Numerical Modeling of the Spread of Cough Saliva Droplets in a Calm Confined Space
Abstract
:1. Introduction
2. Modeling
2.1. The Initial Size Distribution of the Droplets
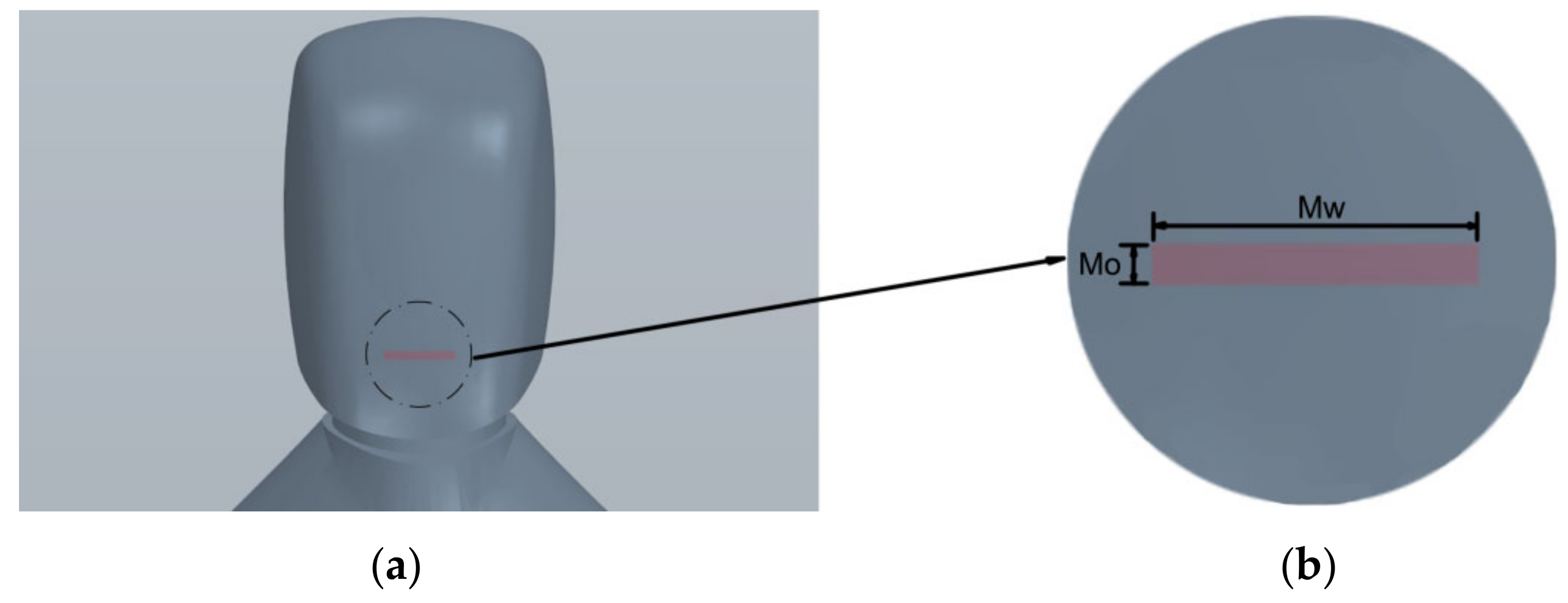
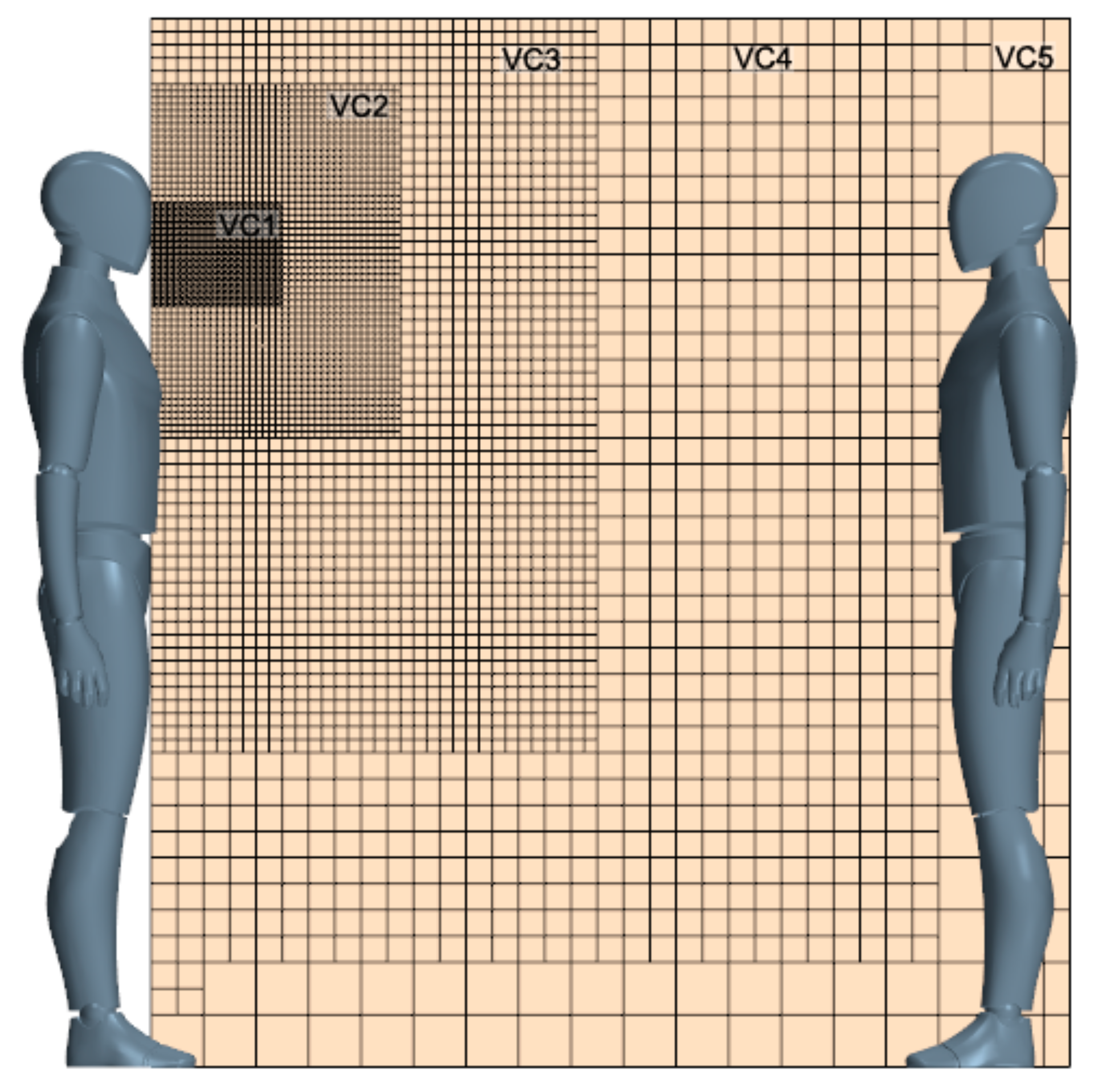
2.2. Initial and Boundary Conditions
2.3. Numerical Set-Up
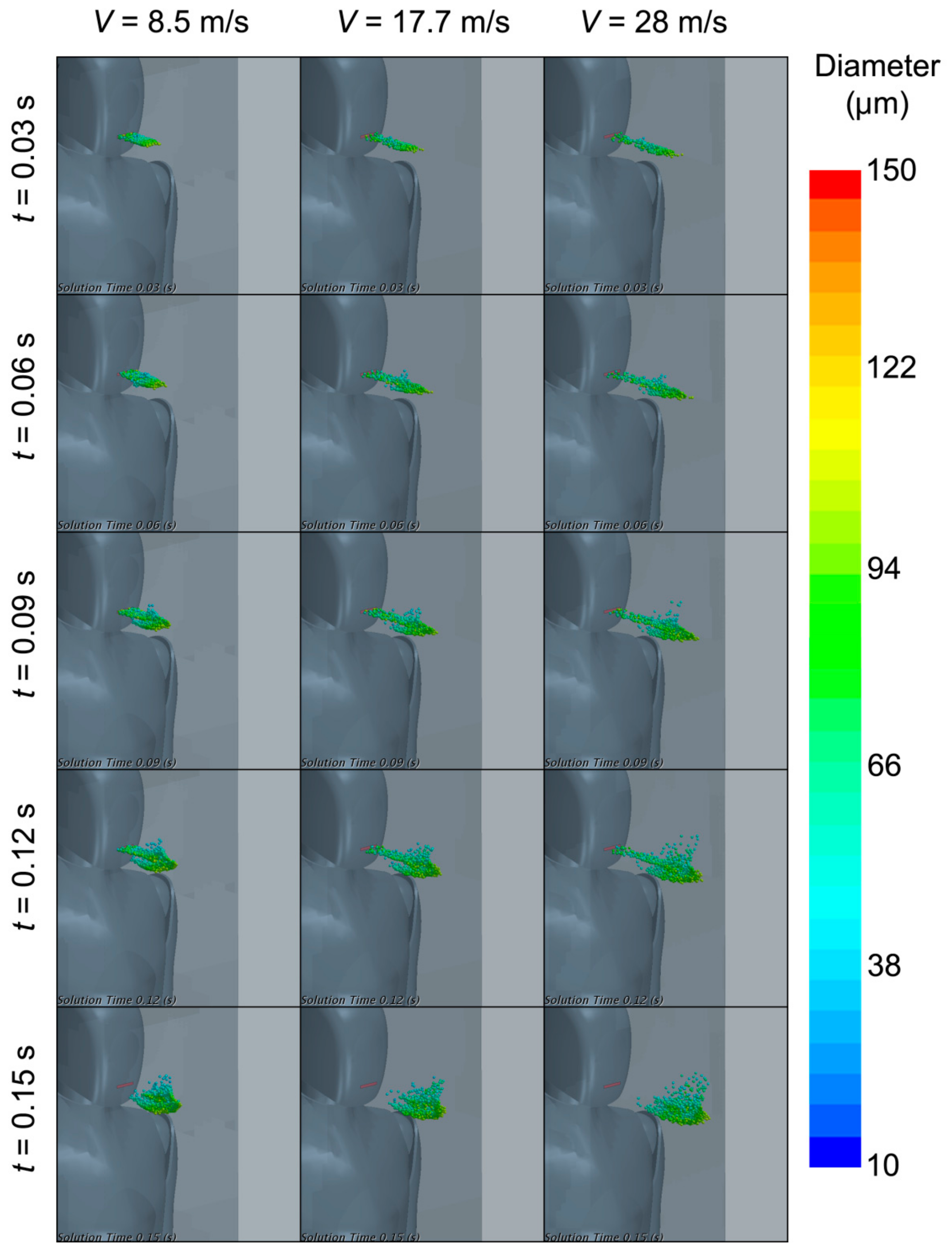
3. Results
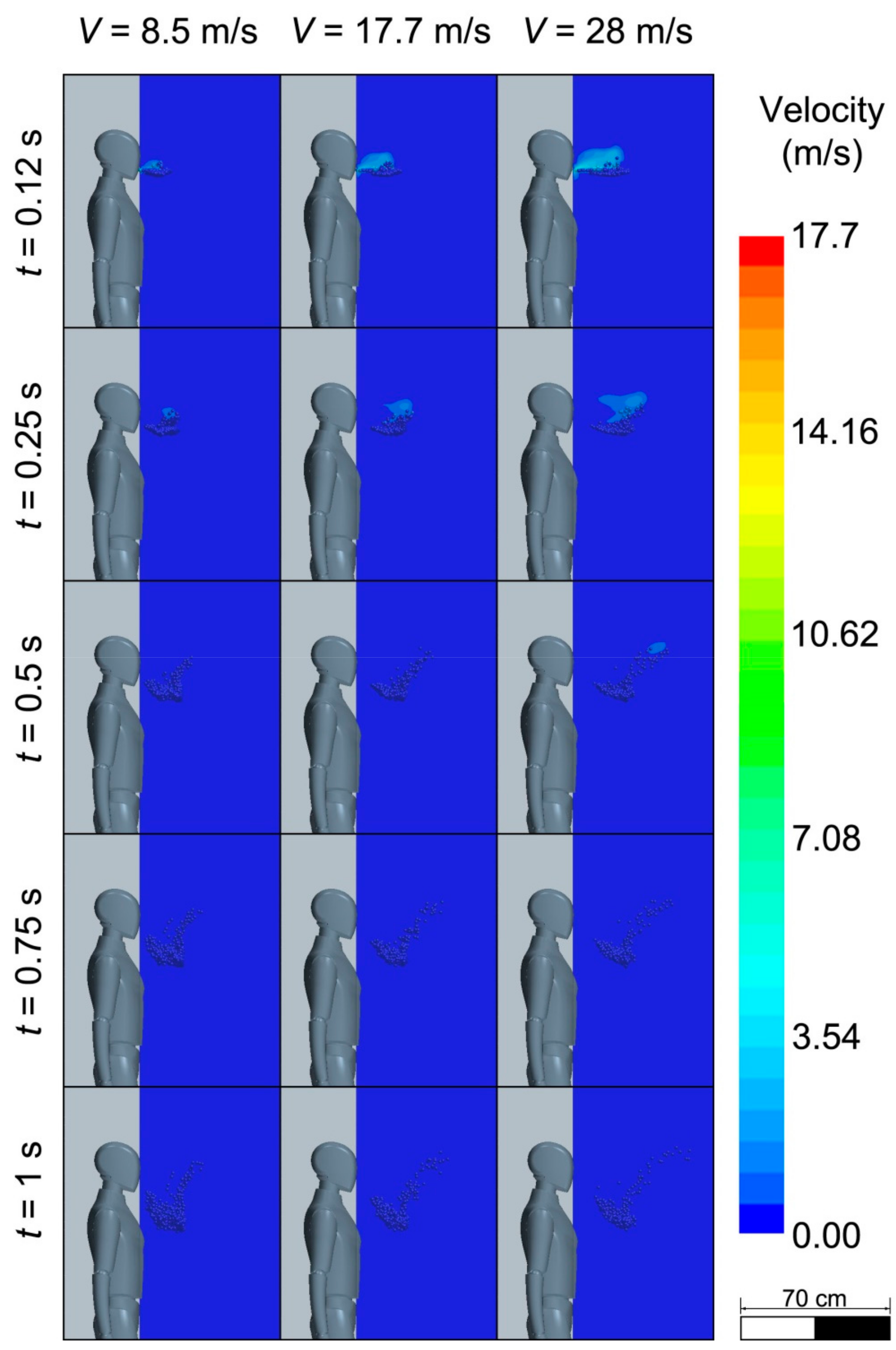
3.1. Initial Size Droplet Dispersion
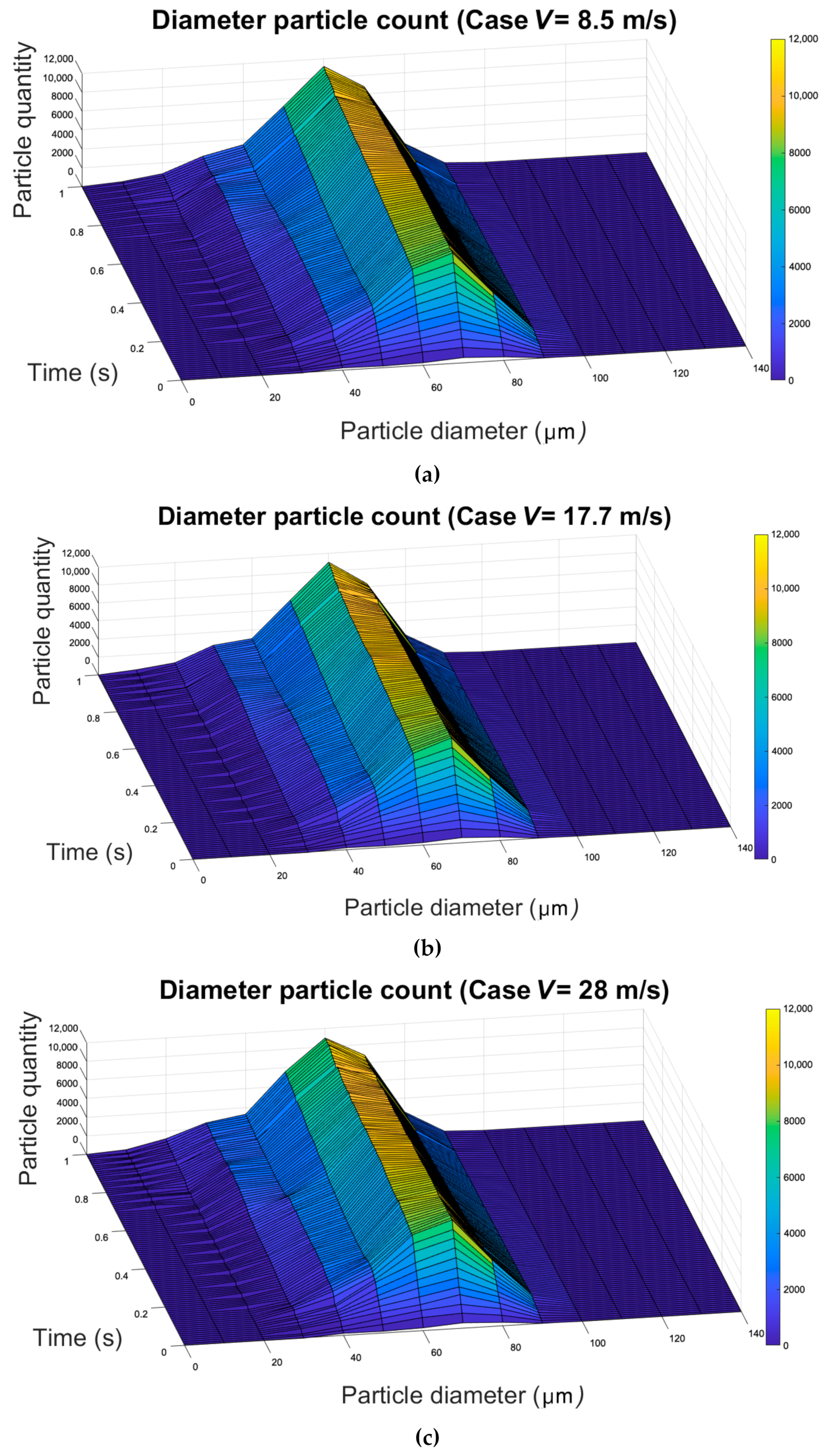
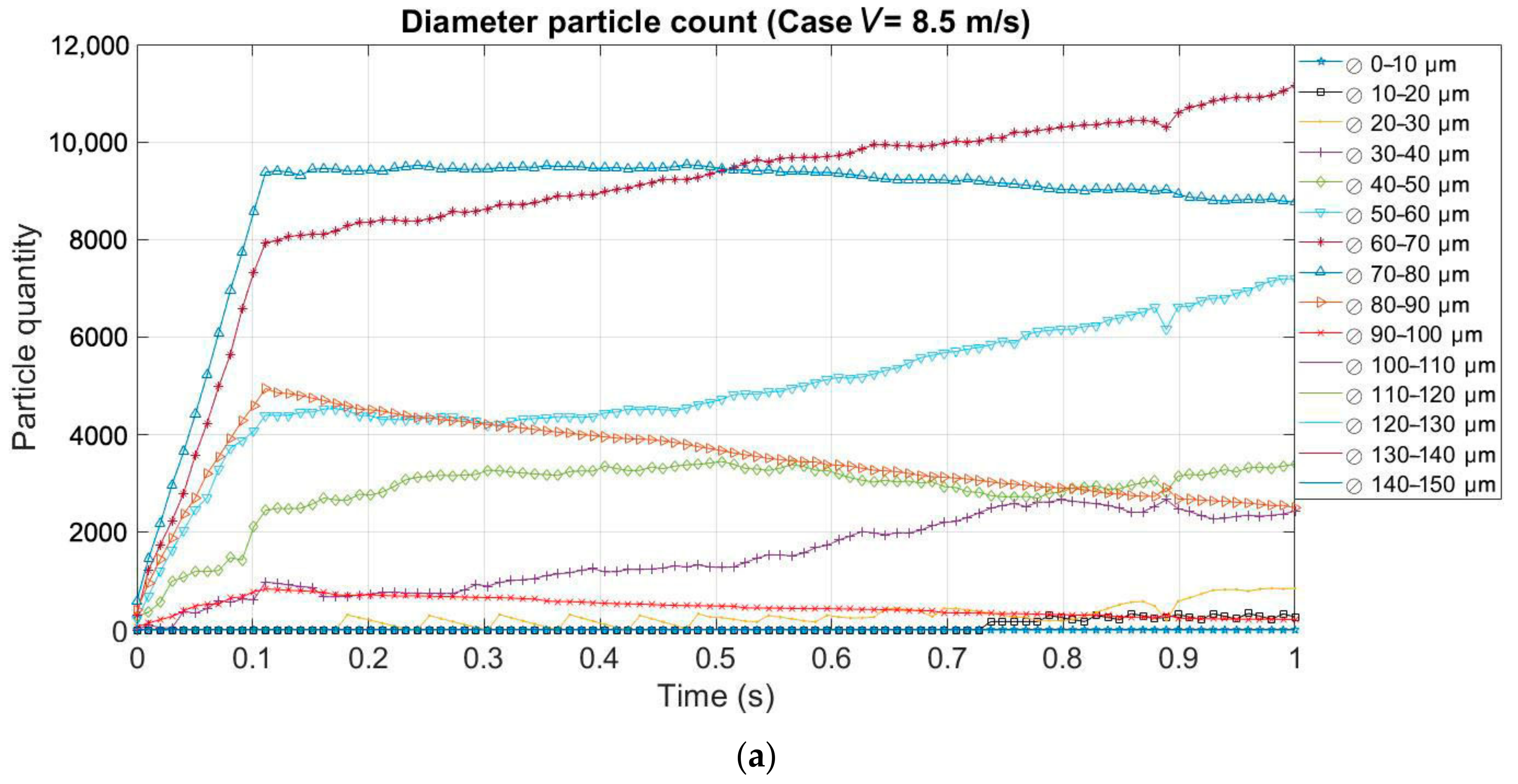
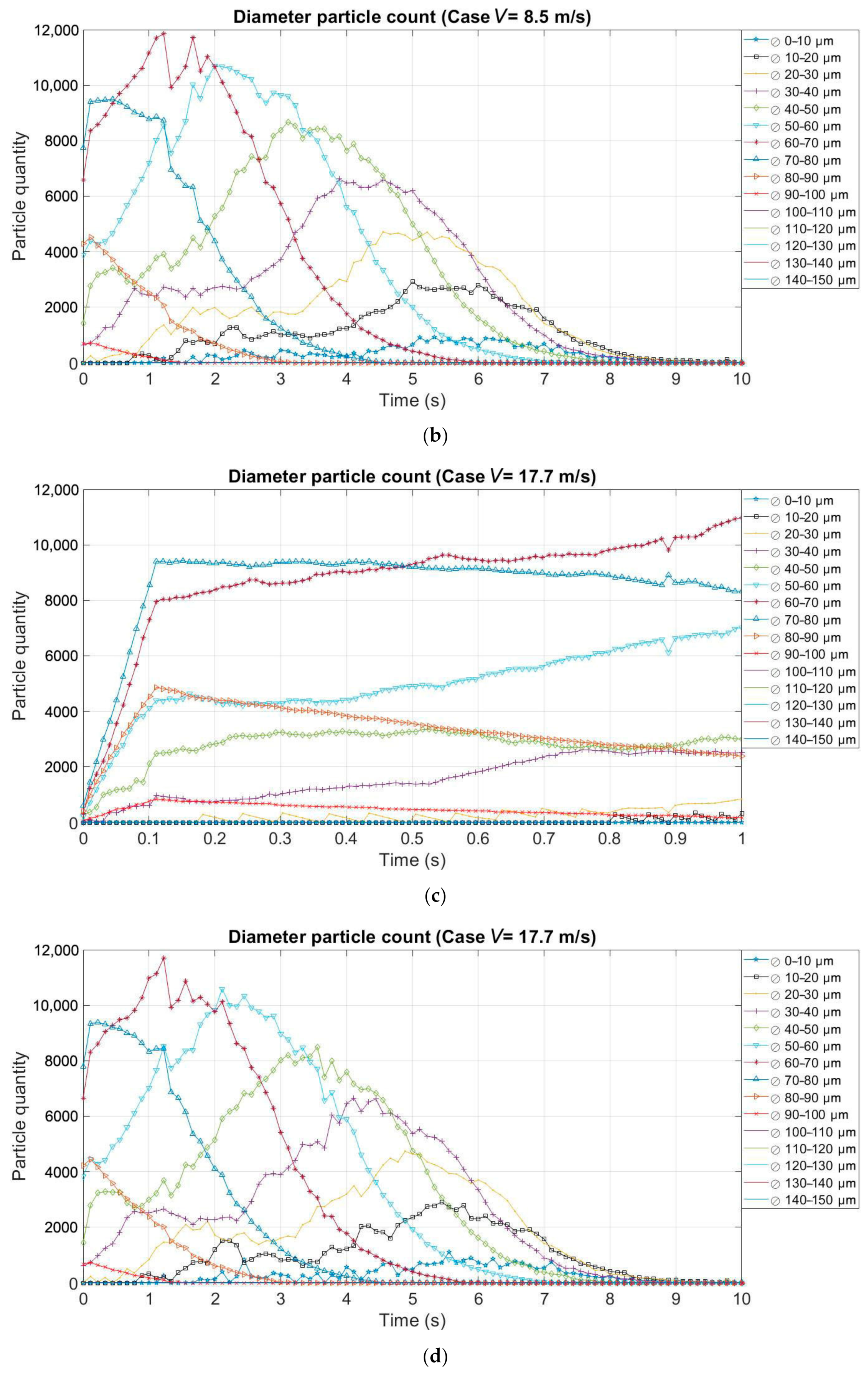
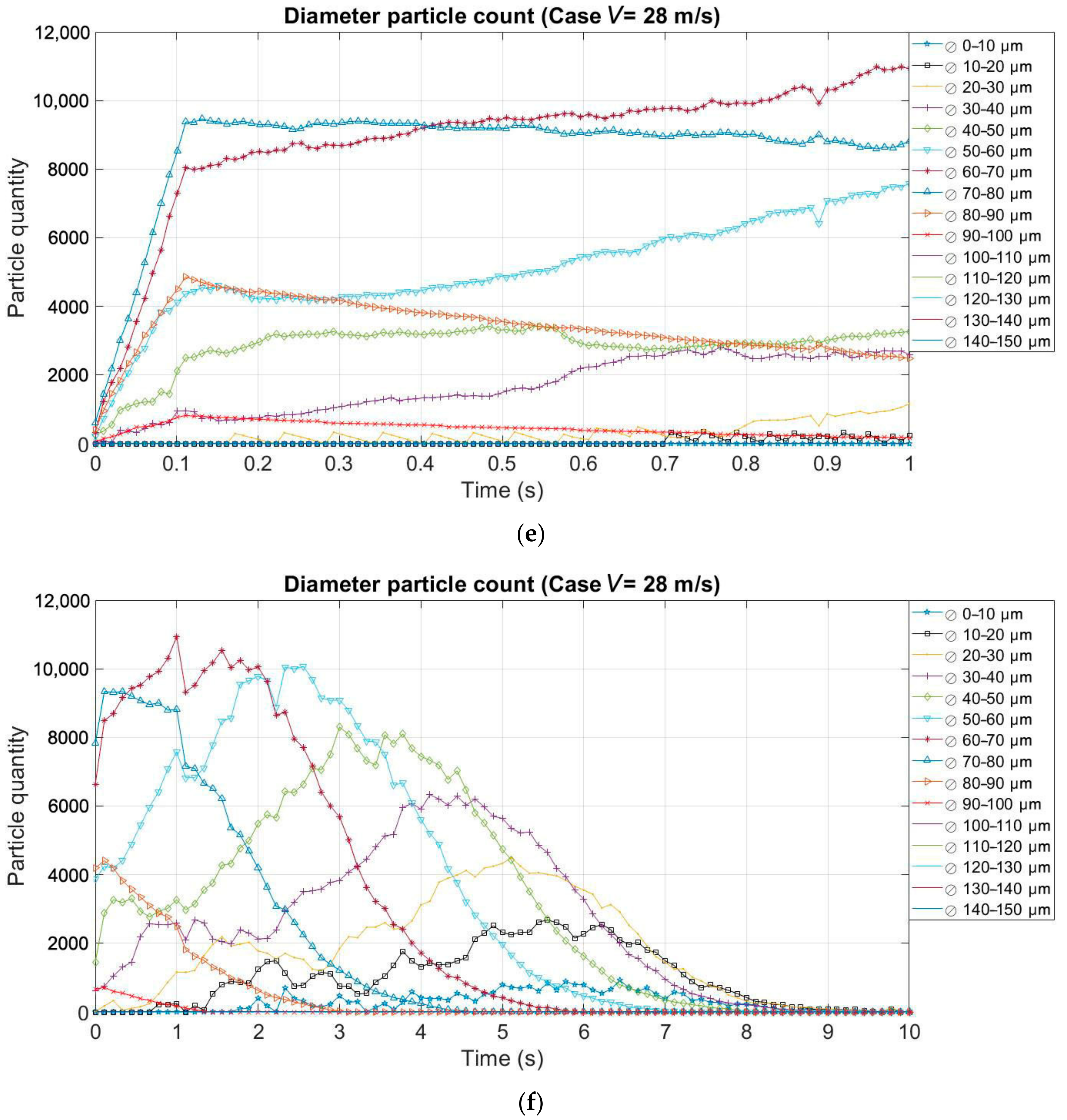
3.2. Effect of the Evaporation on the Size and Quantity of Droplets
3.3. Spreading of Saliva Droplets
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
References
- Hadi, A.G.; Kadhom, M.; Hairunisa, N.; Yousif, E. A Review on COVID-19: Origin, Spread, Symptoms, Treatment, and Prevention. Biointerface Res. Appl. Chem. 2020, 10, 7234–7242. [Google Scholar]
- Moriyama, M.; Hugentobler, W.J.; Iwasaki, A. Seasonality of Respiratory Viral Infections. Annu. Rev. Virol. 2020, 7, 83–101. [Google Scholar] [CrossRef]
- Vuorinen, V.; Aarnio, M.; Alava, M.; Alopaeus, V.; Atanasova, N.; Auvinen, M.; Balasubramanian, N.; Bordbar, H.; Erästö, P.; Grande, R.; et al. Modelling Aerosol Transport and Virus Exposure with Numerical Simulations in Relation to SARS-CoV-2 Transmission by Inhalation Indoors. Saf. Sci. 2020, 130, 104866. [Google Scholar] [CrossRef]
- Morawska, L.; Tang, J.W.; Bahnfleth, W.; Bluyssen, P.M.; Boerstra, A.; Buonanno, G.; Cao, J.; Dancer, S.; Floto, A.; Franchimon, F.; et al. How Can Airborne Transmission of COVID-19 Indoors Be Minimised? Environ. Int. 2020, 142, 105832. [Google Scholar] [CrossRef]
- Van Doremalen, N.; Bushmaker, T.; Morris, D.H.; Holbrook, M.G.; Gamble, A.; Williamson, B.N.; Tamin, A.; Harcourt, J.L.; Thornburg, N.J.; Gerber, S.I.; et al. Aerosol and Surface Stability of SARS-CoV-2 as Compared with SARS-CoV-1. N. Engl. J. Med. 2020, 382, 1564–1567. [Google Scholar] [CrossRef]
- Feng, Y.; Marchal, T.; Sperry, T.; Yi, H. Influence of Wind and Relative Humidity on the Social Distancing Effectiveness to Prevent COVID-19 Airborne Transmission: A Numerical Study. J. Aerosol Sci. 2020, 147, 105585. [Google Scholar] [CrossRef]
- Dbouk, T.; Drikakis, D. On Coughing and Airborne Droplet Transmission to Humans. Phys. Fluids 2020, 32, 53310. [Google Scholar] [CrossRef]
- Dbouk, T.; Drikakis, D. On Respiratory Droplets and Face Masks. Phys. Fluids 2020, 32, 63303. [Google Scholar] [CrossRef]
- Busco, G.; Yang, S.R.; Seo, J.; Hassan, Y.A. Sneezing and Asymptomatic Virus Transmission. Phys. Fluids 2020, 32, 73309. [Google Scholar] [CrossRef]
- Asadi, S.; Wexler, A.S.; Cappa, C.D.; Barreda, S.; Bouvier, N.M.; Ristenpart, W.D. Aerosol Emission and Superemission during Human Speech Increase with Voice Loudness. Sci. Rep. 2019, 9, 2348. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, X.; Ou, C.; Yang, H.; Liu, L.; Song, T.; Kang, M.; Lin, H.; Hang, J. Transmission of Pathogen-Laden Expiratory Droplets in a Coach Bus. J. Hazard. Mater. 2020, 397, 122609. [Google Scholar] [CrossRef]
- Aliabadi, A.A.; Rogak, S.N.; Green, S.I.; Bartlett, K.H. CFD Simulation of Human Coughs and Sneezes: A Study in Droplet Dispersion, Heat, and Mass Transfer. In Proceedings of the Fluid Flow, Heat Transfer and Thermal Systems, Parts A and B, ASMEDC, Vancouver, BC, Canada, 1 January 2010; pp. 1051–1060. [Google Scholar]
- Zhu, S.; Kato, S.; Yang, J.-H. Study on Transport Characteristics of Saliva Droplets Produced by Coughing in a Calm Indoor Environment. Build. Environ. 2006, 41, 1691–1702. [Google Scholar] [CrossRef]
- Wang, B.; Zhang, A.; Sun, J.L.; Liu, H.; Hu, J.; Xu, L.X. Study of SARS Transmission Via Liquid Droplets in Air. J. Biomech. Eng. 2005, 127, 32–38. [Google Scholar] [CrossRef]
- Shao, S.; Zhou, D.; He, R.; Li, J.; Zou, S.; Mallery, K.; Kumar, S.; Yang, S.; Hong, J. Risk Assessment of Airborne Transmission of COVID-19 by Asymptomatic Individuals under Different Practical Settings. J. Aerosol Sci. 2021, 151, 105661. [Google Scholar] [CrossRef] [PubMed]
- Rahiminejad, M.; Haghighi, A.; Dastan, A.; Abouali, O.; Farid, M.; Ahmadi, G. Computer Simulations of Pressure and Velocity Fields in a Human Upper Airway during Sneezing. Comput. Biol. Med. 2016, 71, 115–127. [Google Scholar] [CrossRef] [PubMed]
- Duguid, J.P. The Size and the Duration of Air-Carriage of Respiratory Droplets and Droplet-Nuclei. Epidemiol. Infect. 1946, 44, 471–479. [Google Scholar] [CrossRef] [Green Version]
- Xie, X.; Li, Y.; Sun, H.; Liu, L. Exhaled Droplets Due to Talking and Coughing. J. R. Soc. Interface 2009, 6 (Suppl. 6), S703–S714. [Google Scholar] [CrossRef] [Green Version]
- VanSciver, M.; Miller, S.; Hertzberg, J. Particle Image Velocimetry of Human Cough. Aerosol Sci. Technol. 2011, 45, 415–422. [Google Scholar] [CrossRef]
- Zhang, Y.; Feng, G.; Bi, Y.; Cai, Y.; Zhang, Z.; Cao, G. Distribution of Droplet Aerosols Generated by Mouth Coughing and Nose Breathing in an Air-Conditioned Room. Sustain. Cities Soc. 2019, 51, 101721. [Google Scholar] [CrossRef]
- Van der Reijden, W.A.; Veerman, E.C.I.; Nieuw Amerongen, A.V. Shear Rate Dependent Viscoelastic Behavior of Human Glandular Salivas. BIR 1993, 30, 141–152. [Google Scholar] [CrossRef]
- Richardson, L.F.; Gaunt, J.A. VIII. The deferred approach to the limit. Philos. Trans. R. Soc. Lond. 1927, 226, 299–361. [Google Scholar]
- Aramendia, I.; Fernandez-Gamiz, U.; Lopez-Arraiza, A.; Rey-Santano, C.; Mielgo, V.; Basterretxea, F.; Sancho, J.; Gomez-Solaetxe, M. Experimental and Numerical Modeling of Aerosol Delivery for Preterm Infants. Int. J. Environ. Res. Public Health 2018, 15, 423. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roache, P.J. Perspective: A Method for Uniform Reporting of Grid Refinement Studies. J. Fluids Eng. 1994, 116, 405–413. [Google Scholar] [CrossRef]
- Menter, F.R. Two-Equation Eddy-Viscosity Turbulence Models for Engineering Applications. AIAA J. 1994, 32, 1598–1605. [Google Scholar] [CrossRef] [Green Version]
- Gosman, A.D.; Ioannides, E. Aspects of Computer Simulation of Liquid-Fueled Combustors. J. Energy 1983, 7, 482–490. [Google Scholar] [CrossRef]


| Notation | Meaning | Value |
|---|---|---|
| H | Height | 2 m |
| L | Length | 2.1 m |
| W | Width | 1.2 m |
| Hh | Human height | 1.75 m |
| Hm | Mouth height | 1.56 m |
| Dm | Distance between mouths | 1.55 m |
| Mo | Mouth opening | 5 mm |
| Mw | Mouth width | 40 mm |
| Mesh | Number of Cells | Vaxial (m/s) |
|---|---|---|
| M1 | 526,392 | 5.62 |
| M2 | 263,196 | 5.45 |
| M3 | 131,598 | 4.98 |
| Mesh Level | Vaxial (m/s) | Error (%) |
|---|---|---|
| (Vaxial)h=0 (m/s) | 5.72 | |
| M1 | 5.62 | 1.78 |
| M2 | 5.45 | 4.82 |
| M3 | 4.98 | 13.03 |
| Grid Convergence Index | Domain |
|---|---|
| GCI12 (%) | 2.34 |
| GCI23 (%) | 6.93 |
| GCI23/rpGCI12 (-) | 1.09 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chillón, S.A.; Ugarte-Anero, A.; Aramendia, I.; Fernandez-Gamiz, U.; Zulueta, E. Numerical Modeling of the Spread of Cough Saliva Droplets in a Calm Confined Space. Mathematics 2021, 9, 574. https://doi.org/10.3390/math9050574
Chillón SA, Ugarte-Anero A, Aramendia I, Fernandez-Gamiz U, Zulueta E. Numerical Modeling of the Spread of Cough Saliva Droplets in a Calm Confined Space. Mathematics. 2021; 9(5):574. https://doi.org/10.3390/math9050574
Chicago/Turabian StyleChillón, Sergio A., Ainara Ugarte-Anero, Iñigo Aramendia, Unai Fernandez-Gamiz, and Ekaitz Zulueta. 2021. "Numerical Modeling of the Spread of Cough Saliva Droplets in a Calm Confined Space" Mathematics 9, no. 5: 574. https://doi.org/10.3390/math9050574







