Developmental Origins of Health and Disease: A Lifecourse Approach to the Prevention of Non-Communicable Diseases
Abstract
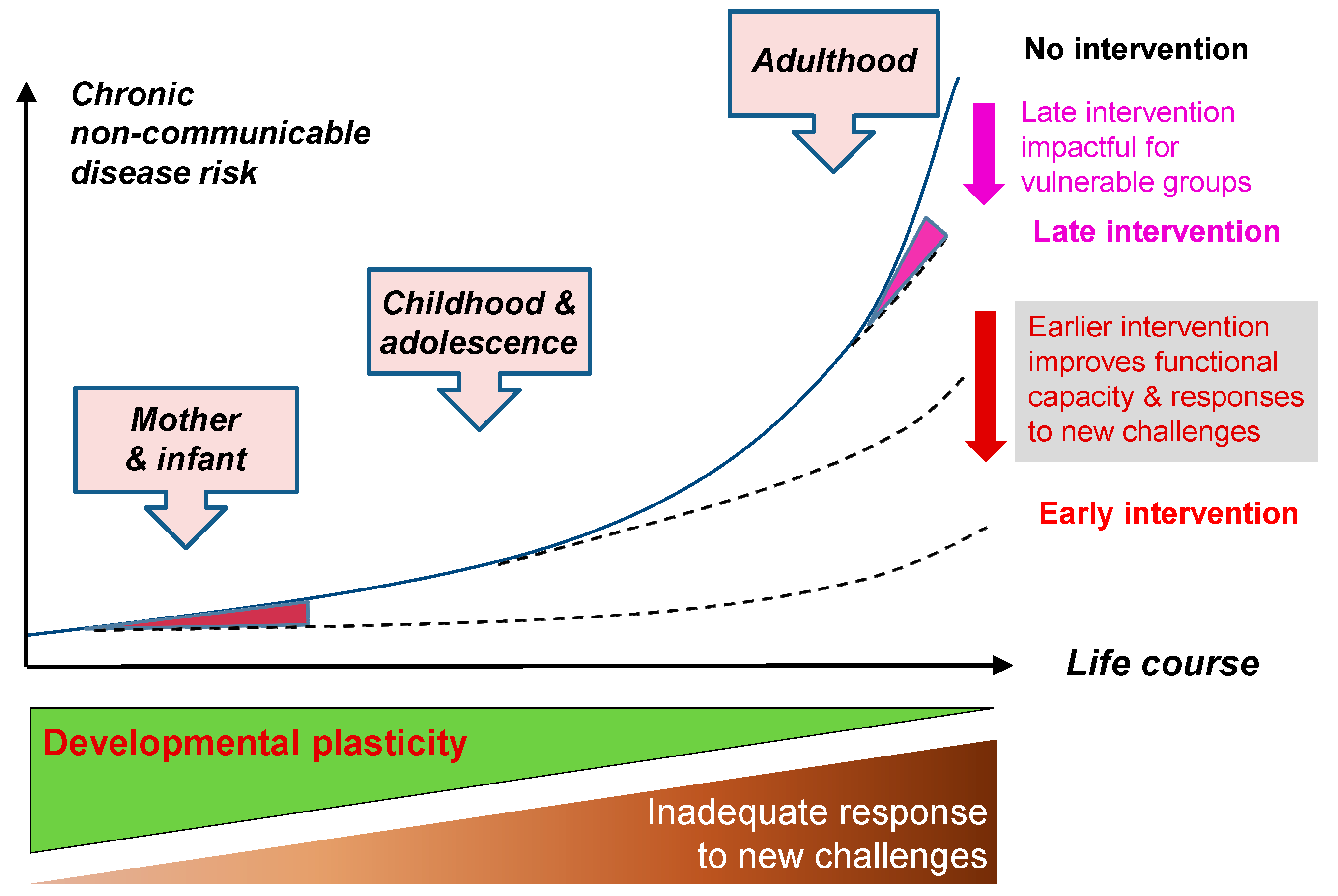
:1. Introduction
2. A Lifecourse Approach
3. Observational Evidence of a Link between Early Development and Later Disease
4. Maternal Nutrition
5. Mechanisms
5.1. Epigenetic Mechanisms
5.2. Behavioural Mechanisms
6. Interventions
6.1. Nutritional Supplementation
6.2. Health Behaviour Change Interventions
7. Conclusions
Acknowledgments
Conflicts of Interest
References
- World Health Organisation. Global Action Plan for the Prevention and Control of Noncommunicable Disease 2013–2020; WHO: Geneva, Switzerland, 2013. [Google Scholar]
- Department of Health. Healthy Lives, Healty People: Our Strategy for Public Health in England; Department of Health: London, UK, 2010.
- Hanson, M.A.; Gluckman, P.D. Early developmental conditioning of later health and disease: Physiology or pathophysiology? Physiol. Rev. 2014, 94, 1027–1076. [Google Scholar] [CrossRef] [PubMed]
- Syddall, H.E.; Sayer, A.A.; Simmonds, S.J.; Osmond, C.; Cox, V.; Dennison, E.M.; Barker, D.J.; Cooper, C. Birth weight, infant weight gain, and cause-specific mortality: The Hertfordshire Cohort Study. Am. J. Epidemiol. 2005, 161, 1074–1080. [Google Scholar] [CrossRef] [PubMed]
- Leon, D.A.; Lithell, H.O.; Vagero, D.; Koupilova, I.; Mohsen, R.; Berglund, L.; Lithell, U.B.; McKeigue, P.M. Reduced fetal growth rate and increased risk of death from ischaemic heart disease: Cohort study of 15,000 Swedish men and women born 1915–1929. BMJ (Clin. Res.) 1998, 317, 241–245. [Google Scholar] [CrossRef]
- Bertram, C.E.; Hanson, M. Animal models and programmeing ofn the metabolic syndrome Type 2 diabetes. Br. Med. Bull. 2001, 60, 101–121. [Google Scholar] [CrossRef]
- Gardner, D.S.; Jackson, A.A.; Langley-Evans, S.C. The effect of prenatal diet and glucocorticoids on growth and systolic blood pressure in the rat. Proc. Nutr. Soc. 1998, 57, 235–240. [Google Scholar] [CrossRef] [PubMed]
- Gluckman, P.D.; Hanson, M.A.; Cooper, C.; Thornberg, K.L. Effect of In Utero and Early-Life Conditions on Adult Health and Disease. N. Engl. J. Med. 2008, 359, 61–73. [Google Scholar] [CrossRef] [PubMed]
- Radford, E.J.; Ito, M.; Shi, H.; Corish, J.A.; Yamazawa, K.; Isganaitis, E.; Seisenberger, S.; Hore, T.A.; Reik, W.; Erkek, S.; et al. In utero effects. In utero undernourishment perturbs the adult sperm methylome and intergenerational metabolism. Science (New York, NY) 2014, 345, 1255903. [Google Scholar] [CrossRef] [PubMed]
- Kuh, D.S. Introduction. In A Life Course Approach to Chronic Disease Epidemiology; Kuh, D., Ed.; Oxford University Press: Oxford, UK, 2004. [Google Scholar]
- Marmot, M.; Atkinson, T.; Bell, J.; Black, C.; Broadfoot, P.; Cunberlege, J.; Diamon, I.; Gilmore, I.; Ham, C.; Meacher, M.; et al. Fair Society, Healthy Lives; The Marmot Review: London, UK, 2010. [Google Scholar]
- Morton, S.B. Maternal nutrition and fetal growth and development. In Developmental Origins of Health and Disease; Gluckman, P.D., Hanson, M.A., Eds.; Cambridge University Press: Cambridge, UK, 2006; pp. 98–129. [Google Scholar]
- Yajnik, C.S.; Deshpande, S.S.; Jackson, A.A.; Refsum, H.; Rao, S.; Fisher, D.J.; Bhat, D.S.; Naik, S.S.; Coyaji, K.J.; Joglekar, C.V.; et al. Vitamin B12 and folate concentrations during pregnancy and insulin resistance in the offspring: The Pune Maternal Nutrition Study. Diabetologia 2008, 51, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Oken, E.; Gillman, M.W. Fetal origins of obesity. Obes. Res. 2003, 11, 496–506. [Google Scholar] [CrossRef] [PubMed]
- Victora, C.G.; Adair, L.; Fall, C.; Hallal, P.C.; Martorell, R.; Richter, L.; Sachdev, H.S. Maternal and child undernutrition: Consequences for adult health and human capital. Lancet 2008, 371, 340–357. [Google Scholar] [CrossRef]
- Black, R.E.; Allen, L.H.; Bhutta, Z.A.; Caulfield, L.E.; de Onis, M.; Ezzati, M.; Mathers, C.; Rivera, J. Maternal and child undernutrition: Global and regional exposures and health consequences. Lancet 2008, 371, 243–260. [Google Scholar] [CrossRef]
- Gluckman, P.D.; Seng, C.Y.; Fukuoka, H.; Beedle, A.S.; Hanson, M.A. Low birthweight and subsequent obesity in Japan. Lancet 2007, 369, 1081–1082. [Google Scholar] [CrossRef]
- Robker, R.L.; Akison, L.K.; Bennett, B.D.; Thrupp, P.N.; Chura, L.R.; Russell, D.L.; Lane, M.; Norman, R.J. Obese women exhibit differences in ovarian metabolites, hormones, and gene expression compared with moderate-weight women. J. Clin. Endocrinol. Metab. 2009, 94, 1533–1540. [Google Scholar] [CrossRef] [PubMed]
- Godfrey, K.; Walker-Bone, K.; Robinson, S.; Taylor, P.; Shore, S.; Wheeler, T.; Cooper, C. Neonatal bone mass: Influence of parental birthweight, maternal smoking, body composition, and activity during pregnancy. J. Bone Miner Res. 2001, 16, 1694–1703. [Google Scholar] [CrossRef] [PubMed]
- Harvey, N.C.; Javaid, M.K.; Arden, N.K.; Poole, J.R.; Crozier, S.R.; Robinson, S.M.; Inskip, H.M.; Godfrey, K.M.; Dennison, E.M.; Cooper, C. Maternal predictors of neonatal bone size and geometry: The Southampton Women’s Survey. J. Dev. Origins Health Dis. 2012, 1, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Zhu, K.; Whitehouse, A.J.; Hart, P.H.; Kusel, M.; Mountain, J.; Lye, S.; Pennell, C.; Walsh, J.P. Maternal vitamin D status during pregnancy and bone mass in offspring at 20 years of age: A prospective cohort study. J. Bone Miner Res. 2014, 29, 1088–1095. [Google Scholar] [CrossRef] [PubMed]
- Hanson, M.; Bustreo, F.; Godfrey, K.; Poston, L.; Stephenson, J. Preconception health. Annual report of the Chief Medical Officer, 2014—The Health of the 51%: Women. Available online: https://www.gov.uk/government/publications/chief-medical-officer-annual-report-2014-womens-health (accessed on 6 March 2017).
- Robinson, S.M.; Crozier, S.R.; Borland, S.E.; Hammond, J.; Barker, D.J.; Inskip, H.M. Impact of educational attainment on the quality of young women’s diets. Eur. J. Clin. Nutr. 2004, 58, 1174–1180. [Google Scholar] [CrossRef] [PubMed]
- Harvey, N.; Dennison, E.; Cooper, C. Osteoporosis: A lifecourse approach. J. Bone Miner Res. 2014, 29, 1917–1925. [Google Scholar] [CrossRef] [PubMed]
- Inskip, H.M.; Crozier, S.R.; Godfrey, K.M.; Borland, S.E.; Cooper, C.; Robinson, S.M. Women's compliance with nutrition and lifestyle recommendations before pregnancy: General population cohort study. BMJ (Clin. Res.) 2009. [Google Scholar] [CrossRef] [PubMed]
- The Wellcome Trust Case Control Consortium. Genome-wide association study of 14,000 cases of seven common diseases and 3000 shared controls. Nature 2007, 447, 661–678. [Google Scholar]
- Morris, A.P.; Voight, B.F.; Teslovich, T.M.; Ferreira, T.; Segre, A.V.; Steinthorsdottir, V.; Strawbridge, R.J.; Khan, H.; Grallert, H.; Mahajan, A.; et al. Large-scale association analysis provides insights into the genetic architecture and pathophysiology of type 2 diabetes. Nat. Genet. 2012, 44, 981–990. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gluckman, P.D.; Hanson, M.A.; Bateson, P.; Beedle, A.S.; Law, C.M.; Bhutta, Z.A.; Anokhin, K.V.; Bougneres, P.; Chandak, G.R.; Dasgupta, P.; et al. Towards a new developmental synthesis: Adaptive developmental plasticity and human disease. Lancet 2009, 373, 1654–1657. [Google Scholar] [CrossRef]
- Heijmans, B.T.; Tobi, E.W.; Stein, A.D.; Tobi, E.W.; Stein, A.D.; Putter, H.; Blauw, G.J.; Susser, E.S.; Slagboom, P.E.; Lumey, L.H. Persistent epigenetic differences associated with prenatal exposure to famine in humans. Proc. Natl. Acad. Sci. USA 2008, 105, 17046–17049. [Google Scholar] [CrossRef] [PubMed]
- Darnton-Hill, I.; Nishida, C.; James, W.P. A life course approach to diet, nutrition and the prevention of chronic diseases. Public Health Nutr. 2004, 7, 101–121. [Google Scholar] [CrossRef] [PubMed]
- Godfrey, K.M.; Sheppard, A.; Gluckman, P.D.; Lillycrop, K.A.; Burdge, G.C.; McLean, C.; Rodford, J.; Slater-Jefferies, J.L.; Garratt, E.; Crozier, S.R.; et al. Epigenetic gene promoter methylation at birth is associated with child's later adiposity. Diabetes 2011, 60, 1528–1534. [Google Scholar] [CrossRef] [PubMed]
- Barlow, J.; Blair, M. Chapter 6: Life stage: Early Years. In: Chief Medical Officer's annual report 2012: Our Children Deserve Better: Prevention Pays. Available online: https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/252656/33571_2901304_CMO_Chapter_6.pdf (accessed on 6 March 2017).
- Viner, R.M. Chapter 8: Life stage: Adolescence. In: Chief Medical Officer's annual report 2012: Our Children Deserve Better: Prevention Pays. Available online: https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/252658/33571_2901304_CMO_Chapter_8.pdf (accessed on 6 March 2017).
- World Health Organisation. Global Health Risks: Mortality and Burden of Disease Attributable to Selected Major Risks; World Health Organisation: Geneva, Switzerland, 2009. [Google Scholar]
- Ezzati, M.; Riboli, E. Behavioral and Dietary Risk Factors for Noncommunicable Diseases. N. Engl. J. Med. 2013, 369, 954–964. [Google Scholar] [CrossRef] [PubMed]
- Hauck, F.R.; Thompson, J.M.; Tanabe, K.O.; Moon, R.Y.; Vennemann, M.M. Breastfeeding and reduced risk of sudden infant death syndrome: A meta-analysis. Pediatrics 2011, 128, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Kramer, M.S.; Kakuma, R. Optimal duration of exclusive breastfeeding. Cochr. Database Syst. Rev. 2002. [Google Scholar] [CrossRef]
- Viner, R.M.; Ozer, E.M.; Denny, S.; Marmot, M.; Resnick, M.; Fatusi, A.; Currie, C. Adolescence and the social determinants of health. Lancet 2012, 379, 1641–1652. [Google Scholar] [CrossRef]
- UNICEF. Progress for Children. A Report Card on Adolescents; UNICEF: New York, NY, USA, 2012. [Google Scholar]
- Hanson, M.A.; Cooper, C.; Aihie Sayer, A.; Eendebak, R.J.; Clough, G.F.; Beard, J.R. Developmental aspects of a life course approach to healthy ageing. J. Physiol. 2016, 594, 2147–2160. [Google Scholar] [CrossRef] [PubMed]
- Nove, A.; Matthews, Z.; Neal, S.; Camacho, A.V. Maternal mortality in adolescents compared with women of other ages: Evidence from 144 countries. Lancet Glob. Health 2014, 2, e155–e164. [Google Scholar] [CrossRef]
- Hanson, M.A.; Bardsley, A.; De-Regil, L.M.; Moore, S.E.; Oken, E.; Poston, L.; Ma, R.C.; McAuliffe, F.M.; Maleta, K.; Purandare, C.N.; et al. The International Federation of Gynecology and Obstetrics (FIGO) recommendations on adolescent, preconception, and maternal nutrition: “Think Nutrition First”. Int. J. Gynaecol. Obstet. 2015, 131, S213–S253. [Google Scholar] [CrossRef]
- De-Regil, L.M.; Palacios, C.; Ansary, A.; Kulier, R.; Pena-Rosas, J.P. Vitamin D supplementation for women during pregnancy. Cochr. Database Syst. Rev. 2012. [Google Scholar] [CrossRef]
- Cooper, C.; Harvey, N.C.; Bishop, N.J.; Kennedy, S.; Papageorghiou, A.T.; Schoenmakers, I.; Fraser, R.; Gandhi, S.V.; Carr, A.; D’Angelo, S.; et al. Maternal gestational vitamin D supplementation and offspring bone health (MAVIDOS): A multicentre, double-blind, randomised placebo-controlled trial. Lancet Diabetes Endocrinol. 2016, 4, 393–402. [Google Scholar] [CrossRef]
- Crozier, S.R.; Harvey, N.C.; Inskip, H.M.; Kennedy, S.; Papageorghiou, A.T.; Schoenmakers, I.; Fraser, R.; Gandhi, S.V.; Carr, A.; D’Angelo, S.; et al. Maternal vitamin D status in pregnancy is associated with adiposity in the offspring: Findings from the Southampton Women's Survey. Am. J. Clin. Nutr. 2012, 96, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Potdar, R.D.; Sahariah, S.A.; Gandhi, M.; Kehoe, S.H.; Brown, N.; Sane, H.; Dayama, M.; Jha, S.; Lawande, A.; Coakley, P.J.; et al. Improving women’s diet quality preconceptionally and during gestation: Effects on birth weight and prevalence of low birth weight—A randomized controlled efficacy trial in India (Mumbai Maternal Nutrition Project). Am. J. Clin. Nutr. 2014, 100, 1257–1268. [Google Scholar] [CrossRef] [PubMed]
- Devakumar, D.; Fall, C.H.; Sachdev, H.S.; Margetts, B.M.; Osmond, C.; Wells, J.C.; Costello, A.; Osrin, D. Maternal antenatal multiple micronutrient supplementation for long-term health benefits in children: A systematic review and meta-analysis. BMC Med. 2016. [Google Scholar] [CrossRef] [PubMed]
- Phelan, S. Pregnancy: A “teachable moment” for weight control and obesity prevention. Am. J. Obstetr. Gynecol. 2010, 202, 135.e1–135.e8. [Google Scholar] [CrossRef] [PubMed]
- Barker, M.; Lawrence, W.; Skinner, T.; Haslam, C.; Robinson, S.; Inskip, H.; Margetts, B.; Jackson, A.; Barker, D.; Cooper, C. Constraints on food choices of women in the UK with lower educational attainment. Public Health Nutr. 2008, 11, 1229–1237. [Google Scholar] [CrossRef] [PubMed]
- Greaves, C.J.; Sheppard, K.E.; Abraham, C.; Sheppard, K.E.; Abraham, C.; Hardeman, W.; Roden, M.; Evans, P.H.; Schwarz, P. Systematic review of reviews of intervention components associated with increased effectiveness in dietary and physical activity interventions. BMC Public Health 2011. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baird, J.; Cooper, C.; Margetts, B.M.; Barker, M.; Inskip, H.M. Changing health behaviour of young women from disadvantaged backgrounds: Evidence from systematic reviews. Proc. Nutr. Soc. 2009, 68, 195–204. [Google Scholar] [CrossRef] [PubMed]
- Michie, S.; Jochelson, K.; Markham, W.A.; Bridle, C. Low-income groups and behaviour change interventions: A review of intervention content, effectiveness and theoretical frameworks. J. Epidemiol. Commun. Health 2009, 63, 610–622. [Google Scholar] [CrossRef] [PubMed]
- Poston, L.; Bell, R.; Croker, H.; Flynn, A.C.; Godfrey, K.M.; Goff, L.; Hayes, L.; Khazaezadeh, N.; Nelson, S.; Otteng-Ntim, E.; et al. Effect of a behavioural intervention in obese pregnant women (the UPBEAT study): A multicentre randomised controlled trial. Lancet Diabetes Endocrinol. 2015, 3, 767–777. [Google Scholar] [CrossRef]
- Dodd, J.M.; Turnbull, D.; McPhee, A.J.; Deussen, A.R.; Grivell, R.M.; Yelland, L.N.; Crowther, C.A.; Wittert, G.; Owens, J.A.; Robinson, J.S. Antenatal lifestyle advice for women who are overweight or obese: LIMIT randomised trial. BMJ (Clin. Res.) 2014. [Google Scholar] [CrossRef] [Green Version]
- Barker, M.; Baird, J.; Lawrence, W.; Jarman, M.; Black, C.; Barnard, K.; Cradock, S.; Davies, J.; Margetts, B.; Inskip, H.; et al. The Southampton Initiative for Health: A complex intervention to improve the diets and increase the physical activity levels of women from disadvantaged communities. J. Health Psychol. 2011, 16, 178–191. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, W.; Black, C.; Tinati, T.; Cradock, S.; Begum, R.; Jarman, M.; Pease, A.; Margetts, B.; Davies, J.; Inskip, H.; et al. ‘Making every contact count’: Evaluation of the impact of an intervention to train health and social care practitioners in skills to support health behaviour change. J. Health Psychol. 2016, 21, 138–151. [Google Scholar] [CrossRef] [PubMed]
- Baird, J.; Jarman, M.; Lawrence, W.; Black, C.; Davies, J.; Tinati, T.; Begum, R.; Mortimore, A.; Robinson, S.; Margetts, B.; et al. The effect of a behaviour change intervention on the diets and physical activity levels of women attending Sure Start Children’s Centres: Results from a complex public health intervention. BMJ Open 2014. [Google Scholar] [CrossRef] [PubMed]
- Woods-Townsend, K.; Bagust, L.; Barker, M.; Christodoulou, A.; Davey, H.; Godfrey, K.; Grace, M.; Griffiths, J.; Hanson, M.; Inskip, H. Engaging teenagers in improving their health behaviours and increasing their interest in science (Evaluation of LifeLab Southampton): Study protocol for a cluster randomized controlled trial. Trials 2015. [Google Scholar] [CrossRef] [PubMed]
- Draper, C.E.; Micklesfield, L.K.; Kahn, K.; Tollman, S.M.; Pettifor, J.M.; Dunger, D.B.; Norris, S.A. Application of Intervention Mapping to develop a community-based health promotion pre-pregnancy intervention for adolescent girls in rural South Africa: Project Ntshembo (Hope). BMC Public Health 2014. [Google Scholar] [CrossRef] [PubMed]
- OFCOM. The Communications Market Report. Available online: https://www.ofcom.org.uk/__data/assets/pdf_file/0022/20668/cmr_uk_2015.pdf (accessed on 6 March 2017).
- Okorodudu, D.E.; Bosworth, H.B.; Corsino, L. Innovative interventions to promote behavioral change in overweight or obese individuals: A review of the literature. Ann. Med. 2015, 47, 179–185. [Google Scholar] [CrossRef] [PubMed]
- Morrison, L. Theory-based strategies for enhancing the impact and usage of digital health behaviour change interventions: A review. Dig. Health 2015. [Google Scholar] [CrossRef]
- Alderman, H.; Behrman, J.R.; Hoddinott, J. Economic and Nutritional Analyses Offer Substantial Synergies for Understanding Human Nutrition. J. Nutr. 2007, 137, 537–544. [Google Scholar] [PubMed]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baird, J.; Jacob, C.; Barker, M.; Fall, C.H.D.; Hanson, M.; Harvey, N.C.; Inskip, H.M.; Kumaran, K.; Cooper, C. Developmental Origins of Health and Disease: A Lifecourse Approach to the Prevention of Non-Communicable Diseases. Healthcare 2017, 5, 14. https://doi.org/10.3390/healthcare5010014
Baird J, Jacob C, Barker M, Fall CHD, Hanson M, Harvey NC, Inskip HM, Kumaran K, Cooper C. Developmental Origins of Health and Disease: A Lifecourse Approach to the Prevention of Non-Communicable Diseases. Healthcare. 2017; 5(1):14. https://doi.org/10.3390/healthcare5010014
Chicago/Turabian StyleBaird, Janis, Chandni Jacob, Mary Barker, Caroline H. D. Fall, Mark Hanson, Nicholas C. Harvey, Hazel M. Inskip, Kalyanaraman Kumaran, and Cyrus Cooper. 2017. "Developmental Origins of Health and Disease: A Lifecourse Approach to the Prevention of Non-Communicable Diseases" Healthcare 5, no. 1: 14. https://doi.org/10.3390/healthcare5010014





