Computational Study of Phase Change Heat Transfer and Latent Heat Energy Storage for Thermal Management of Electronic Components Using Neural Networks
Abstract
:1. Introduction
2. Physical Model
3. Numerical Approach, Mesh Sensitivity Study, and Verification
3.1. The Finite Element Method
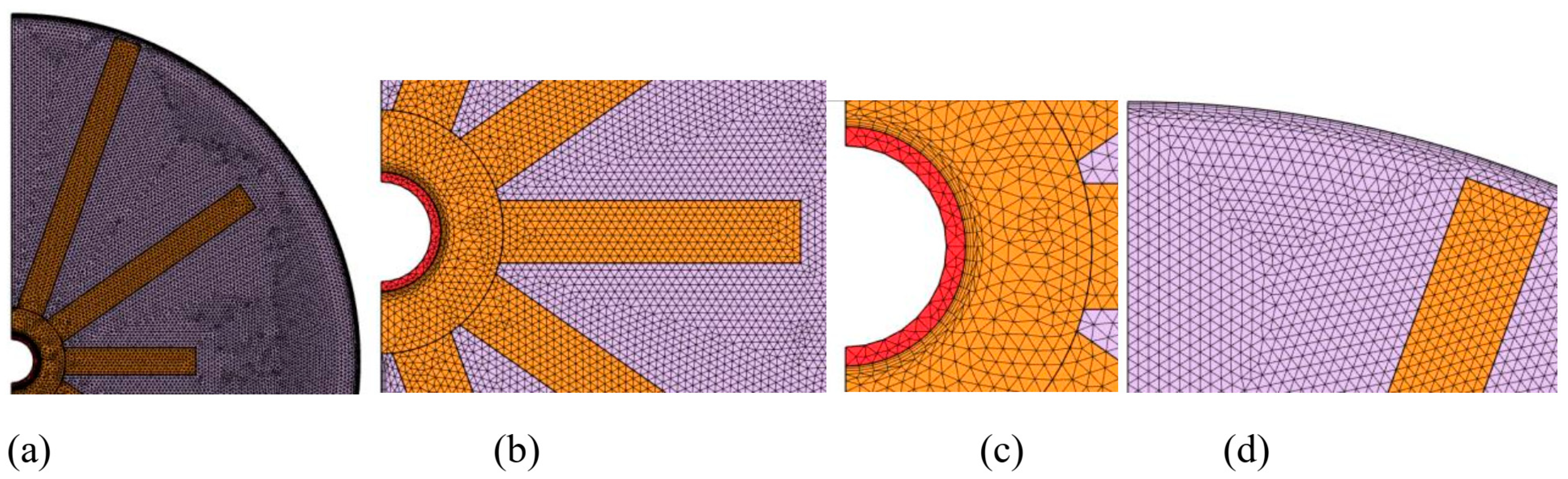
3.2. Mesh Sensitivity
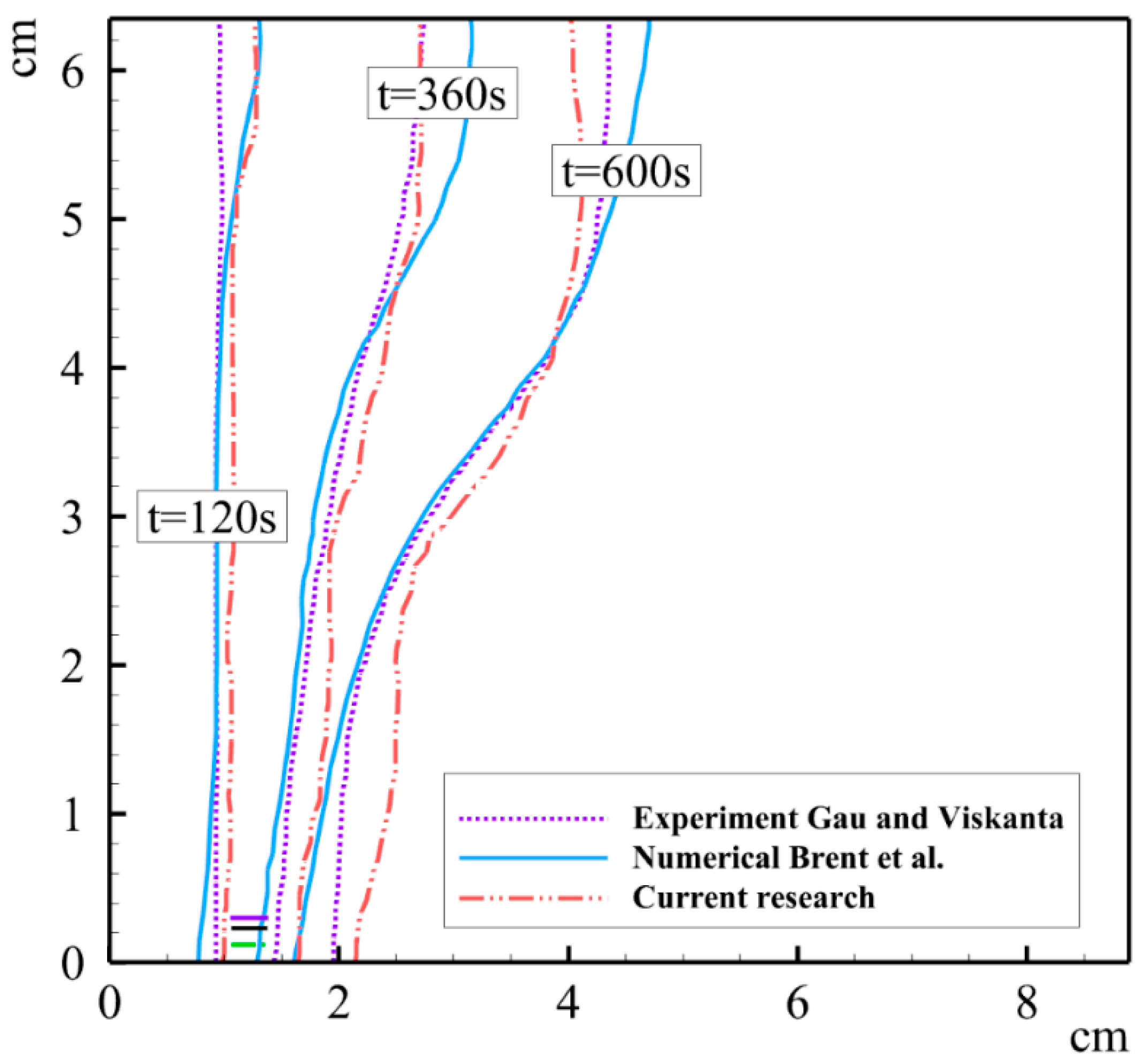
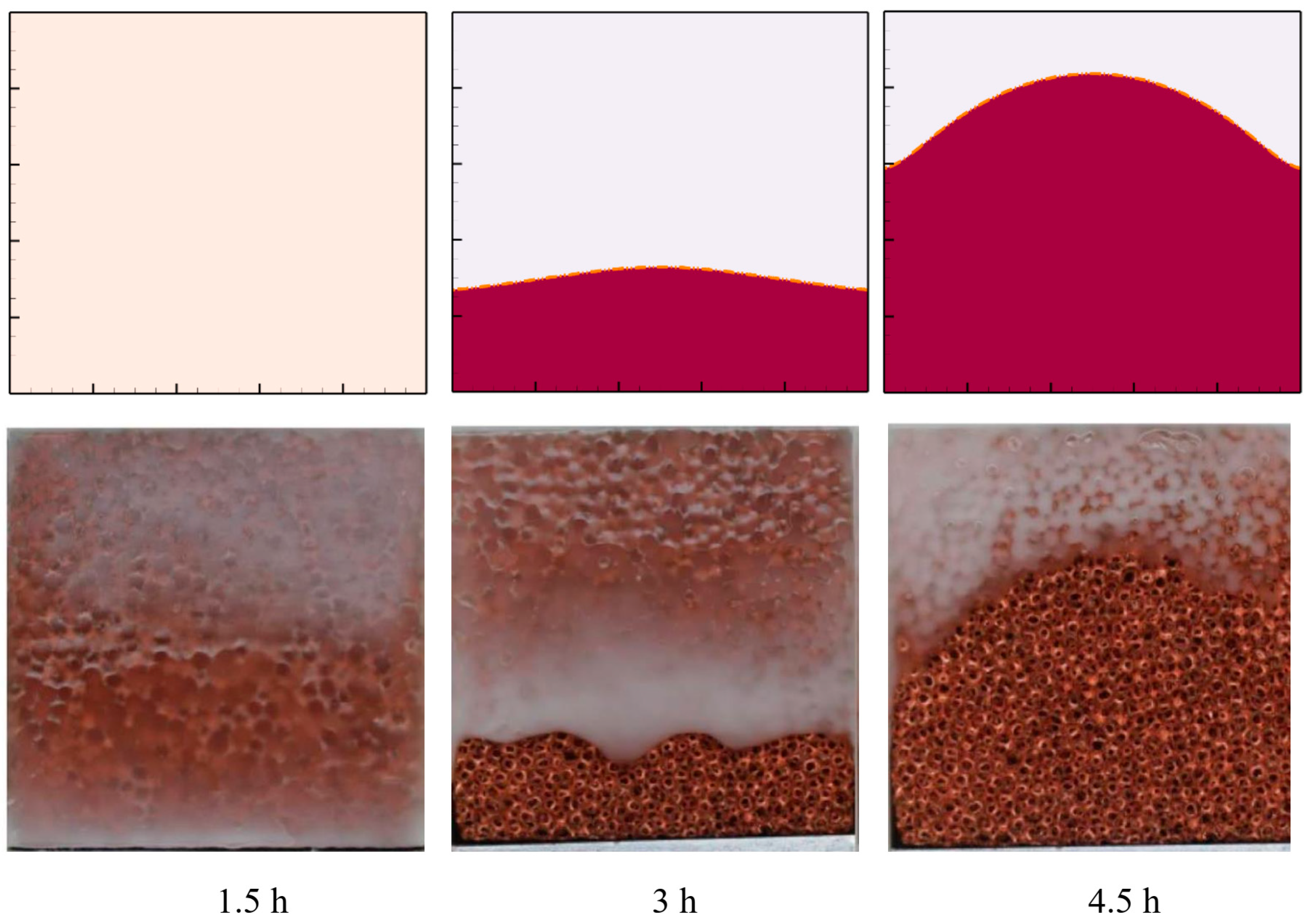
3.3. Validation and Verification
4. Results and Discussion
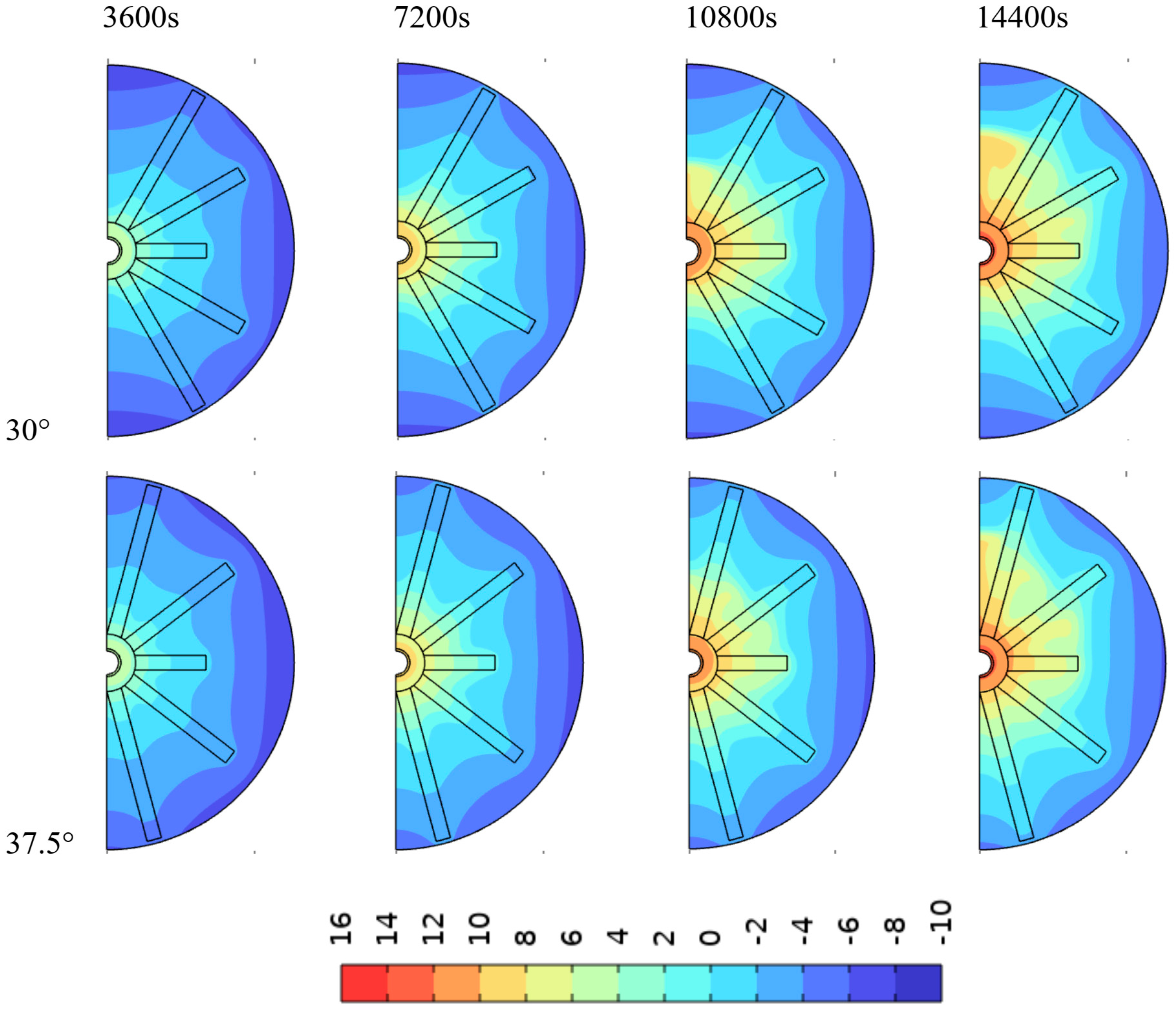
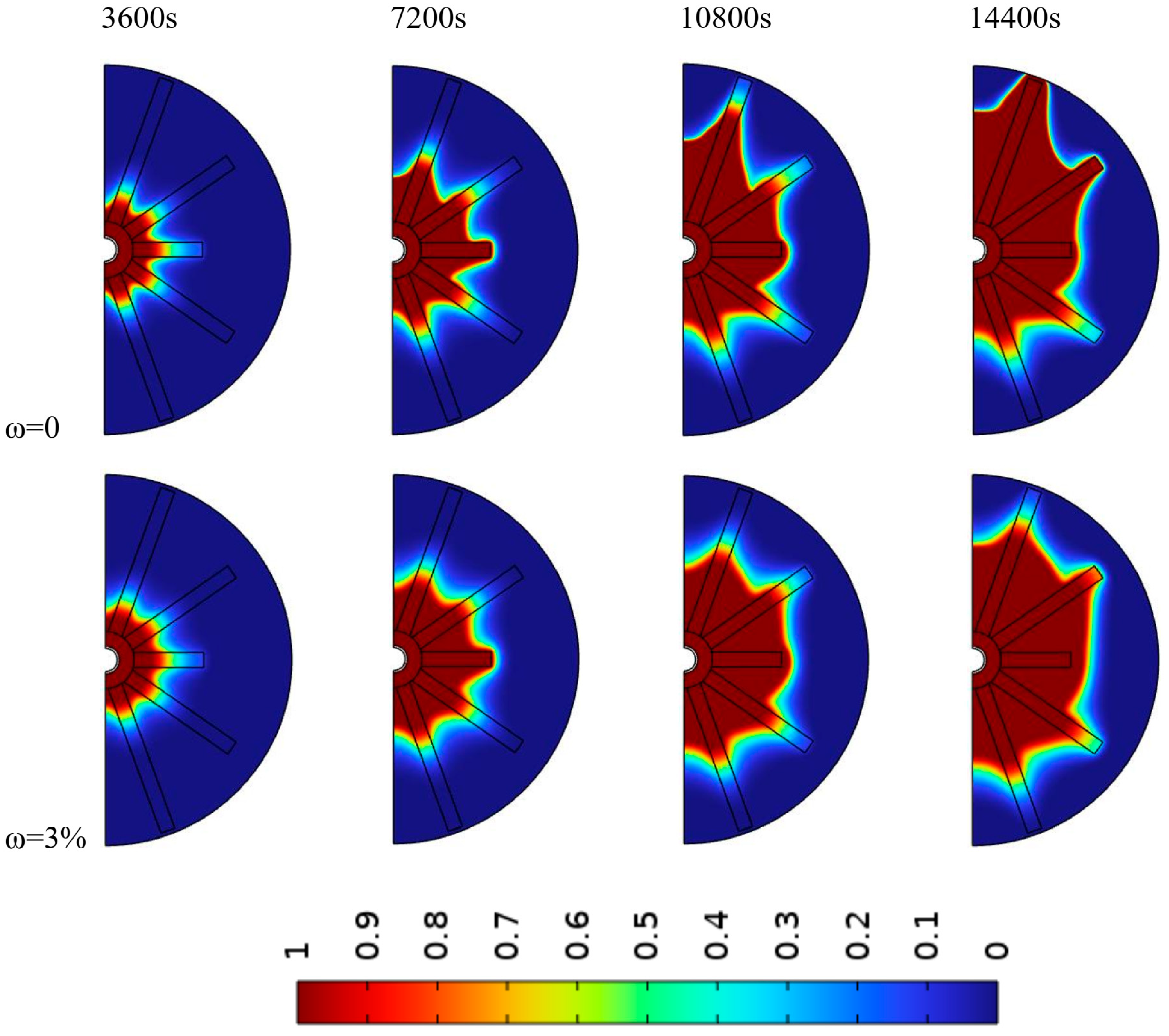
4.1. Impact of Fin Angle
4.2. Impact of Nanoparticles Concentration
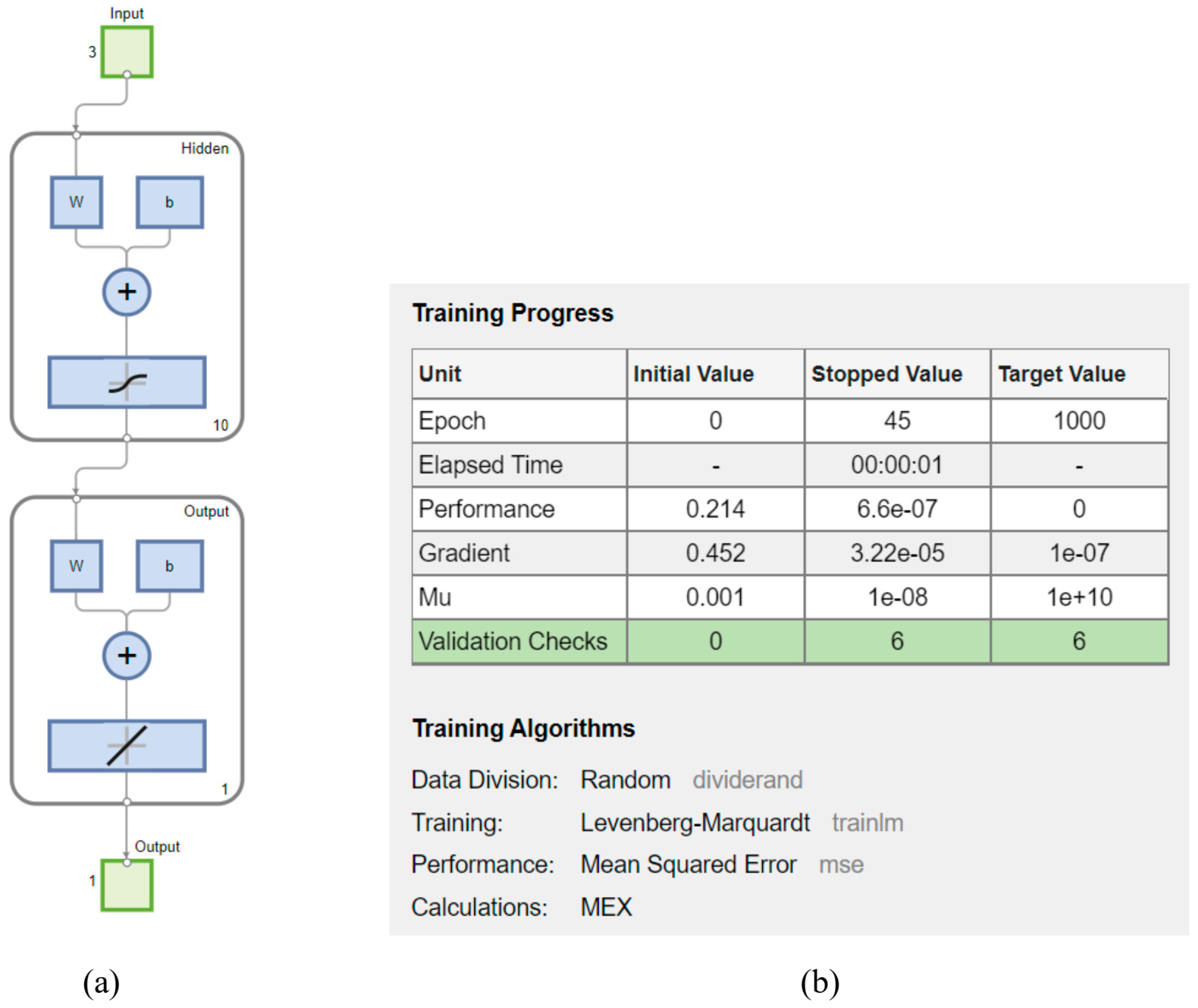
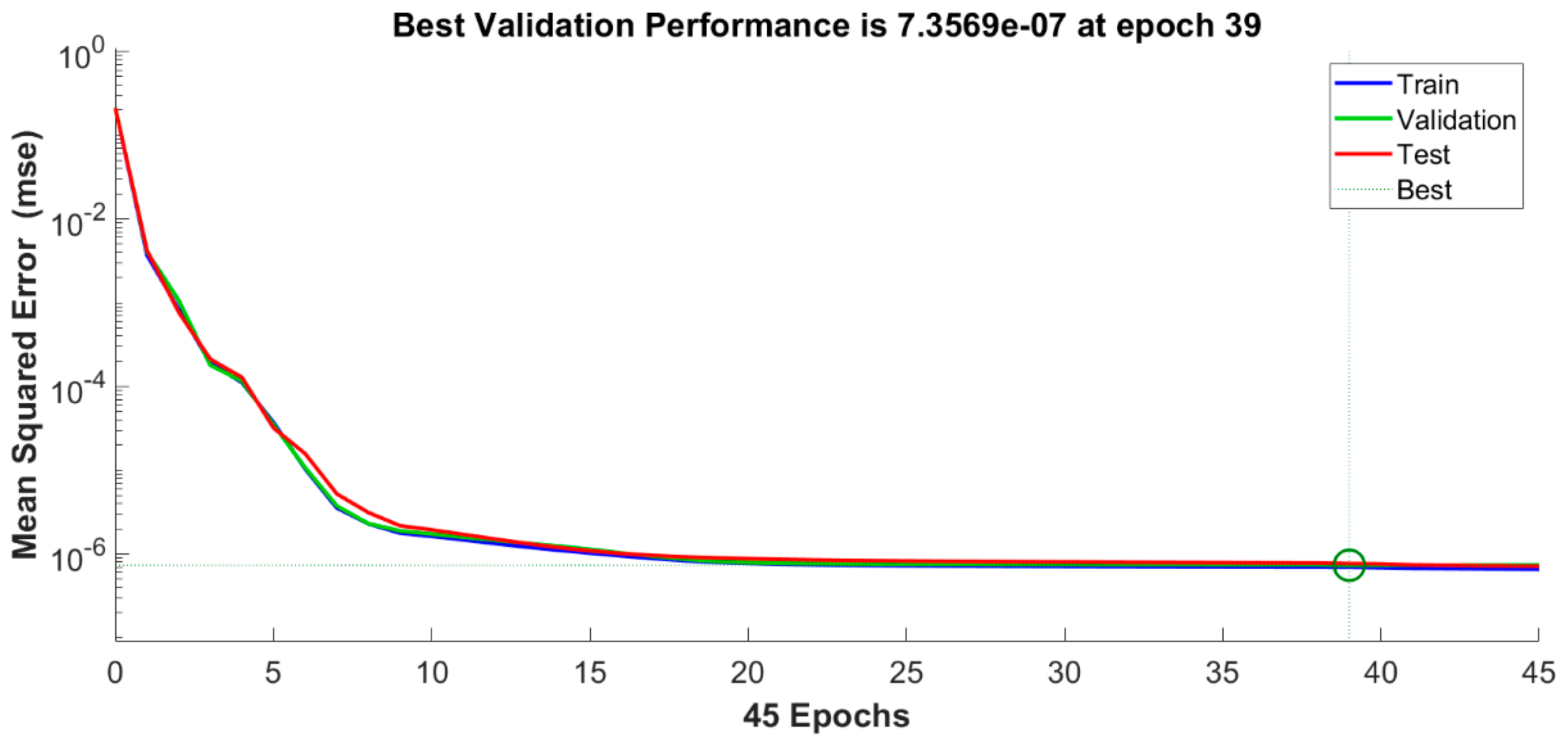
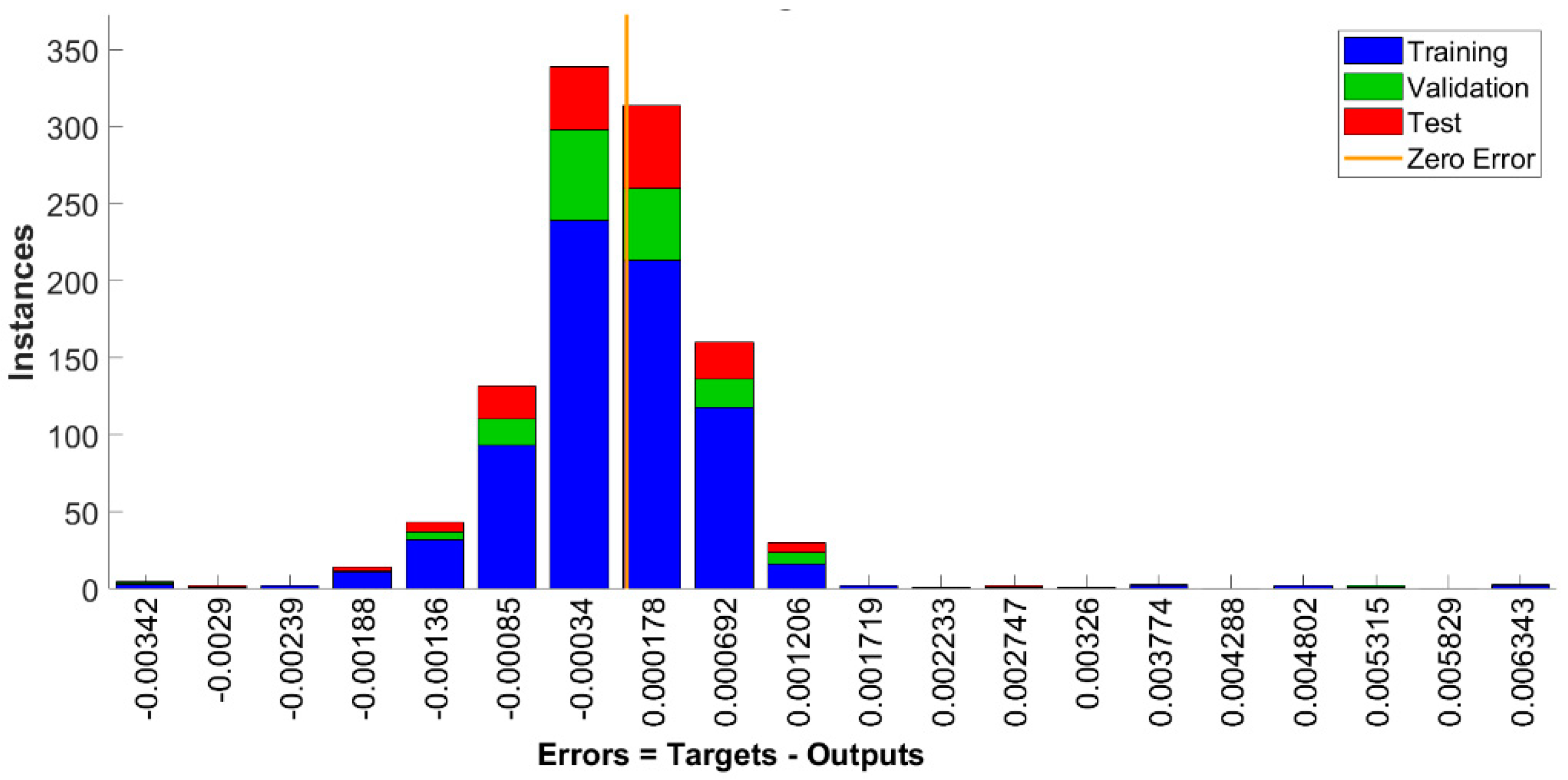
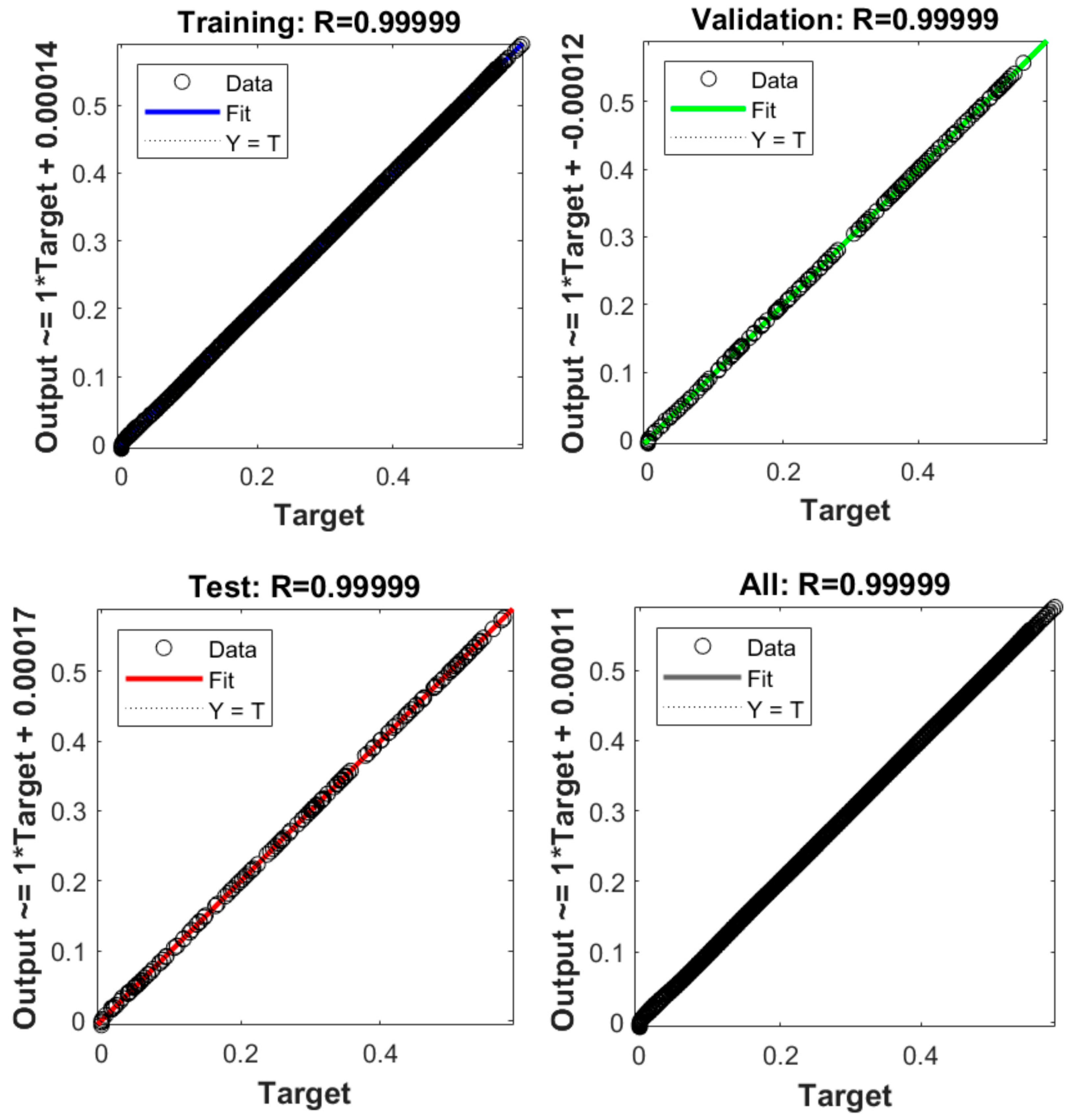
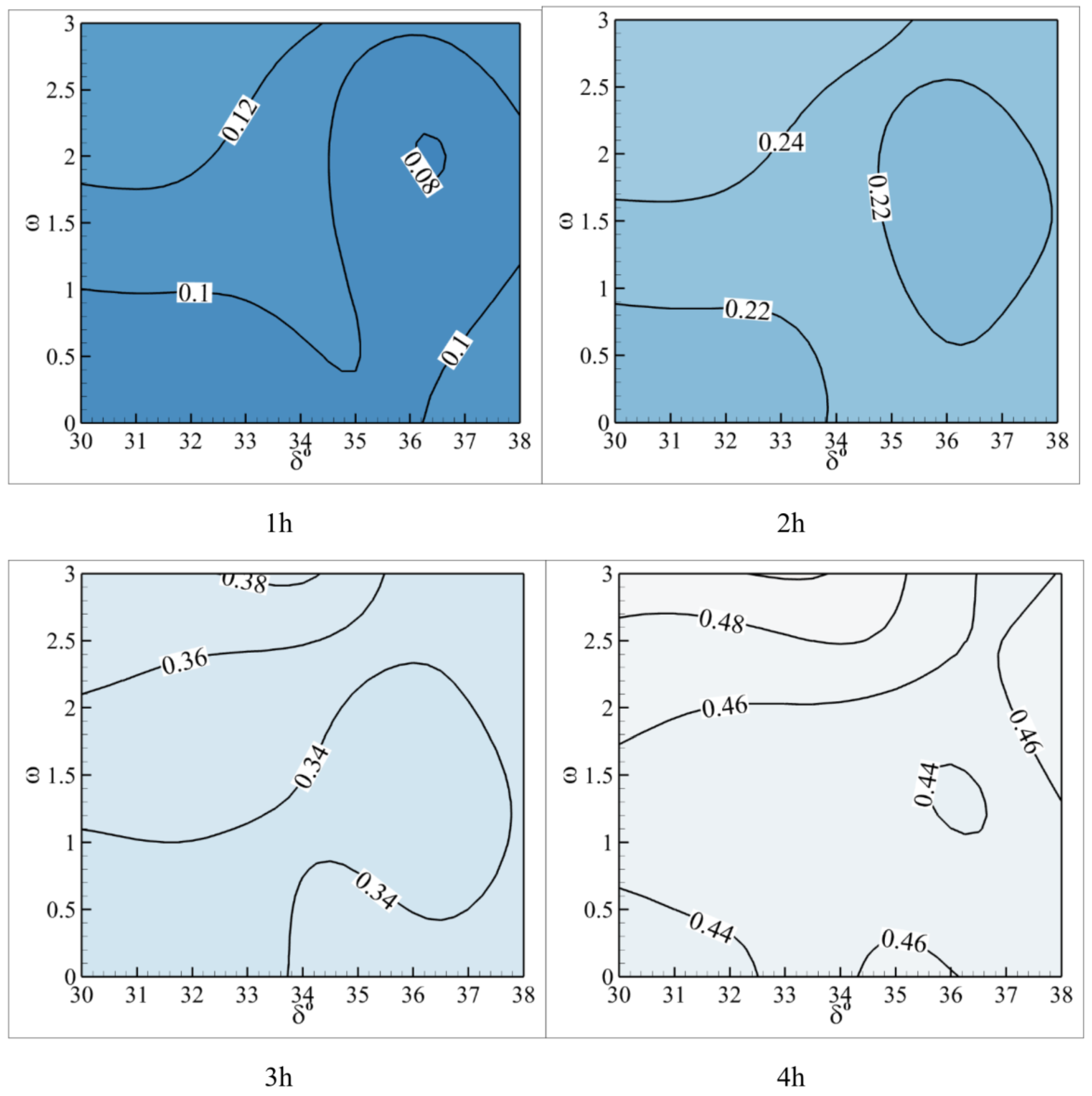
4.3. Neural Network Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shahsavar, A.; Moradi, K.; Yıldız, Ç.; Farhadi, P.; Arıcı, M. Effect of nanoparticle shape on cooling performance of boehmite-alumina nanofluid in a helical heat sink for laminar and turbulent flow regimes. Int. J. Mech. Sci. 2021, 217, 107045. [Google Scholar] [CrossRef]
- Landini, S.; Leworthy, J.; O’Donovan, T.S. A Review of Phase Change Materials for the Thermal Management and Isothermalisation of Lithium-Ion Cells. J. Energy Storage 2019, 25, 100887. [Google Scholar] [CrossRef]
- Heyhat, M.M.; Mousavi, S.; Siavashi, M. Battery thermal management with thermal energy storage composites of PCM, metal foam, fin and nanoparticle. J. Energy Storage 2020, 28, 101235. [Google Scholar] [CrossRef]
- Ghadbeigi, L.; Day, B.; Lundgren, K.; Sparks, T.D. Cold temperature performance of phase change material based battery thermal management systems. Energy Rep. 2018, 4, 303–307. [Google Scholar] [CrossRef]
- Riaz Siddiqui, F.; Tso, C.-Y.; Qiu, H.; Chao, C.Y.H.; Chung Fu, S. Hybrid nanofluid spray cooling performance and its residue surface effects: Toward thermal management of high heat flux devices. Appl. Therm. Eng. 2022, 211, 118454. [Google Scholar] [CrossRef]
- Wei, Y.; Agelin-Chaab, M. Development and experimental analysis of a hybrid cooling concept for electric vehicle battery packs. J. Energy Storage 2019, 25, 100906. [Google Scholar] [CrossRef]
- Feng, L.; Zhou, S.; Li, Y.; Wang, Y.; Zhao, Q.; Luo, C.; Wang, G.; Yan, K. Experimental investigation of thermal and strain management for lithium-ion battery pack in heat pipe cooling. J. Energy Storage 2018, 16, 84–92. [Google Scholar] [CrossRef]
- Jilte, R.D.; Kumar, R.; Ahmadi, M.H.; Chen, L. Battery thermal management system employing phase change material with cell-to-cell air cooling. Appl. Therm. Eng. 2019, 161. [Google Scholar] [CrossRef]
- Mousavi, S.; Siavashi, M.; Heyhat, M.M. Numerical melting performance analysis of a cylindrical thermal energy storage unit using nano-enhanced PCM and multiple horizontal fins. Numer. Heat Transf. Part A Appl. 2019, 75, 560–577. [Google Scholar] [CrossRef]
- El-Khouly, M.M.; El Bouz, M.A.; Sultan, G.I. Experimental and computational study of using nanofluid for thermal management of electronic chips. J. Energy Storage 2021, 39, 102630. [Google Scholar] [CrossRef]
- Selimefendigil, F.; Oztop, H.F.; Chamkha, A.J. Natural convection in a CuO–water nanofluid filled cavity under the effect of an inclined magnetic field and phase change material (PCM) attached to its vertical wall. J. Therm. Anal. Calorim. 2018, 135, 1577–1594. [Google Scholar] [CrossRef]
- Selimefendigil, F.; Öztop, H.F. Effects of an inner stationary cylinder having an elastic rod-like extension on the mixed convection of CNT-water nanofluid in a three dimensional vented cavity. Int. J. Heat Mass Transf. 2019, 137, 650–668. [Google Scholar] [CrossRef]
- Selimefendigil, F.; Öztop, H.F. Corrugated conductive partition effects on MHD free convection of CNT-water nanofluid in a cavity. Int. J. Heat Mass Transf. 2019, 129, 265–277. [Google Scholar] [CrossRef]
- Yang, X.; Wei, P.; Cui, X.; Jin, L.; He, Y.-L. Thermal response of annuli filled with metal foam for thermal energy storage: An experimental study. Appl. Energy 2019, 250, 1457–1467. [Google Scholar] [CrossRef]
- Yang, X.; Bai, Q.; Guo, Z.; Niu, Z.; Yang, C.; Jin, L.; Lu, T.J.; Yan, J. Comparison of direct numerical simulation with volume-averaged method on composite phase change materials for thermal energy storage. Appl. Energy 2018, 229, 700–714. [Google Scholar] [CrossRef]
- Yang, X.; Yu, J.; Guo, Z.; Jin, L.; He, Y.-L. Role of porous metal foam on the heat transfer enhancement for a thermal energy storage tube. Appl. Energy 2019, 239, 142–156. [Google Scholar] [CrossRef]
- Bayrak, F.; Oztop, H.F.; Selimefendigil, F. Effects of different fin parameters on temperature and efficiency for cooling of photovoltaic panels under natural convection. Sol. Energy 2019, 188, 484–494. [Google Scholar] [CrossRef]
- Selimefendigil, F.; Öztop, H.F. Forced convection in a branching channel with partly elastic walls and inner L-shaped conductive obstacle under the influence of magnetic field. Int. J. Heat Mass Transf. 2019, 144, 118598. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, H.; Xia, X. Experimental investigation on the thermal behavior of cylindrical battery with composite paraffin and fin structure. Int. J. Heat Mass Transf. 2017, 109, 958–970. [Google Scholar] [CrossRef]
- Sushobhan, B.R.; Kar, S.P. Thermal Modeling of Melting of Nano based Phase Change Material for Improvement of Thermal Energy Storage. Energy Procedia 2017, 109, 385–392. [Google Scholar] [CrossRef]
- Khodadadi, M.; Sheikholeslami, M. Heat transfer efficiency and electrical performance evaluation of photovoltaic unit under influence of NEPCM. Int. J. Heat Mass Transf. 2022, 183, 122232. [Google Scholar] [CrossRef]
- Leong, K.Y.; Abdul Rahman, M.R.; Gurunathan, B.A. Nano-enhanced phase change materials: A review of thermo-physical properties, applications and challenges. J. Energy Storage 2019, 21, 18–31. [Google Scholar] [CrossRef]
- Li, Z.-R.; Hu, N.; Liu, J.; Zhang, R.-H.; Fan, L.-W. Revisiting melting heat transfer of nano-enhanced phase change materials (NePCM) in differentially-heated rectangular cavities using thermochromic liquid crystal (TLC) thermography. Int. J. Heat Mass Transf. 2020, 159, 120119. [Google Scholar] [CrossRef]
- Li, Z.-R.; Fu, G.-T.; Fan, L.-W. Synergistic effects of nano-enhanced phase change material (NePCM) and fin shape on heat storage performance of a finned shell-and-tube unit: An experimental study. J. Energy Storage 2022, 45, 103772. [Google Scholar] [CrossRef]
- Li, D.; Wu, Y.; Liu, C.; Zhang, G.; Arıcı, M. Numerical investigation of thermal and optical performance of window units filled with nanoparticle enhanced PCM. Int. J. Heat Mass Transf. 2018, 125, 1321–1332. [Google Scholar] [CrossRef]
- Ho, C.J.; Guo, Y.-W.; Yang, T.-F.; Rashidi, S.; Yan, W.-M. Numerical study on forced convection of water-based suspensions of nanoencapsulated PCM particles/Al2O3 nanoparticles in a mini-channel heat sink. Int. J. Heat Mass Transf. 2020, 157, 119965. [Google Scholar] [CrossRef]
- Li, Z.; Shahsavar, A.; Al-Rashed, A.A.A.A.; Talebizadehsardari, P. Effect of porous medium and nanoparticles presences in a counter-current triple-tube composite porous/nano-PCM system. Appl. Therm. Eng. 2020, 167, 114777. [Google Scholar] [CrossRef]
- Ji, C.; Qin, Z.; Dubey, S.; Choo, F.H.; Duan, F. Simulation on PCM melting enhancement with double-fin length arrangements in a rectangular enclosure induced by natural convection. Int. J. Heat Mass Transf. 2018, 127, 255–265. [Google Scholar] [CrossRef]
- Jmal, I.; Baccar, M. Numerical investigation of PCM solidification in a finned rectangular heat exchanger including natural convection. Int. J. Heat Mass Transf. 2018, 127, 714–727. [Google Scholar] [CrossRef]
- Abdi, A.; Martin, V.; Chiu, J.N.W. Numerical investigation of melting in a cavity with vertically oriented fins. Appl. Energy 2019, 235, 1027–1040. [Google Scholar] [CrossRef]
- Yazici, M.Y.; Avci, M.; Aydin, O. Combined effects of inclination angle and fin number on thermal performance of a PCM-based heat sink. Appl. Therm. Eng. 2019, 159, 113956. [Google Scholar] [CrossRef]
- Nakhchi, M.E.; Esfahani, J.A. Improving the melting performance of PCM thermal energy storage with novel stepped fins. J. Energy Storage 2020, 30, 101424. [Google Scholar] [CrossRef]
- Masoumi, H.; Haghighi Khoshkhoo, R. Investigation of melting of nanoparticle-enhanced phase change materials (NePCMs) in a shell-and-tube heat exchanger with longitudinal fins. Heat Mass Transf. 2020, 57, 681–701. [Google Scholar] [CrossRef]
- Bahiraei, M.; Mazaheri, N.; Daneshyar, M.R. Employing elliptical pin-fins and nanofluid within a heat sink for cooling of electronic chips regarding energy efficiency perspective. Appl. Therm. Eng. 2021, 183, 116159. [Google Scholar] [CrossRef]
- Amani, Y.; Takahashi, A.; Chantrenne, P.; Maruyama, S.; Dancette, S.; Maire, E. Thermal conductivity of highly porous metal foams: Experimental and image based finite element analysis. Int. J. Heat Mass Transf. 2018, 122, 1–10. [Google Scholar] [CrossRef]
- Masoumi, H.; Haghighi Khoshkhoo, R.; Mirfendereski, S.M. Experimental and numerical investigation of melting/solidification of nano-enhanced phase change materials in shell & tube thermal energy storage systems. J. Energy Storage 2022, 47, 103561. [Google Scholar] [CrossRef]
- Guo, J.; Liu, Z.; Du, Z.; Yu, J.; Yang, X.; Yan, J. Effect of fin-metal foam structure on thermal energy storage: An experimental study. Renew. Energy 2021, 172, 57–70. [Google Scholar] [CrossRef]
- Zhang, C.; Yu, M.; Fan, Y.; Zhang, X.; Zhao, Y.; Qiu, L. Numerical study on heat transfer enhancement of PCM using three combined methods based on heat pipe. Energy 2020, 195, 116809. [Google Scholar] [CrossRef]
- Joshi, V.; Rathod, M.K. Experimental and numerical assessments of thermal transport in fins and metal foam infused latent heat thermal energy storage systems: A comparative evaluation. Appl. Therm. Eng. 2020, 178, 115518. [Google Scholar] [CrossRef]
- Senobar, H.; Aramesh, M.; Shabani, B. Nanoparticles and metal foams for heat transfer enhancement of phase change materials: A comparative experimental study. J. Energy Storage 2020, 32, 101911. [Google Scholar] [CrossRef]
- Sheikholeslami, M.; Keshteli, A.N.; Shafee, A. Melting and solidification within an energy storage unit with triangular fin and CuO nano particles. J. Energy Storage 2020, 32, 101716. [Google Scholar] [CrossRef]
- Kok, B. Examining effects of special heat transfer fins designed for the melting process of PCM and Nano-PCM. Appl. Therm. Eng. 2020, 170, 114989. [Google Scholar] [CrossRef]
- Pássaro, J.; Rebola, A.; Coelho, L.; Conde, J.; Evangelakis, G.A.; Prouskas, C.; Papageorgiou, D.G.; Zisopoulou, A.; Lagaris, I.E. Effect of fins and nanoparticles in the discharge performance of PCM thermal storage system with a multi pass finned tube heat exchange. Appl. Therm. Eng. 2022, 212, 118569. [Google Scholar] [CrossRef]
- Ren, Q.; Meng, F.; Guo, P. A comparative study of PCM melting process in a heat pipe-assisted LHTES unit enhanced with nanoparticles and metal foams by immersed boundary-lattice Boltzmann method at pore-scale. Int. J. Heat Mass Transf. 2018, 121, 1214–1228. [Google Scholar] [CrossRef]
- Singh, R.P.; Xu, H.; Kaushik, S.C.; Rakshit, D.; Romagnoli, A. Charging performance evaluation of finned conical thermal storage system encapsulated with nano-enhanced phase change material. Appl. Therm. Eng. 2019, 151, 176–190. [Google Scholar] [CrossRef]
- Mahdi, J.M.; Mohammed, H.I.; Hashim, E.T.; Talebizadehsardari, P.; Nsofor, E.C. Solidification enhancement with multiple PCMs, cascaded metal foam and nanoparticles in the shell-and-tube energy storage system. Appl. Energy 2020, 257, 113993. [Google Scholar] [CrossRef]
- Li, W.Q.; Guo, S.J.; Tan, L.; Liu, L.L.; Ao, W. Heat transfer enhancement of nano-encapsulated phase change material (NEPCM) using metal foam for thermal energy storage. Int. J. Heat Mass Transf. 2021, 166, 120737. [Google Scholar] [CrossRef]
- Nield, D.A.; Bejan, A. Convection in Porous Media; Springer: Berlin/Heidelberg, Germany, 2006; Volume 3. [Google Scholar]
- Bondareva, N.S.; Buonomo, B.; Manca, O.; Sheremet, M.A. Heat transfer performance of the finned nano-enhanced phase change material system under the inclination influence. Int. J. Heat Mass Transf. 2019, 135, 1063–1072. [Google Scholar] [CrossRef]
- Ghalambaz, M.; Mehryan, S.A.M.; Hajjar, A.; Younis, O.; Sheremet, M.A.; Pour, M.S.; Hulme-Smith, C. Phase-Transition Thermal Charging of a Channel-Shape Thermal Energy Storage Unit: Taguchi Optimization Approach and Copper Foam Inserts. Molecules 2021, 26, 1235. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.-Q.; Liu, M.-J.; Hu, N.; Huang, Y.-K.; Fan, L.-W.; Yu, Z.-T.; Ge, J. Inward solidification heat transfer of nano-enhanced phase change materials in a spherical capsule: An experimental study. J. Heat Transf. 2018, 140, 022301. [Google Scholar] [CrossRef]
- Bhattacharya, A.; Calmidi, V.V.; Mahajan, R.L. Thermophysical properties of high porosity metal foams. Int. J. Heat Mass Transf. 2002, 45, 1017–1031. [Google Scholar] [CrossRef]
- Xiao, X.; Zhang, P.; Li, M. Preparation and thermal characterization of paraffin/metal foam composite phase change material. Appl. Energy 2013, 112, 1357–1366. [Google Scholar] [CrossRef]
- Zienkiewicz, O.C.; Taylor, R.L.; Nithiarasu, P. The Finite Element Method for Fluid Dynamics, 7th ed.; Butterworth-Heinemann: Oxford, UK, 2014. [Google Scholar]
- Reddy, J.N.; Gartling, D.K. The Finite Element Method in Heat Transfer and Fluid Dynamics; CRC Press: Boca Raton, FL, USA, 2010. [Google Scholar]
- Zienkiewicz, O.C.; Taylor, R.L.; Nithiarasu, P. The Finite Element Method for Fluid Dynamics; Elsevier: Amsterdam, The Netherlands, 2015; Volume 6. [Google Scholar]
- Schenk, O.; Gärtner, K. Solving unsymmetric sparse systems of linear equations with PARDISO. Future Gener. Comput. Syst. 2004, 20, 475–487. [Google Scholar] [CrossRef]
- Wriggers, P. Nonlinear Finite Element Methods; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2008. [Google Scholar]
- Verbosio, F.; De Coninck, A.; Kourounis, D.; Schenk, O. Enhancing the scalability of selected inversion factorization algorithms in genomic prediction. J. Comput. Sci. 2017, 22, 99–108. [Google Scholar] [CrossRef]
- Söderlind, G.; Wang, L. Adaptive time-stepping and computational stability. J. Comput. Appl. Math. 2006, 185, 225–243. [Google Scholar] [CrossRef] [Green Version]
- Gau, C.; Viskanta, R. Melting and solidification of a pure metal on a vertical wall. Heat Transf. 1986, 108, 174–181. [Google Scholar] [CrossRef]
- Brent, A.; Voller, V.R.; Reid, K. Enthalpy-porosity technique for modeling convection-diffusion phase change: Application to the melting of a pure metal. Numer. Heat Transf. Part A Appl. 1988, 13, 297–318. [Google Scholar]
- Zheng, H.; Wang, C.; Liu, Q.; Tian, Z.; Fan, X. Thermal performance of copper foam/paraffin composite phase change material. Energy Convers. Manag. 2018, 157, 372–381. [Google Scholar] [CrossRef]
- Heckerman, D.E.; Shortliffe, E.H. From certainty factors to belief networks. Artif. Intell. Med. 1992, 4, 35–52. [Google Scholar] [CrossRef]
- Jimison, H.B.; Fagan, L.M.; Shachter, R.D.; Shortliffe, E.H. Patient-specific explanation in models of chronic disease. Artif. Intell. Med. 1992, 4, 191–205. [Google Scholar] [CrossRef]
- Kolokythas, K.; Argiriou, A. An application of a feedforward neural network model for wind speed predictions. Int. J. Sustain. Energy 2022, 41, 323–340. [Google Scholar] [CrossRef]
- Hussain, I.; Bibi, F.; Bhat, S.A.; Sajjad, U.; Sultan, M.; Ali, H.M.; Azam, W.; Kaushal, S.K.; Hussain, S.; Yan, W.-M. Evaluating the parameters affecting the direct and indirect evaporative cooling systems. Eng. Anal. Bound. Elem. 2022, 145, 211–223. [Google Scholar] [CrossRef]
- Sajjad, U.; Hussain, I.; Hamid, K.; Ali, H.M.; Wang, C.-C.; Yan, W.-M. Liquid-to-vapor phase change heat transfer evaluation and parameter sensitivity analysis of nanoporous surface coatings. Int. J. Heat Mass Transf. 2022, 194, 123088. [Google Scholar] [CrossRef]
- Bhat, S.A.; Huang, N.-F.; Hussain, I.; Bibi, F.; Sajjad, U.; Sultan, M.; Alsubaie, A.S.; Mahmoud, K.H. On the Classification of a Greenhouse Environment for a Rose Crop Based on AI-Based Surrogate Models. Sustainability 2021, 13, 12166. [Google Scholar] [CrossRef]
- Rajput, G.; Raut, G.; Chandra, M.; Vishvakarma, S.K. VLSI implementation of transcendental function hyperbolic tangent for deep neural network accelerators. Microprocess. Microsyst. 2021, 84, 104270. [Google Scholar] [CrossRef]
- Edalatifar, M.; Tavakoli, M.B.; Ghalambaz, M.; Setoudeh, F. Using deep learning to learn physics of conduction heat transfer. J. Therm. Anal. Calorim. 2021, 146, 1435–1452. [Google Scholar] [CrossRef]






| Thermophysical Features | 3 wt% | 1 wt% | 0.5 wt% | 0 wt% | Cu Foam |
|---|---|---|---|---|---|
| Thermal expansion coefficient, β (1/K) | 0.000987 | 0.001008 | 0.001018 | 0.001018 | |
| Thermal conductivity (solid) W/(m K) | 0.540 | 0.451 | 0.350 | 0.252 | 8900 |
| Thermal conductivity (liquid) W/(m K) | 0.320 | 0.260 | 0.180 | 0.159 | 380 |
| Density (solid) kg/m3 | 907.9 | 896.9 | 894.1 | 891.4 | |
| Density (liquid) kg/m3 | 837.6 | 826.9 | 824.3 | 821.6 | |
| Dynamic viscosity mPa·s | 194.01 | 59.5 | 23.45 | 13.23 | |
| Melting point (°C) | 37 | 37 | 37 | 37 | |
| Specific heat capacity (solid) J/Kg K | 1910 | 1990 | 2020 | 2040 | 386 |
| Specific heat capacity (Liquid) J/Kg K | 2190 | 2300 | 2330 | 2360 | |
| Latent heat | 183.5 | 212.2 | 219.5 | 227.8 |
| Nmesh | Elements | Edges | MVF @5 h | Tavg − Tf (°C) @5 h | Computational Time (s) |
|---|---|---|---|---|---|
| 1.125 | 2263 | 355 | 0.541 | 12.523 | 321 |
| 0.875 | 3598 | 407 | 0.546 | 12.545 | 327 |
| 0.5 | 9335 | 629 | 0.550 | 12.625 | 535 |
| 0.25 | 34,190 | 1190 | 0.549 | 12.532 | 1442 |
| 0.125 | 133,266 | 2351 | 0.541 | 12.523 | 4907 |
| IW | LW | Basis | ||
|---|---|---|---|---|
| −1.75662 | −2.87270 | −1.37869 | −0.07141 | 2.67276 |
| −0.77097 | 0.59404 | −0.38713 | −0.25546 | 0.96215 |
| −3.08972 | −0.29039 | 0.20247 | 0.07288 | 1.34066 |
| 0.06595 | −0.04451 | −1.03930 | −0.82122 | 0.02993 |
| −2.84475 | 1.69511 | 0.64577 | 0.02496 | −0.50272 |
| 0.81239 | 2.27725 | −1.81946 | 0.01689 | 0.32215 |
| 0.04182 | −0.04045 | −2.86907 | −0.16864 | −1.93782 |
| −1.38479 | −2.23744 | −0.91333 | −0.03850 | −1.95756 |
| 0.29314 | −0.29432 | −1.85788 | −0.22229 | 2.00081 |
| 1.53578 | −0.67536 | 2.60817 | 0.00454 | 2.74161 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shafi, J.; Sheremet, M.; Fteiti, M.; Saeed, A.M.; Ghalambaz, M. Computational Study of Phase Change Heat Transfer and Latent Heat Energy Storage for Thermal Management of Electronic Components Using Neural Networks. Mathematics 2023, 11, 356. https://doi.org/10.3390/math11020356
Shafi J, Sheremet M, Fteiti M, Saeed AM, Ghalambaz M. Computational Study of Phase Change Heat Transfer and Latent Heat Energy Storage for Thermal Management of Electronic Components Using Neural Networks. Mathematics. 2023; 11(2):356. https://doi.org/10.3390/math11020356
Chicago/Turabian StyleShafi, Jana, Mikhail Sheremet, Mehdi Fteiti, Abdulkafi Mohammed Saeed, and Mohammad Ghalambaz. 2023. "Computational Study of Phase Change Heat Transfer and Latent Heat Energy Storage for Thermal Management of Electronic Components Using Neural Networks" Mathematics 11, no. 2: 356. https://doi.org/10.3390/math11020356






