Bistability and Chaos Emergence in Spontaneous Dynamics of Astrocytic Calcium Concentration
Abstract
:1. Introduction
2. Description of the Mathematical Model
3. Input Calcium Flow
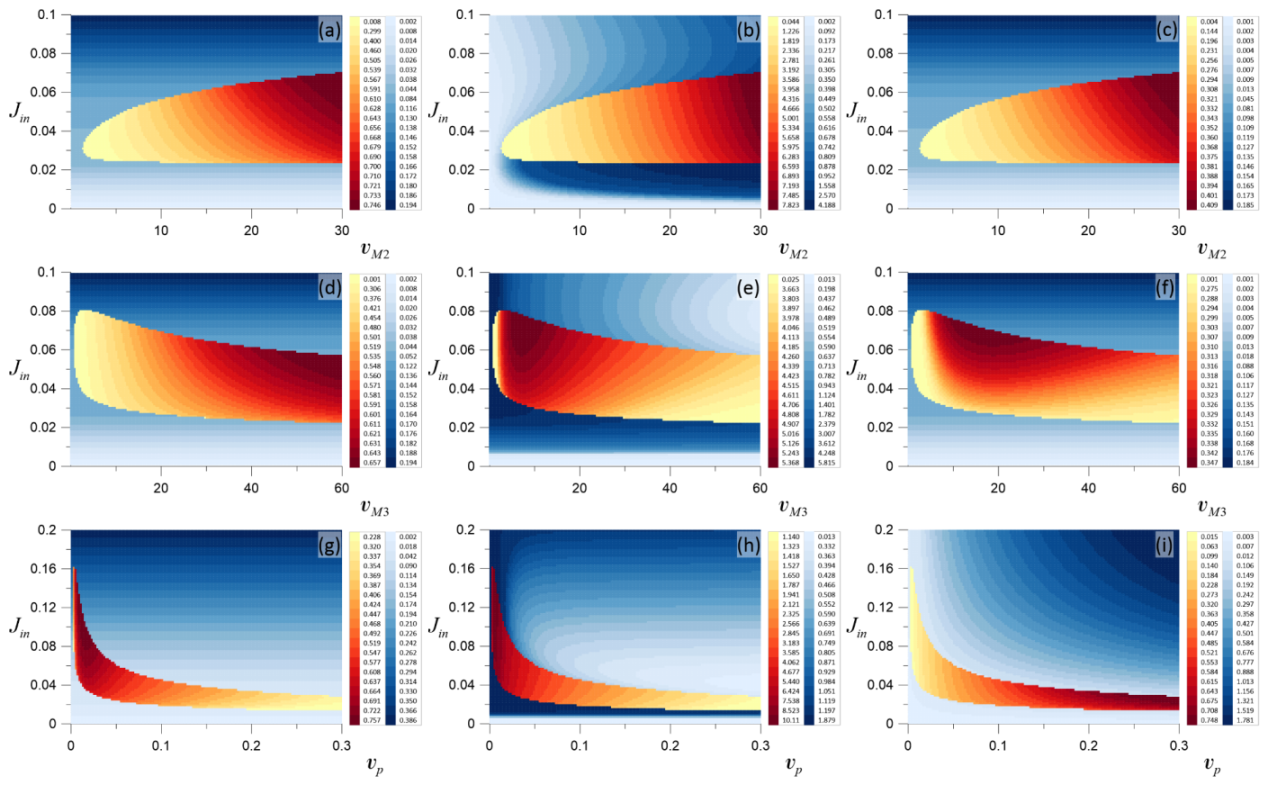
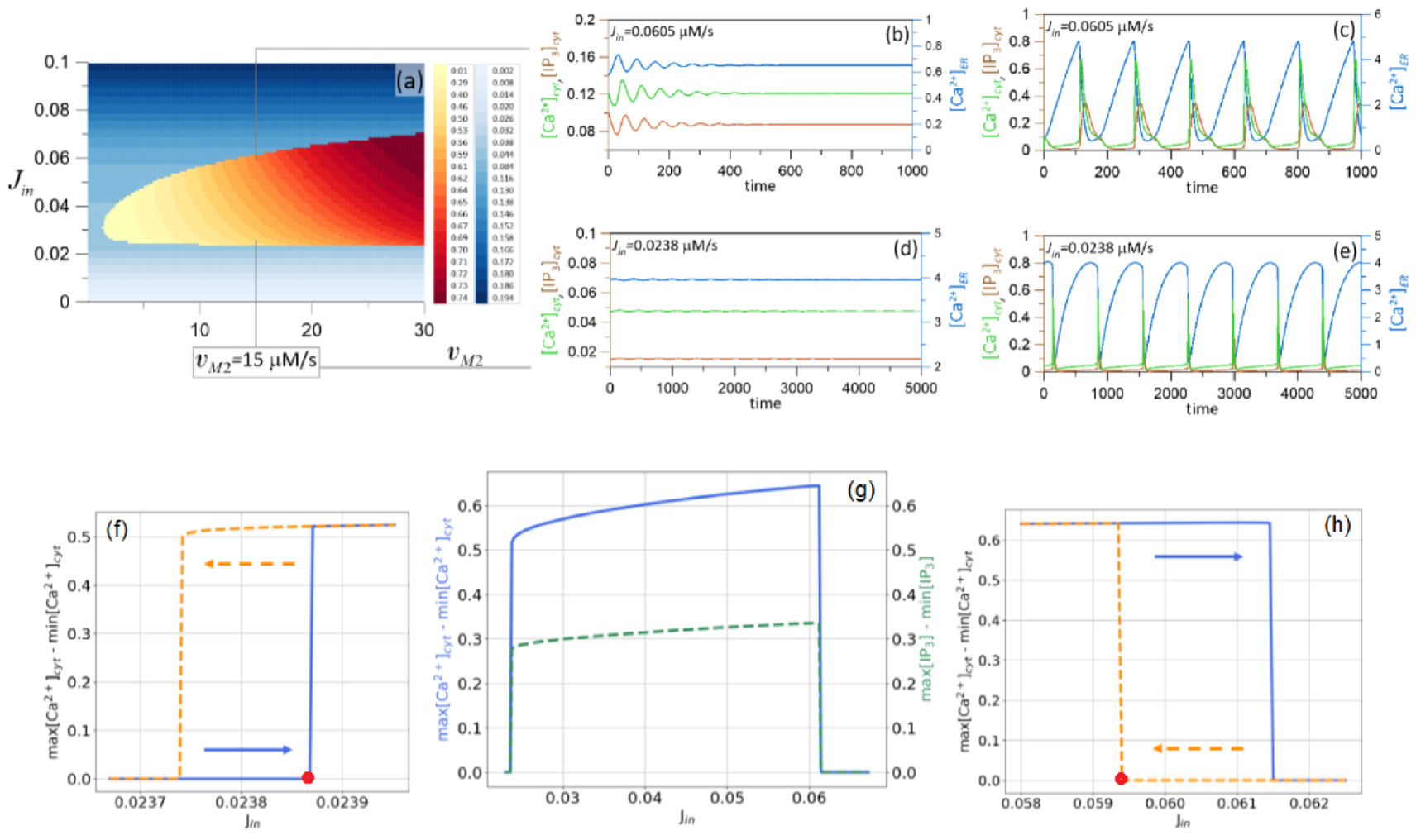
3.1. Steady States and Oscillatory Modes
3.2. Bistability Emergence: Coexistence of Steady State and Oscillatory Mode
- (a)
- The change of extracellular calcium flow reveals the existence of two -values where the Andronov–Hopf bifurcation occurs;
- (b)
- Both the Andronov–Hopf bifurcations are subcritical, i.e., the change of the equilibrium stability occurs with an unstable limit cycle emergence;
- (c)
- These unstable limit cycles coalesce with the stable cycles and disappear via the fold limit cycle bifurcations with further change of extracellular calcium flow. The -parameter ranges between the Andronov–Hopf and the fold limit cycle bifurcations define the ranges of bistability, where the coexistence of an oscillatory and steady state modes is observed.
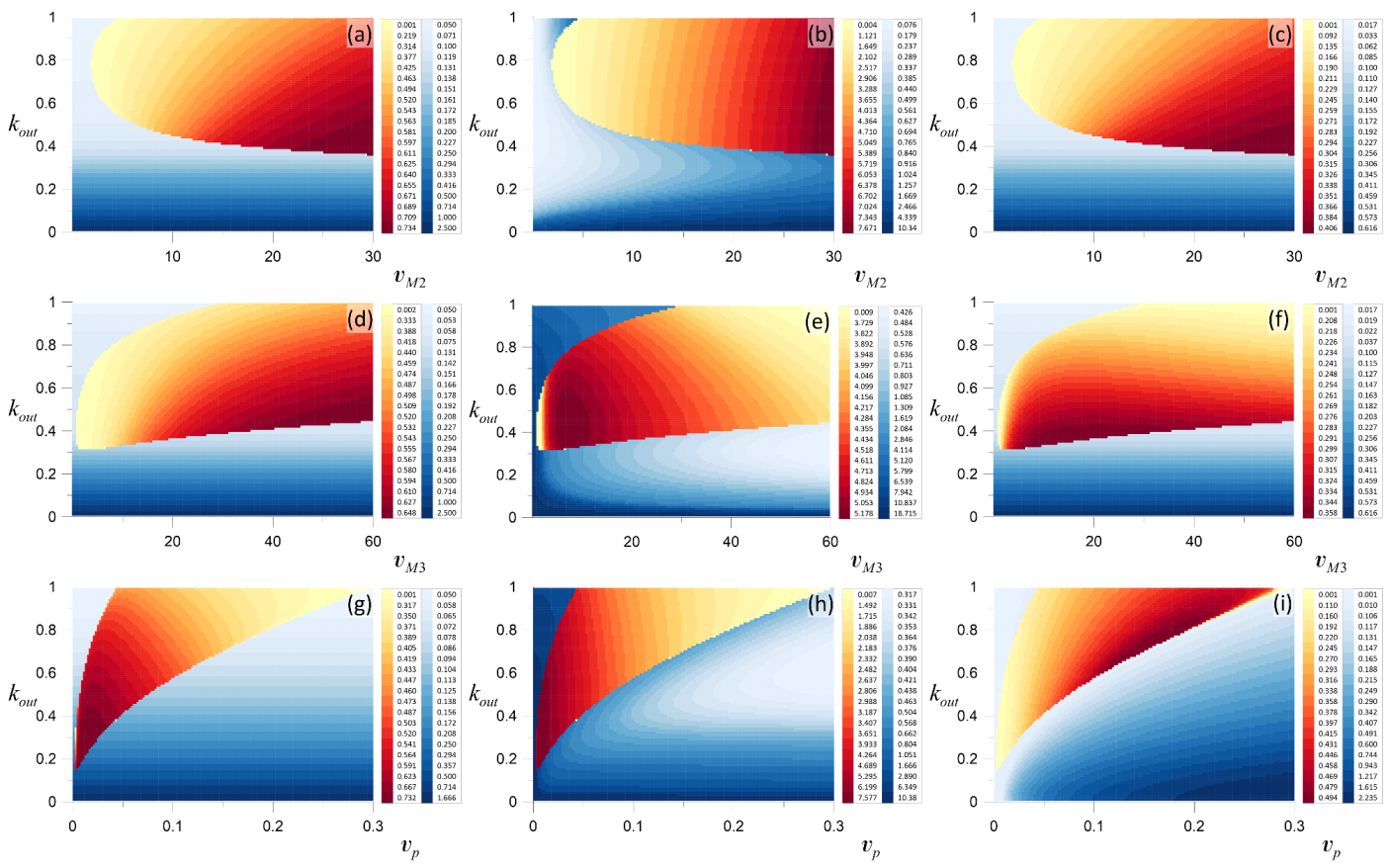
4. Output Calcium Flow
4.1. Steady States and Oscillatory Modes
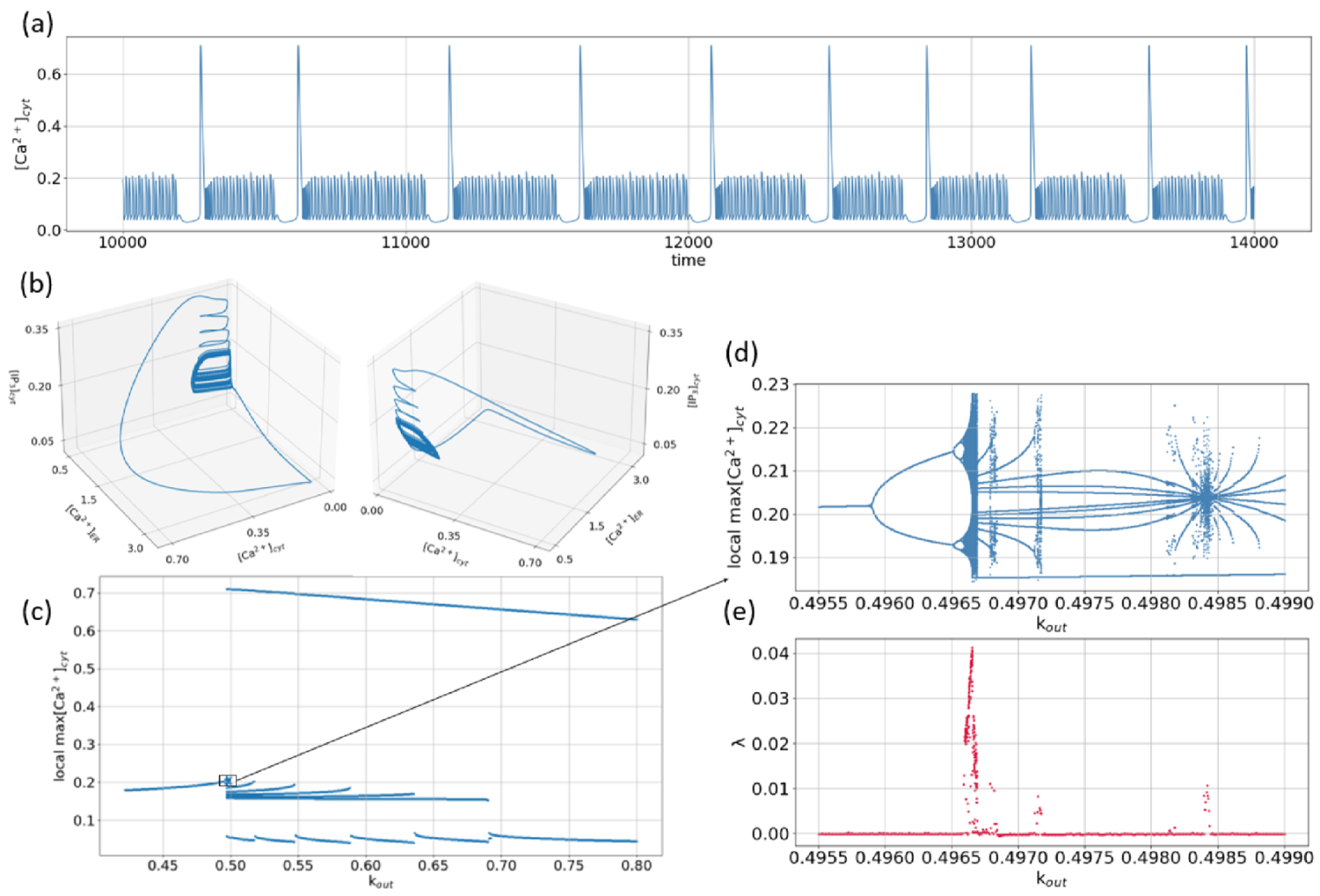
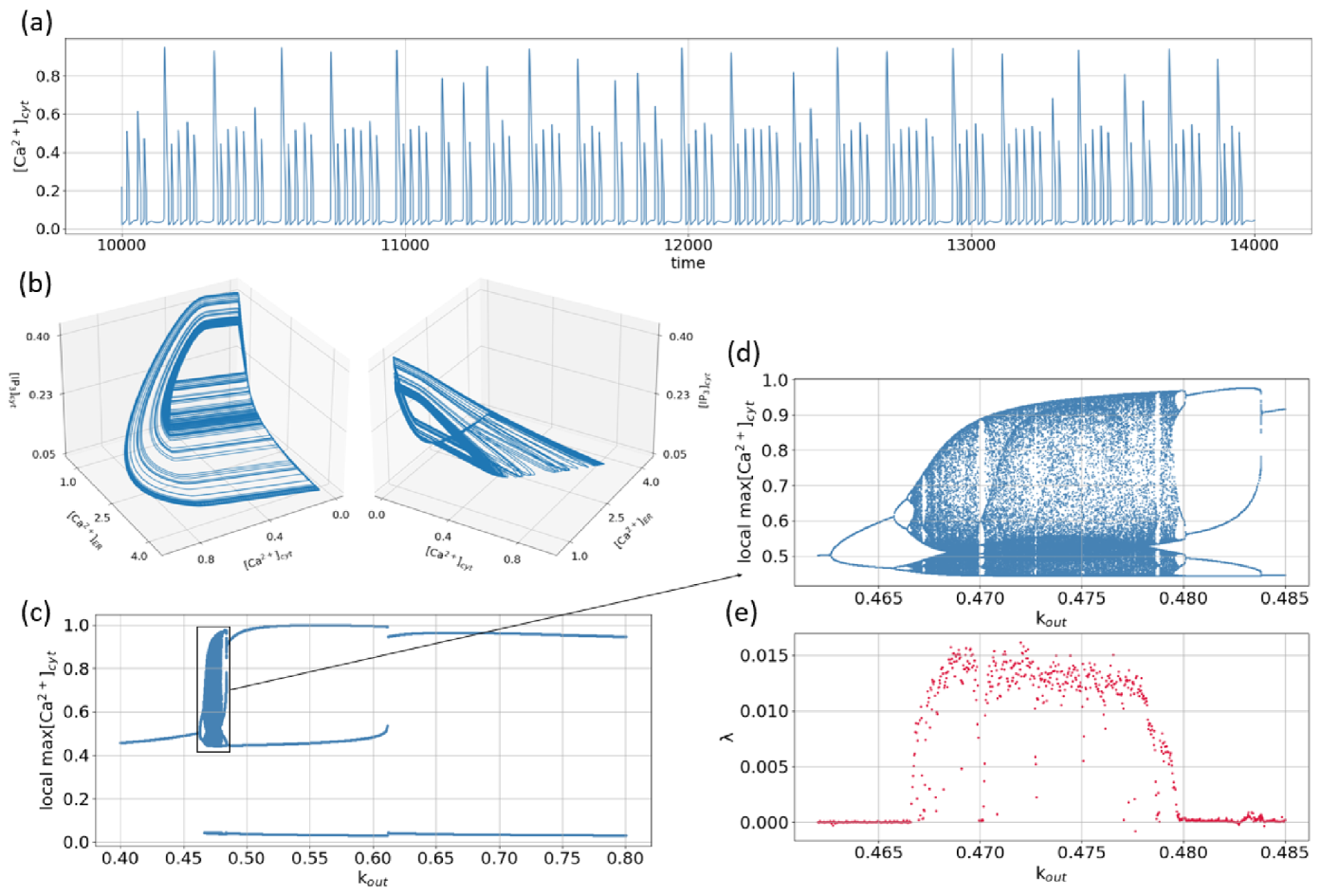
4.2. Chaotic Spontaneous Calcium Dynamics
4.2.1. Burst-Type Dynamics: Small-Peaks Irregularity
4.2.2. Small-Amplitude Chaotic Chemical Activity
4.2.3. Burst-Type Dynamics: All-Peaks Irregularity
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
References
- Parri, H.R.; Crunelli, V. The role of Ca2+ in the generation of spontaneous astrocytic Ca2+ oscillations. Neuroscience 2003, 120, 979–992. [Google Scholar] [CrossRef]
- Perea, G.; Araque, A. Properties of synaptically evoked astrocyte calcium signal reveal synaptic information processing by astrocytes. J. Neurosci. 2005, 25, 2192–2203. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Lou, N.; Xu, Q.; Tian, G.F.; Peng, W.G.; Han, X.; Kang, J.; Takano, T.; Nedergaard, M. Astrocytic Ca2+ signaling evoked by sensory stimulation in vivo. Nat. Neurosci. 2006, 9, 816–823. [Google Scholar] [CrossRef] [PubMed]
- Periasamy, M.; Kalyanasundaram, A. SERCA pump isoforms: Their role in calcium transport and disease. Muscle Nerve 2007, 35, 430–442. [Google Scholar] [CrossRef]
- Wu, Y.-W.; Tang, X.; Arizono, M.; Bannai, H.; Shih, P.-Y.; Dembitskaya, Y.; Kazantsev, V.; Tanaka, M.; Itohara, S.; Mikoshiba, K.; et al. Spatiotemporal calcium dynamics in single astrocytes and its modulation by neuronal activity. Cell Calcium 2014, 55, 119–129. [Google Scholar] [CrossRef]
- Stammers, A.N.; Susser, S.E.; Hamm, N.C.; Hlynsky, M.W.; Kimber, D.E.; Kehler, D.S.; Duhamel, T.A. The regulation of sarco(endo)plasmic reticulum calcium-ATPases (SERCA). Can. J. Physiol. Pharmacol. 2015, 93, 843–854. [Google Scholar] [CrossRef]
- Bazargani, N.; Attwell, D. Astrocyte calcium signaling: The third wave. Nat. Neurosci. 2016, 19, 182–189. [Google Scholar] [CrossRef]
- Semyanov, A. Spatiotemporal pattern of calcium activity in astrocytic network. Cell Calcium. 2019, 78, 15–25. [Google Scholar] [CrossRef]
- Verkhratsky, A.; Nedergaard, M. Physiology of astroglia. Physiol. Rev. 2018, 98, 239–389. [Google Scholar] [CrossRef]
- Wang, F.; Smith, N.A.; Xu, Q.; Fujita, T.; Baba, A.; Matsuda, T.; Takano, T.; Bekar, L.; Nedergaard, M. Astrocytes modulate neural network activity by Ca2+-dependent uptake of extracellular K+. Sci. Signal. 2012, 5, ra26. [Google Scholar] [CrossRef] [Green Version]
- Petzold, G.C.; Murthy, V.N. Role of astrocytes in neurovascular coupling. Neuron 2011, 71, 782–797. [Google Scholar] [CrossRef] [Green Version]
- Heller, J.P.; Rusakov, D.A. Morphological plasticity of astroglia: Understanding synaptic microenvironment. Glia 2015, 63, 2133–2151. [Google Scholar] [CrossRef]
- Araque, A.; Carmignoto, G.; Haydon, P.G.; Oliet, S.H.; Robitaille, R.; Volterra, A. Gliotransmitters travel in time and space. Neuron 2014, 81, 728–739. [Google Scholar] [CrossRef] [Green Version]
- Semyanov, A.; Henneberger, C.; Agarwal, A. Making sense of astrocytic calcium signals—From acquisition to interpretation. Nat. Rev. Neurosci. 2020, 21, 551–564. [Google Scholar] [CrossRef]
- Wang, T.-F.; Zhou, C.; Tang, A.-H.; Wang, S.-Q.; Chai, Z. Cellular mechanism for spontaneous calcium oscillations in astrocytes. ActaPharmacol. Sin. 2006, 27, 861–868. [Google Scholar]
- Nett, W.J.; Oloff, S.H.; McCarthy, K.D. Hippocampal astrocytes in situ exhibit calcium oscillations that occur independent of neuronal activity. J. Neurophysiol. 2002, 87, 528–537. [Google Scholar] [CrossRef]
- Sun, M.-Y.; Devaraju, P.; Xie, A.X.; Holman, I.; Samones, E.; Murphy, T.R.; Fiacco, T.A. Astrocyte calcium microdomainsare inhibited by bafilomycin A1 and cannot be replicated by low-level Schaffer collateral stimulation in situ. Cell Calcium 2014, 55, 1–16. [Google Scholar] [CrossRef]
- Srinivasan, R.; Huang, B.S.; Venugopal, S.; Johnston, A.D.; Chai, H.; Zeng, H.; Golshani, P.; Khakh, B.S. Ca2+ signaling in astrocytes from IP3R2−/− mice in brain slices and during startle responses in vivo. Nat. Neurosci. 2015, 18, 708–717. [Google Scholar] [CrossRef] [Green Version]
- Agarwal, A.; Wu, P.-H.; Hughes, E.G.; Fukaya, M.; Tischfield, M.A.; Langseth, A.J.; Wirtz, D.; Bergles, D.E. Transient opening of the mitochondrial permeability transition pore induces microdomain calcium transients in astrocyte processes. Neuron 2017, 93, 587–605.e587. [Google Scholar] [CrossRef] [Green Version]
- Aguado, F.; Espinosa-Parrilla, J.F.; Carmona, M.A.; Soriano, E. Neuronal activity regulates correlated network properties of spontaneous calcium transients in astrocytes in situ. J. Neurosci. 2002, 22, 9430–9444. [Google Scholar] [CrossRef] [Green Version]
- Tashiro, A.; Goldberg, J.; Yuste, R. Calcium oscillations in neocortical astrocytes under epileptiform conditions. J. Neurobiol. 2002, 50, 45–55. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Braun, J.; Mattia, M. Attractors and noise: Twin drivers of decisions and multistability. NeuroImage 2010, 52, 740–751. [Google Scholar] [CrossRef] [PubMed]
- Foss, J.; Moss, F.; Milton, J. Noise, multistability, and delayed recurrent loops. Phys. Rev. E 1997, 55, 4536–4543. [Google Scholar] [CrossRef]
- Newman, J.P.; Butera, R.J. Mechanism, dynamics and biological existence of multistability in a large class of bursting neurons. Chaos 2010, 20, 023118. [Google Scholar] [CrossRef] [Green Version]
- Pisarchik, A.N.; Feudel, U. Control of multistability. Phys. Rep. 2014, 540, 167–218. [Google Scholar] [CrossRef]
- Rungta, R.L.; Bernier, L.-P.; Dissing-Olesen, L.; Groten, C.J.; LeDue, J.M.; Ko, R.; Drissler, S.; MacVicar, B.A. Ca2+ transients in astrocyte fine processes occur via Ca2+ influx in the adult mouse hippocampus. Glia 2016, 64, 2093–2103. [Google Scholar] [CrossRef]
- Wu, Y.-W.; Gordleeva, S.; Tang, X.; Shih, P.-Y.; Dembitskaya, Y.; Semyanov, A. Morphological profile determines the frequency of spontaneous calcium events in astrocytic processes. Glia 2019, 67, 246–262. [Google Scholar] [CrossRef]
- Meyer, T.; Stryer, L. Calcium Spiking. Annu. Rev. Biophys. Biophys. Chem. 1991, 20, 153–174. [Google Scholar] [CrossRef]
- Foskett, J.K.; White, C.; Cheung, K.-H.; Mak, D.-O.D. Inositol trisphosphate receptor Ca2+ release channels. Physiol. Rev. 2007, 87, 593–658. [Google Scholar] [CrossRef] [Green Version]
- Zeng, S.; Li, B.; Zeng, S.; Chen, S. Simulation of spontaneous Ca2+ oscillations in astrocytes mediated by voltage-gated calcium channels. Biophys. J. 2009, 97, 2429–2437. [Google Scholar] [CrossRef] [Green Version]
- Lavrentovich, M.; Hemkin, S. A mathematical model of spontaneous calcium (II) oscillations in astrocytes. J. Theor. Biol. 2008, 251, 553–560. [Google Scholar] [CrossRef]
- Goto, I.; Kinoshita, S.; Natsume, K. The model of glutamate-induced intracellular Ca2+ oscillation and intercellular Ca2+ wave in brain astrocytes. Neurocomputing 2004, 58–60, 461–467. [Google Scholar] [CrossRef]
- Kazantsev, V.B. Spontaneous calcium signals induced by gap junctions in a network model of astrocytes. Phys. Rev. E 2009, 79, 010901. [Google Scholar] [CrossRef]
- Matrosov, V.V.; Kazantsev, V.B. Bifurcation mechanisms of regular and chaotic network signaling in brain astrocytes. Chaos 2011, 21, 023103. [Google Scholar] [CrossRef]
- Matrosov, V.; Gordleeva, S.; Boldyreva, N.; Ben-Jacob, E.; Kazantsev, V.; De Pittà, M. Emergence of Regular and Complex Calcium Oscillations by Inositol 1,4,5-Trisphosphate Signaling in Astrocytes. In Computational Glioscience; Springer Series in Computational Neuroscience; De Pittà, M., Berry, H., Eds.; Springer: Cham, Switzerland, 2019. [Google Scholar]
- Manninen, T.; Havela, R.; Linne, M.-L. Computational Models for Calcium-Mediated Astrocyte Functions. Front. Comput. Neurosci. 2018, 12, 14. [Google Scholar] [CrossRef] [Green Version]
- Sinitsina, M.S.; Gordleeva, S.Y.; Kazantsev, V.B.; Pankratova, E.V. Emergence of complicated regular and irregular spontaneous Ca2+ oscillations in astrocytes. In Proceedings of the 4th Scientific School on Dynamics of Complex Networks and their Application in Intellectual Robotics (DCNAIR), Innopolis, Russia, 7–9 September 2020. [Google Scholar]
- Zuo, H.; Ye, M. Bifurcation and numerical simulations of Ca2+ oscillatory behavior in astrocytes. Front. Phys. 2020, 8, 258. [Google Scholar] [CrossRef]
- Ye, M.; Zuo, H. Stability analysis of regular and chaotic Ca2+ oscillations in astrocytes. Discret. Dyn. Nat. Soc. 2020, 2020, 9279315. [Google Scholar] [CrossRef]
- Ji, Q.; Ye, M. Control of chaotic calcium oscillations in biological cells. Complexity 2021, 2021, 8861465. [Google Scholar] [CrossRef]
- Sinitsina, M.S.; Gordleeva, S.Y.; Kazantsev, V.B.; Pankratova, E.V. Calcium concentration in astrocytes: Emergence of complicated spontaneous oscillations and their cessation. Izvestiya VUZ. Appl. Nonlinear Dyn. 2021, 29, 440–448. [Google Scholar]
- Wolf, A.; Swift, J.B.; Swinney, H.L.; Vastano, J.A. Determining lyapunov exponents from a time series. Phys. D Nonlinear Phenom. 1985, 16, 285–317. [Google Scholar] [CrossRef] [Green Version]
- Malashchenko, T.; Shilnikov, A.; Cymbalyuk, G. Six types of multistability in a neuronal model based on slow calcium current. PLoS ONE 2011, 6, e21782. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ngouonkadi, M.E.B.; Fotsin, H.B.; Fotso, L.P.; Tamba, K.V.; Cerdeira, H.A. Bifurcations and multistability in the extended Hindmarsh–Rose neuronal oscillator. Chaos Solitons Fract. 2016, 85, 151–163. [Google Scholar] [CrossRef] [Green Version]
- Stankevich, N.V.; Volkov, E.I. Multistability in a three-dimensional oscillator: Tori, resonant cycles and chaos. Nonlinear Dyn. 2018, 94, 2455–2467. [Google Scholar] [CrossRef]
- Lazarevich, I.; Stasenko, S.; Rozhnova, M.; Pankratova, E.; Dityatev, A.; Kazantsev, V. Activity-dependent switches between dynamic regimes of extracellular matrix expression. PLoS ONE 2020, 15, e0227917. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rozhnova, M.; Pankratova, E.; Stasenko, S.; Kazantsev, V. Bifurcation analysis of multistability and oscillation emergence in a model of brain extracellular matrix. Chaos Solitons Fractals 2021, 151, 111253. [Google Scholar] [CrossRef]
- Sinitsina, M.S.; Gordleeva, S.Y.; Kazantsev, V.B.; Pankratova, E.V. Quiescence-to-oscillations transition features in dynamics of spontaneous astrocytic calcium concentration. In Communications in Computer & Information Science, Mathematical Modeling and Supercomputer Technologies; Springer: Cham, Switzerland, 2021; Volume 1413, pp. 129–137. [Google Scholar]
- Swillens, S.; Dupont, G.; Combettes, L.; Champeil, P. From calcium blips to calcium puffs: Theoretical analysis of the requirements for interchannel communication. Proc. Natl. Acad. Sci. USA 1999, 96, 13750–13755. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Belykh, V.N.; Pankratova, E.V. Chaotic synchronization in ensembles of coupled neurons modeled by the FitzHugh-Rinzel system. Radiophys. Quantum Electron. 2006, 49, 910–921. [Google Scholar] [CrossRef]
- Iglesias, C.; Meunier, C.; Manuel, M.; Timofeeva, Y.; Delestrée, N.; Zytnicki, D. Mixed Mode Oscillations in Mouse Spinal Motoneurons Arise from a Low Excitability State. J. Neurosci. 2011, 31, 5829–5840. [Google Scholar] [CrossRef] [Green Version]
- Bacak, B.J.; Kim, T.; Smith, J.C.; Rubin, J.E.; Rybak, I.A. Mixed-mode oscillations and population bursting in the pre-Bötzinger complex. eLife 2016, 5, e13403. [Google Scholar] [CrossRef] [Green Version]
- Pankratova, E.V.; Kalyakulina, A.I. Environmentally induced amplitude death and firing provocation in large-scale networks of neuronal systems. Regul. Chaotic Dyn. 2016, 21, 840–848. [Google Scholar] [CrossRef]
- Sadhu, S.; Thakur, S.C. Uncertainty and predictability in population dynamics of a bitrophic ecological model: Mixed-mode oscillations, bistability and sensitivity to parameters. Ecol. Complex. 2017, 32 Pt B, 196–208. [Google Scholar] [CrossRef]
- Baldemir, H.; Avitabile, D.; Tsaneva-Atanasova, K. Pseudo-plateau bursting and mixed-mode oscillations in a model of developing inner hair cells. Commun. Nonlinear Sci. Numer. Simul. 2020, 80, 104979. [Google Scholar] [CrossRef]
- Inaba, N.; Tsubone, T. Nested mixed-mode oscillations, part II: Experimental and numerical study of a classical Bonhoeffer–van der Pol oscillator. Phys. D Nonlinear Phenom. 2020, 406, 132493. [Google Scholar] [CrossRef]
- Chen, Z.; Chen, F. Mixed mode oscillations induced by bi-stability and fractal basins in the FGP plate under slow parametric and resonant external excitations. Chaos Solitons Fractals 2020, 137, 109814. [Google Scholar] [CrossRef]
- An, X.; Qiao, S. The hidden, period-adding, mixed-mode oscillations and control in a HR neuron under electromagnetic induction. Chaos Solitons Fractals 2021, 143, 110587. [Google Scholar] [CrossRef]
- Rebecchi, M.J.; Pentyala, S.N. Structure, function, and control of phosphoinositide-specific phospholipase C. Physiol Rev. 2000, 80, 1291–1335. [Google Scholar] [CrossRef]
- Bindocci, E.; Savtchouk, I.; Liaudet, N.; Becker, D.; Carriero, G.; Volterra, A. Three-dimensional Ca2+ imaging advances understanding of astrocyte biology. Science 2017, 356, eaai8185. [Google Scholar] [CrossRef] [Green Version]
- Santello, M.; Toni, N.; Volterra, A. Astrocyte function frominformation processing to cognition and cognitive impairment. Nat. Neurosci. 2019, 22, 154–166. [Google Scholar] [CrossRef] [Green Version]
- Gordleeva, S.Y.; Ermolaeva, A.V.; Kastalskiy, I.A.; Kazantsev, V.B. Astrocyte as spatiotemporal integrating detector of neuronal activity. Front. Physiol. 2019, 10, 294. [Google Scholar] [CrossRef]
- Pankratova, E.V.; Kalyakulina, A.I.; Stasenko, S.V.; Gordleeva, S.Y.; Lazarevich, I.A.; Kazantsev, V.B. Neuronal synchronization enhanced by neuron-astrocyte interaction. Nonlinear Dyn. 2019, 97, 647–662. [Google Scholar] [CrossRef]
- Makovkin, S.Y.; Shkerin, I.V.; Gordleeva, S.Y.; Ivanchenko, M.V. Astrocyte-induced intermittent synchronization of neurons in a minimalnetwork. Chaos Solitons Fractals 2020, 138, 109951. [Google Scholar] [CrossRef]
- Gordleeva, S.Y.; Lebedev, S.A.; Rumyantseva, M.A.; Kazantsev, V.B. Astrocyte as a detector of synchronous events of a neural network. JETP Lett. 2018, 107, 440–445. [Google Scholar] [CrossRef]
- Kanakov, O.; Gordleeva, S.; Ermolaeva, A.; Jalan, S.; Zaikin, A. Astrocyte-induced positive integrated information in neuron-astrocyte ensembles. Phys. Rev. E 2019, 99, 012418. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, J.; Mcdaid, L.J.; Harkin, J.; Karim, S.; Johnson, A.P.; Millard, A.G.; Hilder, J.; Halliday, D.M.; Tyrrell, A.M.; Timmis, J. Exploring self-repair in a coupled spiking astrocyte neural network. IEEE Trans. Neural Netw. Learn. Syst. 2019, 30, 865–875. [Google Scholar] [CrossRef] [Green Version]
- Nazari, S.; Amiri, M.; Faez, K.; Hulle, M.M.V. Information transmitted from bioinspired neuron–astrocyte network improves cortical spiking networks pattern recognition performance. IEEE Trans. Neural Netw. Learn. Syst. 2020, 31, 464–474. [Google Scholar] [CrossRef]
- Kanakov, O.; Gordleeva, S.; Zaikin, A. Integrated informationin the spiking–bursting stochastic model. Entropy 2020, 22, 1334. [Google Scholar] [CrossRef]
- Abrego, L.; Gordleeva, S.; Kanakov, O.; Krivonosov, M.; Zaikin, A. Estimating integrated information in bidirectional neuron-astrocyte communication. Phys. Rev. E 2021, 103, 022410. [Google Scholar] [CrossRef]
- Wade, J.J.; McDaid, L.J.; Harkin, J.; Crunelli, V.; Kelso, J.A.S. Bidirectional coupling between astrocytes and neurons mediates learning and dynamic coordination in the brain: A multiple modeling approach. PLoS ONE 2011, 6, e29445. [Google Scholar] [CrossRef]
- Tewari, S.; Parpura, V. A possible role of astrocytes in contextualmemory retrieval: An analysis obtained using a quantitative framework. Front. Comput. Neurosci. 2013, 7, 145. [Google Scholar] [CrossRef] [Green Version]
- Gordleeva, S.Y.; Lotareva, Y.A.; Krivonosov, M.I.; Zaikin, A.A.; Ivanchenko, M.V.; Gorban, A.N. Astrocytes Organizeassociative Memory; Springer International Publishing: Berlin/Heidelberg, Germany, 2019; pp. 384–391. [Google Scholar]
- Gordleeva, S.Y.; Tsybina, Y.A.; Krivonosov, M.I.; Ivanchenko, M.V.; Zaikin, A.A.; Kazantsev, V.B.; Gorban, A.N. Modeling working memory in a spiking neuron network accompanied by astrocytes. Front. Cell. Neurosci. 2021, 15, 86. [Google Scholar] [CrossRef]
- Tsybina, Y.; Kastalskiy, I.; Krivonosov, M.; Zaikin, A.; Kazantsev, V.; Gorban, A.; Gordleeva, S. Astrocytesmediateanalogousmemory in amulti-layer neuron-astrocytic network. arXiv 2021, arXiv:2108.13414. [Google Scholar]
- Lines, J.; Baraibar, A.M.; Fang, C.; Martin, E.D.; Aguilar, J.; Lee, M.K.; Araque, A.; Kofuji, P. Astrocyte-neuronal network interplay is disrupted in Alzheimer’s disease mice. Glia 2021, 70, 368–378. [Google Scholar] [CrossRef]
- Whitwell, H.J.; Bacalini, M.G.; Blyuss, O.; Chen, S.; Garagnani, P.; Gordleeva, S.Y.; Jalan, S.; Ivanchenko, M.; Kanakov, O.; Kustikova, V.; et al. The human body as a super network: Digital methods to analyze the propagation of aging. Front. Aging Neurosci. 2020, 12, 136. [Google Scholar] [CrossRef]
- Gordleeva, S.; Kanakov, O.; Ivanchenko, M.; Zaikin, A.; Franceschi, C. Brain aging and garbage cleaning. Semin. Immunopathol. 2020, 42, 647–665. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pankratova, E.V.; Sinitsina, M.S.; Gordleeva, S.; Kazantsev, V.B. Bistability and Chaos Emergence in Spontaneous Dynamics of Astrocytic Calcium Concentration. Mathematics 2022, 10, 1337. https://doi.org/10.3390/math10081337
Pankratova EV, Sinitsina MS, Gordleeva S, Kazantsev VB. Bistability and Chaos Emergence in Spontaneous Dynamics of Astrocytic Calcium Concentration. Mathematics. 2022; 10(8):1337. https://doi.org/10.3390/math10081337
Chicago/Turabian StylePankratova, Evgeniya V., Maria S. Sinitsina, Susanna Gordleeva, and Victor B. Kazantsev. 2022. "Bistability and Chaos Emergence in Spontaneous Dynamics of Astrocytic Calcium Concentration" Mathematics 10, no. 8: 1337. https://doi.org/10.3390/math10081337





