco, V.; Costa, L. Smart Additive Manufacturing: The Path to the Digital Value Chain. Technologies 2021, 9, 88. [Google Scholar] [CrossRef]
- Floyd, E.L.; Wang, J.; Regens, J.L. Fume emissions from a low-cost 3-D printer with various filaments. J. Occup. Environ. Hyg. 2017, 14, 523–533. [Google Scholar] [CrossRef] [PubMed]
- Anzalone, G.C.; Zhang, C.; Wijnen, B.; Sanders, P.G.; Pearce, J.M. A Low-Cost Open-Source Metal 3-D Printer. IEEE Access 2013, 1, 803–810. [Google Scholar] [CrossRef]
- Duong, T. Launch of low-cost metal powder bed 3D printer. Met. Powder Rep. 2014, 69, 42. [Google Scholar] [CrossRef]
- Budding, A.; Vaneker, T.; Winnubst, A. Open Source Powder based Rapid Prototyping Machine for Ceramics. Procedia CIRP 2013, 6, 533–538. [Google Scholar] [CrossRef]
- Robinson, T.M.; Talebian, S.; Foroughi, J.; Yue, Z.; Fay, C.D.; Wallace, G.G. Fabrication of Aligned Biomimetic Gellan Gum-Chitosan Microstructures through 3D Printed Microfluidic Channels and Multiple In Situ Cross-Linking Mechanisms. Acs Biomater. Sci. Eng. 2020, 6, 3638–3648. [Google Scholar] [CrossRef] [PubMed]
- Fay, C.D.; Jeiranikhameneh, A.; Sayyar, S.; Talebian, S.; Nagle, A.; Cheng, K.; Fleming, S.; Mukherjee, P.; Wallace, G.G. Development of a customised 3D printer as a potential tool for direct printing of patient-specific facial prosthesis. Int. J. Adv. Manuf. Technol. 2022, 120, 7143–7155. [Google Scholar] [CrossRef]
- Shoushtari Zadeh Naseri, A.; Fay, C.; Nattestad, A.; Ryder, G.; Sayyar, S.; Yue, Z.; Liu, X.; Officer, D.L.; Wallace, G.G. A Novel Cryogenic Approach to 3D Printing Cytocompatible, Conductive, Hydrogel-Based Inks. 3D Print. Addit. Manuf. 2022; ahead of print. [Google Scholar] [CrossRef]
- Calcagnile, P.; Cacciatore, G.; Demitri, C.; Montagna, F.; Esposito Corcione, C. A Feasibility Study of Processing Polydimethylsiloxane–Sodium Carboxymethylcellulose Composites by a Low-Cost Fused Deposition Modeling 3D Printer. Materials 2018, 11, 1578. [Google Scholar] [CrossRef] [PubMed]
- Gharaie, S.; Zolfagharian, A.; Moghadam, A.A.A.; Shukur, N.; Bodaghi, M.; Mosadegh, B.; Kouzani, A. Direct 3D printing of a two-part silicone resin to fabricate highly stretchable structures. In Progress in Additive Manufacturing; Springer: Berlin/Heidelberg, Germany, 2023. [Google Scholar]
- Fernandez-Vicente, M.; Calle, W.; Ferrandiz, S.; Conejero, A. Effect of Infill Parameters on Tensile Mechanical Behavior in Desktop 3D Printing. 3D Print. Addit. Manuf. 2016, 3, 183–192. [Google Scholar] [CrossRef]
- Muthusamy, M.; Safaee, S.; Chen, R.K. Additive Manufacturing of Overhang Structures Using Moisture-Cured Silicone with Support Material. J. Manuf. Mater. Process. 2018, 2, 24. [Google Scholar] [CrossRef]
- Muenks, D.; Kyosev, Y. Productivity Comparison Between Vat Polymerization and Fused Filament Fabrication Methods for Additive Manufacturing of Polymers. 3D Print. Addit. Manuf. 2023, 10, 40–49. [Google Scholar] [CrossRef]
- Zolfagharian, A.; Gharaie, S.; Kouzani, A.Z.; Lakhi, M.; Ranjbar, S.; Dezaki, M.L.; Bodaghi, M. Silicon-based soft parallel robots 4D printing and multiphysics analysis. Smart Mater. Struct. 2022, 31, 115030. [Google Scholar] [CrossRef]
- Mohammadi, M.; Zolfagharian, A.; Bodaghi, M.; Xiang, Y.; Kouzani, A.Z. 4D printing of soft orthoses for tremor suppression. Bio-Des. Manuf. 2022, 5, 786–807. [Google Scholar] [CrossRef]
- Zolfagharian, A.; Khosravani, M.R.; Duong Vu, H.; Nguyen, M.K.; Kouzani, A.Z.; Bodaghi, M. AI-Based Soft Module for Safe Human– Robot Interaction towards 4D Printing. Polymers 2022, 14, 3302. [Google Scholar] [CrossRef] [PubMed]
- Zolfagharian, A.; Lakhi, M.; Ranjbar, S.; Sayah Irani, M.; Nafea, M.; Bodaghi, M. 4D printing parameters optimisation for bi-stable soft robotic gripper design. J. Braz. Soc. Mech. Sci. Eng. 2023, 45, 224. [Google Scholar] [CrossRef]
- Au, A.K.; Huynh, W.; Horowitz, L.F.; Folch, A. 3D-Printed Microfluidics. Angew. Chem. Int. Ed. 2016, 55, 3862–3881. [Google Scholar] [CrossRef]
- Rogers, C.I.; Qaderi, K.; Woolley, A.T.; Nordin, G.P. 3D printed microfluidic devices with integrated valves. Biomicrofluidics 2015, 9, 016501. [Google Scholar] [CrossRef]
- Wu, L.; Beirne, S.; Cabot, J.M.; Paull, B.; Wallace, G.G.; Innis, P.C. Fused filament fabrication 3D printed polylactic acid electroosmotic pumps. Lab Chip 2021, 21, 3338–3351. [Google Scholar] [CrossRef]
- Diamond, D.; Collins, F.; Cleary, J.; Zuliani, C.; Fay, C. Distributed Environmental Monitoring. In Autonomous Sensor Networks: Collective Sensing Strategies for Analytical Purposes; Filippini, D., Ed.; Springer: Berlin/Heidelberg, Germany, 2013; pp. 321–363. [Google Scholar]
- Wu, L.; Manchanda, A.; Gupta, V.; Paull, B. Graphene Oxide-Functionalized Thread-Based Electrofluidic Approach for DNA Hybridization. ACS Omega 2023, 8, 13569–13577. [Google Scholar] [CrossRef]
- Kummari, S.; Panicker, L.R.; Rao Bommi, J.; Karingula, S.; Sunil Kumar, V.; Mahato, K.; Goud, K.Y. Trends in Paper-Based Sensing Devices for Clinical and Environmental Monitoring. Biosensors 2023, 13, 420. [Google Scholar] [CrossRef] [PubMed]
- Fay, C.D.; Wu, L. Critical importance of RGB color space specificity for colorimetric bio/chemical sensing: A comprehensive study. Talanta 2024, 266, 124957. [Google Scholar] [CrossRef] [PubMed]
- Benito-Lopez, F.; Coyle, S.; Byrne, R.; Smeaton, A.; O’Connor, N.E.; Diamond, D. Pump Less Wearable Microfluidic Device for Real Time pH Sweat Monitoring. Procedia Chem. 2009, 1, 1103–1106. [Google Scholar] [CrossRef]
- Fay, C.; Lau, K.T.; Beirne, S.; Ó Conaire, C.; McGuinness, K.; Corcoran, B.; O’Connor, N.E.; Diamond, D.; McGovern, S.; Coleman, G.; et al. Wireless aquatic navigator for detection and analysis (WANDA). Sensors Actuators B Chem. 2010, 150, 425–435. [Google Scholar] [CrossRef]
- Fay, C.D.; Nattestad, A. LED PEDD Discharge Photometry: Effects of Software Driven Measurements for Sensing Applications. Sensors 2022, 22, 1526. [Google Scholar] [CrossRef] [PubMed]
- Fay, C.D.; Nattestad, A. Advances in Optical Based Turbidity Sensing Using LED Photometry (PEDD). Sensors 2022, 22, 254. [Google Scholar] [CrossRef] [PubMed]
- Curto, V.F.; Coyle, S.; Byrne, R.; Angelov, N.; Diamond, D.; Benito-Lopez, F. Concept and development of an autonomous wearable micro-fluidic platform for real time pH sweat analysis. Sensors Actuators B Chem. 2012, 175, 263–270. [Google Scholar] [CrossRef]
- Murray, E.; Roche, P.; Briet, M.; Moore, B.; Morrin, A.; Diamond, D.; Paull, B. Fully automated, low-cost ion chromatography system for in-situ analysis of nitrite and nitrate in natural waters. Talanta 2020, 216, 120955. [Google Scholar] [CrossRef]
- Kajtez, J.; Buchmann, S.; Vasudevan, S.; Birtele, M.; Rocchetti, S.; Pless, C.J.; Heiskanen, A.; Barker, R.A.; Martínez-Serrano, A.; Parmar, M.; et al. 3D-Printed Soft Lithography for Complex Compartmentalized Microfluidic Neural Devices. Adv. Sci. 2020, 7, 2001150. [Google Scholar] [CrossRef] [PubMed]
- Fay, C.D. Computer-Aided Design and Manufacturing (CAD/CAM) for Bioprinting. In 3D Bioprinting: Principles and Protocols; Crook, J.M., Ed.; Springer: New York, NY, USA, 2020; pp. 27–41. [Google Scholar] [CrossRef]
- Ruiz-Cantu, L.; Gleadall, A.; Faris, C.; Segal, J.; Shakesheff, K.; Yang, J. Multi-material 3D bioprinting of porous constructs for cartilage regeneration. Mater. Sci. Eng. C 2020, 109, 110578. [Google Scholar] [CrossRef]
- Yang, G.H.; Kim, W.; Kim, J.; Kim, G. A skeleton muscle model using GelMA-based cell-aligned bioink processed with an electric-field assisted 3D/4D bioprinting. Theranostics 2021, 11, 48–63. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Sun, Y.; Gao, Q.; He, C.; Yao, K.; Wang, T.; Xie, M.; Yu, K.; Nie, J.; Chen, Y.; et al. Gelatin Methacryloyl Hydrogel, from Standardization, Performance, to Biomedical Application. Adv. Healthc. Mater. 2023, 12, 2300395. [Google Scholar] [CrossRef] [PubMed]







| Parameter | Value | Unit |
|---|---|---|
| Print speed (XYZ movement) | 5 | mm.s |
| Syringe size | 10 | cc |
| XYZ motor driver control | 600 | mV |
| Micro-step resolution | 16 | Steps |
| Layer height | 0.6 | mm |
| Extrusion feed rate | 40 | .s |
| Retraction distance | 4 | mm |
| Retraction speed | 3 | mm.s |
| Layer to LED distance | 50 | mm |
| LED irradiance | 25 | W.cm |
| Tip diameter/layer height | 580 | |
| Room temperature | 20 | °C |
| ID | Component | Supplier | Number/Identifier |
|---|---|---|---|
| 1 | Printer frame | ME 3D | ME2 |
| 2 | Extruder motor | RobotDigg | 42HS48-2504 |
| 3 | Extruder frame | RobotDigg | 100-WL-81 |
| 4 | Curing LED | Mouser (AU) | LZ1-10UV00-0000 |
| 5 | Curing heat sink | Mouser (AU) | 6238PB-MT5 |
| 6 | DC–DC regulator | Jaycar | XC4514 |
| 7 | SyringeBungHolder.step | Customised | 10 |
| 8 | CarriageMount.step | Customised | 12 |
| 9 | MountingBracket.step | Customised | 13 (mnt) |
| 10 | LEDArm.step | Customised | 15 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fay, C.D.; Wu, L. Cost-Effective 3D Printing of Silicone Structures Using an Advanced Intra-Layer Curing Approach. Technologies 2023, 11, 179. https://doi.org/10.3390/technologies11060179
Fay CD, Wu L. Cost-Effective 3D Printing of Silicone Structures Using an Advanced Intra-Layer Curing Approach. Technologies. 2023; 11(6):179. https://doi.org/10.3390/technologies11060179
Chicago/Turabian StyleFay, Cormac D., and Liang Wu. 2023. "Cost-Effective 3D Printing of Silicone Structures Using an Advanced Intra-Layer Curing Approach" Technologies 11, no. 6: 179. https://doi.org/10.3390/technologies11060179






