Research Progress on Bionic Recognition and Biosensors for the Detection of Biomarkers of Diabetic Nephropathy
Abstract
:1. Introduction
2. Application of BRBS in the Detection of Diabetic Nephropathy Biomarkers
2.1. Urinary Albumin
2.2. Creatinine (Crn)
2.3. Cystatin C (Cys-C)
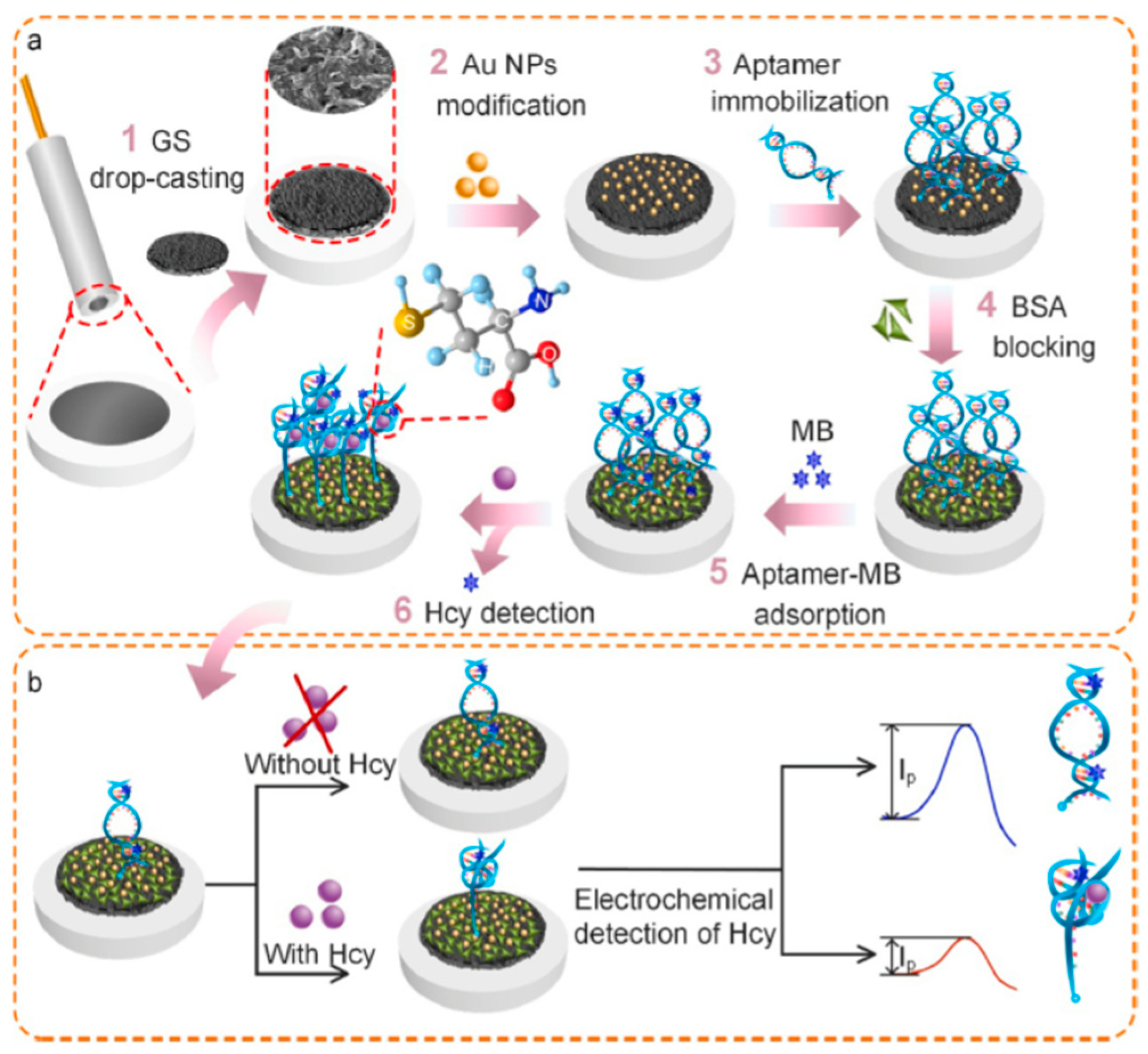
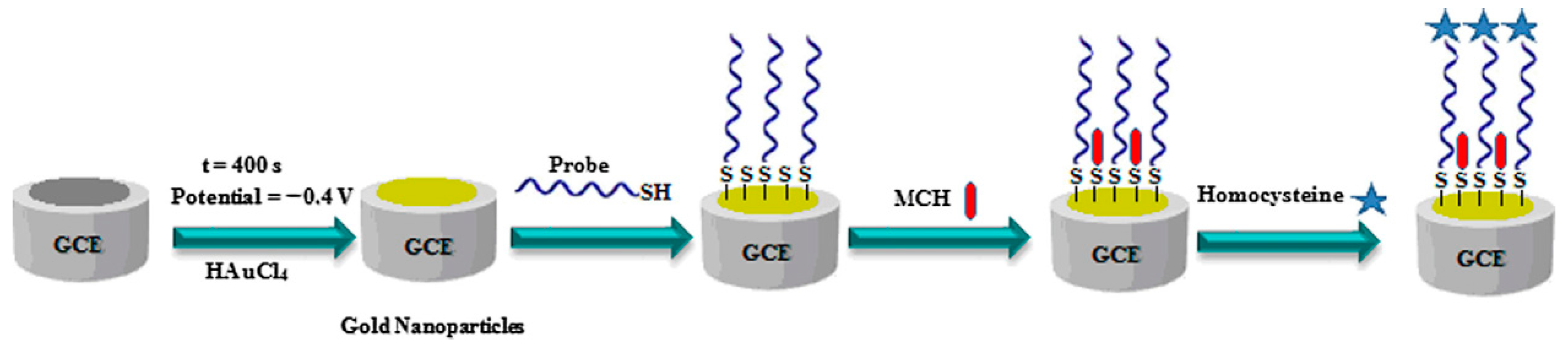
2.4. Homocysteine (Hcy)
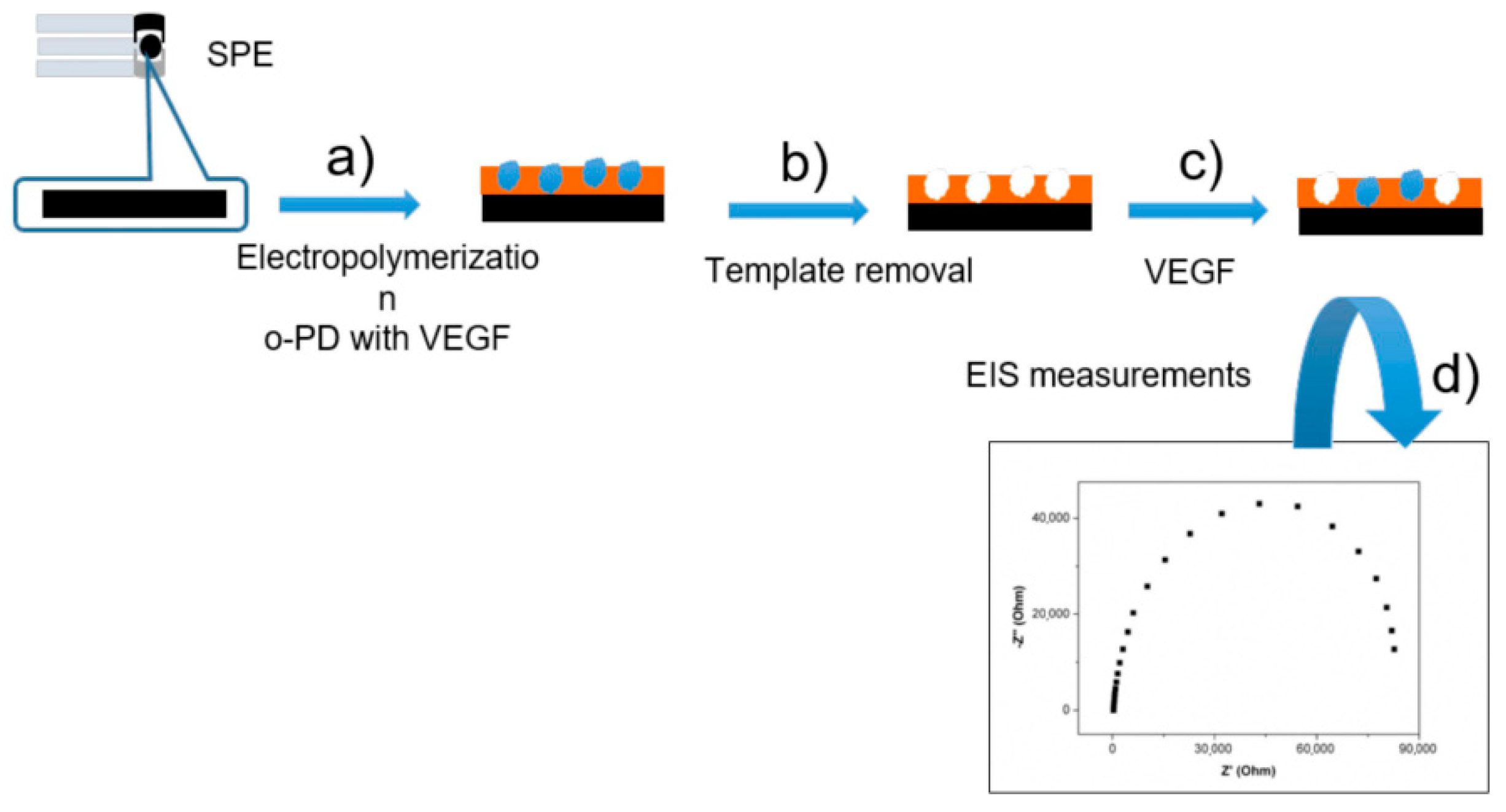
2.5. Vascular Endothelial Growth Factor (VEGF)
2.6. Epidermal Growth Factor Receptor (EGFR)
2.7. mRNA-21
2.8. Ceruloplasmin (Cp)
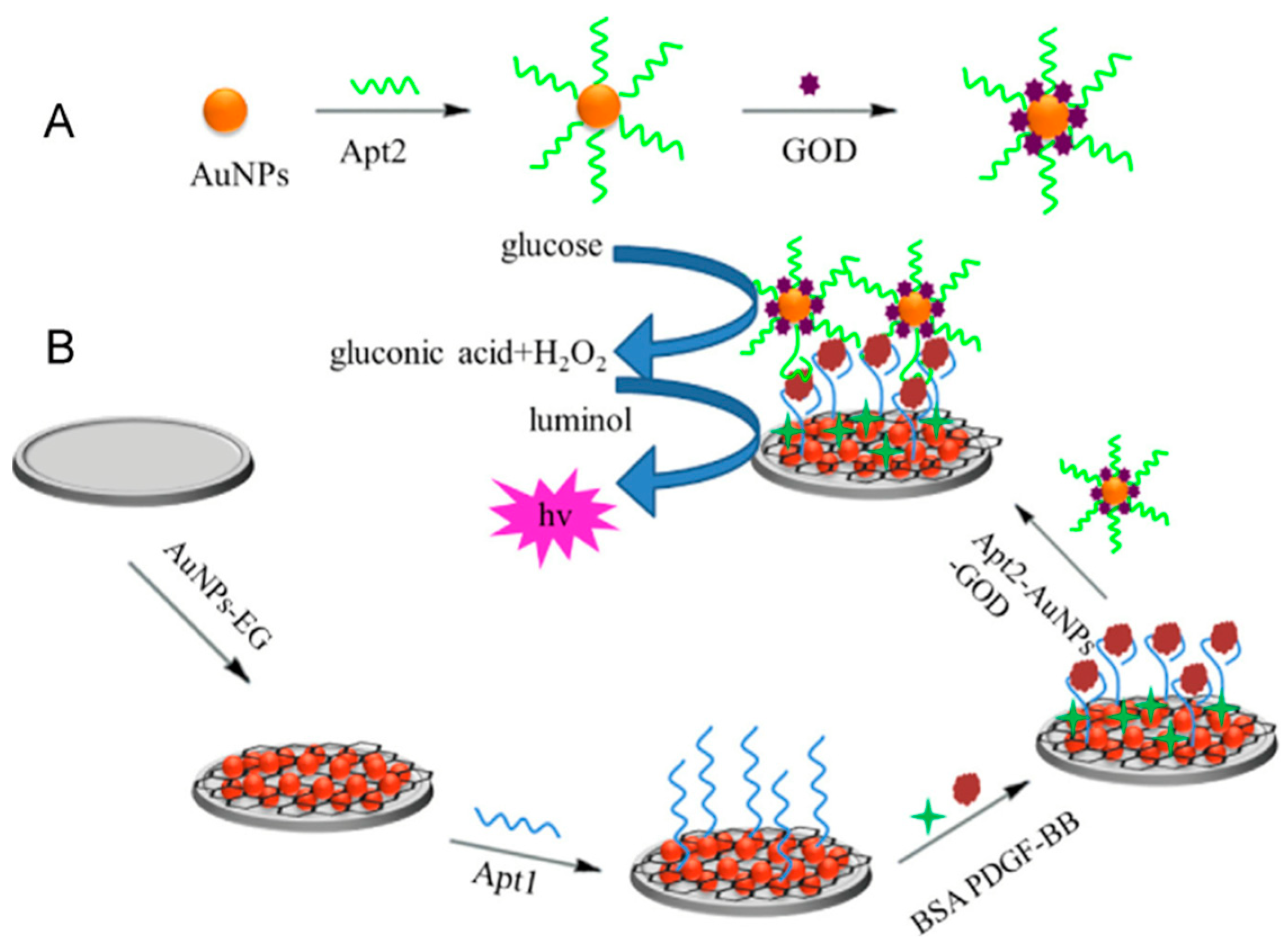
2.9. Platelet-Derived Growth Factor (PDGF)
3. Conclusions and Future Perspectives
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Fawzy, M.S.; Al Beladi, F.I. Association of Circulating Vitamin D, VDBP, and Vitamin D Receptor Expression with Severity of Diabetic Nephropathy in a Group of Saudi Type 2 Diabetes Mellitus Patients. Clin. Lab. 2018, 64, 1623–1633. [Google Scholar] [CrossRef] [PubMed]
- Hayes, A.; Arima, H.; Woodward, M.; Chalmers, J.; Poulter, N.; Hamet, P.; Clarke, P. Changes in Quality of Life Associated with Complications of Diabetes: Results from the ADVANCE Study. Value Health 2016, 19, 36–41. [Google Scholar] [CrossRef] [PubMed]
- Thorn, L.M.; Gordin, D.; Harjutsalo, V.; Hagg, S.; Masar, R.; Saraheimo, M.; Tolonen, N.; Waden, J.; Groop, P.H.; Forsblom, C.M.; et al. The Presence and Consequence of Nonalbuminuric Chronic Kidney Disease in Patients With Type 1 Diabetes. Diabetes Care 2015, 38, 2128–2133. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Xu, B.J.; Deng, X.; Wu, X.W.; Li, Y.J.; Wang, S.R.; Wang, Y.N.; Ji, S.; Guo, M.Z.; Yang, D.Z.; et al. Predictive metabolic signatures for the occurrence and development of diabetic nephropathy and the intervention of Ginkgo biloba leaves extract based on gas or liquid chromatography with mass spectrometry. J. Pharm. Biomed. Anal. 2019, 166, 30–39. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, H.; Loh, T.P.; Liu, Q.; Teo, T.L.; Lee, T.K.; Sethi, S.K. Measurement of urine albumin by liquid chromatography-isotope dilution tandem mass spectrometry and its application to value assignment of external quality assessment samples and certification of reference materials. Clin. Chem. Lab. Med. 2021, 59, 711–720. [Google Scholar] [CrossRef]
- Ji, H.; Shen, L.; Shi, X.; Su, J.; Tang, Z.; Wang, H.; Ju, S.; Wang, J. Establishment of an absolute quantitative method for measurement of urinary cystatin C by stable isotope dilution ultra high performance liquid chromatography tandem mass spectrometry. Anal. Methods 2018, 10, 5236–5241. [Google Scholar] [CrossRef]
- Harlan, R.; Clarke, W.; Di Bussolo, J.M.; Kozak, M.; Straseski, J.; Meany, D.L. An automated turbulent flow liquid chromatography-isotope dilution mass spectrometry (LC-IDMS) method for quantitation of serum creatinine. Clin. Chim. Acta. 2010, 411, 1728–1734. [Google Scholar] [CrossRef]
- Wang, H.; Chai, Y.; Li, H.; Yuan, R. Sensitive electrochemiluminescent immunosensor for diabetic nephropathy analysis based on tris(bipyridine) ruthenium(II) derivative with binary intramolecular self-catalyzed property. Biosens. Bioelectron. 2018, 100, 35–40. [Google Scholar] [CrossRef]
- Li, Y.; Wang, Y.; Bai, L.; Lv, H.; Huang, W.; Liu, S.; Ding, S.; Zhao, M. Ultrasensitive electrochemiluminescent immunosensing based on trimetallic Au-Pd-Pt/MoS(2) nanosheet as coreaction accelerator and self-enhanced ABEI-centric complex. Anal. Chim. Acta. 2020, 1125, 86–93. [Google Scholar] [CrossRef]
- Xu, H.; Kou, F.; Ye, H.; Wang, Z.; Huang, S.; Liu, X.; Zhu, X.; Lin, Z.; Chen, G. Highly sensitive antibody-aptamer sensor for vascular endothelial growth factor based on hybridization chain reaction and pH meter/indicator. Talanta 2017, 175, 177–182. [Google Scholar] [CrossRef]
- Wang, H.; Ma, Y.; Guo, C.; Yang, Y.; Peng, Z.; Liu, Z.; Zhang, Z. Templated seed-mediated derived Au nanoarchitectures embedded with nanochitosan: Sensitive electrochemical aptasensor for vascular endothelial growth factor and living MCF-7 cell detection. Appl. Surf. Sci. 2019, 481, 505–514. [Google Scholar] [CrossRef]
- Hassanzadeh, M.; Ghaemy, M.; Amininasab, S.M.; Shami, Z. Molecularly imprinted polymer capped near infrared fluorescent emitting Ag2S-functionalized-COOH quantum dots for detection of creatinine as a nanosensor with high sensitivity and selectivity. Sens. Actuators A Phys. 2021, 331, 112936. [Google Scholar] [CrossRef]
- Zhuang-Fei, J.; Qin, L.; Qing-Yao, L.; Hui-Xian, X.; Jia-Yuan, H.; Chong-Zhi, W.; Lian-Di, Z.; Qi-Hui, Z.; Ling, L.; Chun-Su, Y. Fast exhaustive enrichment and electrochemical quantitative detection of anthocyanins from natural products by using dual responsive and dummy molecularly imprinted polymers. Microchem. J. 2022, 179, 107545. [Google Scholar]
- Taghdisi, S.M.; Danesh, N.M.; Ramezani, M.; Alibolandi, M.; Nameghi, M.A.; Gerayelou, G.; Abnous, K. A novel electrochemical aptasensor for ochratoxin a sensing in spiked food using strand-displacement polymerase reaction. Talanta 2021, 223 Pt 1, 121705. [Google Scholar] [CrossRef] [PubMed]
- Yan, S.R.; Foroughi, M.M.; Safaei, M.; Jahani, S.; Ebrahimpour, N.; Borhani, F.; Rezaei Zade Baravati, N.; Aramesh-Boroujeni, Z.; Foong, L.K. A review: Recent advances in ultrasensitive and highly specific recognition aptasensors with various detection strategies. Int. J. Biol. Macromol. 2020, 155, 184–207. [Google Scholar] [CrossRef]
- Kimura-Suda, H.; Petrovykh, D.Y.; Tarlov, M.J.; Whitman, L.J. Base-dependent competitive adsorption of single-stranded DNA on gold. J. Am. Chem. Soc. 2003, 125, 9014–9015. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Cai, X.; Wang, L.; Li, J.; Li, Q.; Zuo, X.; Shi, J.; Huang, Q.; Fan, C. DNA orientation-specific adhesion and patterning of living mammalian cells on self-assembled DNA monolayers. Chem. Sci. 2016, 7, 2722–2727. [Google Scholar] [CrossRef]
- McTaggart, M.P.; Price, C.P.; Pinnock, R.G.; Stevens, P.E.; Newall, R.G.; Lamb, E.J. The Diagnostic Accuracy of a Urine Albumin-Creatinine Ratio Point-of-Care Test for Detection of Albuminuria in Primary Care. Am. J. Kidney Dis. 2012, 60, 787–794. [Google Scholar] [CrossRef]
- MacIsaac, R.J.; Ekinci, E.I.; Jerums, G. Markers of and Risk Factors for the Development and Progression of Diabetic Kidney Disease. Am. J. Kidney Dis. 2014, 63, S39–S62. [Google Scholar] [CrossRef]
- Zhang, G.; Yu, Y.; Guo, M.; Lin, B.; Zhang, L. A sensitive determination of albumin in urine by molecularly imprinted electrochemical biosensor based on dual-signal strategy. Sens. Actuators B Chem. 2019, 288, 564–570. [Google Scholar] [CrossRef]
- Boon, E.M.; Ceres, D.M.; Drummond, T.G.; Hill, M.G.; Barton, J.K. Mutation detection by electrocatalysis at DNA-modified electrodes. Nat. Biotechnol. 2000, 18, 1096–1102. [Google Scholar] [CrossRef]
- Kintzel, P.E. Anticancer drug-induced kidney disorders. Drug Saf. 2001, 24, 19–38. [Google Scholar] [CrossRef]
- Levey, A.S.; Perrone, R.D.; Madias, N.E. Serum Creatinine and Renal Function. Annu. Rev. Med. 1988, 39, 465–490. [Google Scholar] [CrossRef]
- Musa, N.; Ramzy, T.; Hamdy, A.; Arafa, N.; Hassan, M. Assessment of urinary podocalyxin as a marker of glomerular injury in obesity-related kidney disease in children and adolescents with obesity compared to urinary albumin creatinine ratio. Clin. Obes. 2021, 11, e12452. [Google Scholar] [CrossRef] [PubMed]
- Prabhu, S.N.; Mukhopadhyay, S.C.; Gooneratne, C.P.; Davidson, A.S.; Liu, G. Molecularly Imprinted Polymer-based detection of creatinine towards smart sensing. Med. Devices Sens. 2020, 3, e10133. [Google Scholar] [CrossRef]
- Canfarotta, F.; Poma, A.; Guerreiro, A.; Piletsky, S. Solid-phase synthesis of molecularly imprinted nanoparticles. Nat. Protoc. 2016, 11, 443–455. [Google Scholar] [CrossRef]
- Gutiérrez-Climente, R.; Gómez-Caballero, A.; Unceta, N.; Aránzazu Goicolea, M.; Barrio, R.J. A new potentiometric sensor based on chiral imprinted nanoparticles for the discrimination of the enantiomers of the antidepressant citalopram. Electrochim. Acta 2016, 196, 496–504. [Google Scholar] [CrossRef]
- Meng, C.; Knežević, S.; Du, F.; Guan, Y.; Kanoufi, F.; Sojic, N.; Xu, G. Recent advances in electrochemiluminescence imaging analysis. eScience 2022, 2, 591–605. [Google Scholar] [CrossRef]
- Quan, S.; Ji, K.; Liu, F.; Barkae, T.H.; Halawa, M.I.; Hanif, S.; Lou, B.; Li, J.; Xu, G. Chemiluminescence of lucigenin-tetracycline and itsapplication for sensitive determination of procyanidin. J. Food Drug Anal. 2022, 30, 293–302. [Google Scholar] [CrossRef]
- Mostafa, I.M.; Gilani, M.; Chen, Y.; Lou, B.; Li, J.; Xu, G. Lucigenin-pyrogallol chemiluminescence for the multiple detection of pyrogallol, cobalt ion, and tyrosinase. J. Food Drug Anal. 2021, 29, 510–520. [Google Scholar] [CrossRef]
- Babamiri, B.; Salimi, A.; Hallaj, R.; Hasanzadeh, M. Nickel nanoclusters as a novel emitter for molecularly imprinted electrochemiluminescence based sensor toward nanomolar detection of creatinine. Biosens. Bioelectron. 2018, 107, 272–279. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Wang, X.; Lu, W.; Wu, X.; Li, J. Molecular imprinting: Perspectives and applications. Chem. Soc. Rev. 2016, 45, 2137–2211. [Google Scholar] [CrossRef]
- Li, Y.; Luo, L.; Nie, M.; Davenport, A.; Li, Y.; Li, B.; Choy, K.L. A graphene nanoplatelet-polydopamine molecularly imprinted biosensor for Ultratrace creatinine detection. Biosens. Bioelectron. 2022, 216, 114638. [Google Scholar] [CrossRef]
- Amer, A.H.; Haridas, N. Early Diagnostic Markers in Diabetic Nephropathy Patients. J. Clin. Diagn. Res. 2018, 12, BC05–BC09. [Google Scholar] [CrossRef]
- Gupta, K.; Nayyar, S.B.; Sachdeva, J.K.; Kumar, P. Cystatin C in the early diagnosis of diabetic nephropathy and its correlation with albuminuria. Int. J. Adv. Med. 2017, 4, 56–59. [Google Scholar] [CrossRef]
- Dingding, D.; Jun, W.; Pengxin, H.; Xin, L.; Luhang, Z.; Shenao, M. Dual-monomer molecularly imprinted electrochemical sensor based on amino-functionalized MOFs and graphene for trace determination of taurine. Mikrochim. Acta 2023, 190, 126–162. [Google Scholar]
- Hoefer, M.; Bandaru, P.R. Determination and enhancement of the capacitance contributions in carbon nanotube based electrode systems. Appl. Phys. Lett. 2009, 95, 183108. [Google Scholar] [CrossRef]
- Ferreira, P.A.B.; Araujo, M.C.M.; Prado, C.M.; de Lima, R.A.; Rodriguez, B.A.G.; Dutra, R.F. An ultrasensitive Cystatin C renal failure immunosensor based on a PPy/CNT electrochemical capacitor grafted on interdigitated electrode. Colloids Surf. B Biointerfaces 2020, 189, 110834. [Google Scholar] [CrossRef]
- Canobre, S.C.; Xavier FF, S.; Fagundes, W.S.; de Freitas, A.C.; Amaral, F.A. Performance of the Chemical and Electrochemical Composites of PPy/CNT as Electrodes in Type I Supercapacitors. J. Nanomater. 2015, 2015, 560164. [Google Scholar] [CrossRef]
- Gomes, R.S.; Gomez-Rodriguez, B.A.; Fernandes, R.; Sales, M.G.F.; Moreira, F.T.C.; Dutra, R.F. Plastic Antibody of Polypyrrole/Multiwall Carbon Nanotubes on Screen-Printed Electrodes for Cystatin C Detection. Biosensors 2021, 11, 175. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Zhang, H.; Zhong, M.; Wang, S.; Xu, Q.; Cho, D.-H.; Qiu, H. A novel off-on fluorescent probe for specific detection and imaging of cysteine in live cells and in vivo. Chin. Chem. Lett. 2020, 31, 133–135. [Google Scholar] [CrossRef]
- Refsum, H.; Ueland, P.M.; Nygard, O.; Vollset, S.E. Homocysteine and Cardiovascular Disease. Annu. Rev. Med. 1998, 49, 31–62. [Google Scholar] [CrossRef] [PubMed]
- Sedgwick, A.C.; Wu, L.; Han, H.H.; Bull, S.D.; He, X.P.; James, T.D.; Sessler, J.L.; Tang, B.Z.; Tian, H.; Yoon, J. Excited-state intramolecular proton-transfer (ESIPT) based fluorescence sensors and imaging agents. Chem. Soc. Rev. 2018, 47, 8842–8880. [Google Scholar] [CrossRef] [PubMed]
- Ben Messaoud, N.; Ghica, M.E.; Dridi, C.; Ben Ali, M.; Brett CM, A. Electrochemical sensor based on multiwalled carbon nanotube and gold nanoparticle modified electrode for the sensitive detection of bisphenol A. Sens. Actuators B Chem. 2017, 253, 513–522. [Google Scholar] [CrossRef]
- Plowman, B.J.; Sidhureddy, B.; Sokolov, S.V.; Young, N.P.; Chen, A.; Compton, R.G. Electrochemical Behavior of Gold-Silver Alloy Nanoparticles. ChemElectroChem 2016, 3, 1039–1043. [Google Scholar] [CrossRef]
- Kumar, N.; Goyal, R.N. Silver nanoparticles decorated graphene nanoribbon modified pyrolytic graphite sensor for determination of histamine. Sens. Actuators B Chem. 2018, 268, 383–391. [Google Scholar] [CrossRef]
- Zhou, Y.-C.; Zhao, M.; Yu, Y.-Q.; Lei, Y.-M.; Chai, Y.-Q.; Yuan, R.; Zhuo, Y. Three-dimensional nano-network composed of Pt nanoparticles functionalized Mn-doped CeO2 and hemin/G-quadruplex as electrocatalysts for cardiovascular biomarker detection. Sens. Actuators B Chem. 2017, 246, 1–8. [Google Scholar] [CrossRef]
- Yang, L.; Xu, B.; Ye, H.; Zhao, F.; Zeng, B. A novel quercetin electrochemical sensor based on molecularly imprinted poly(para-aminobenzoic acid) on 3D Pd nanoparticles-porous graphene-carbon nanotubes composite. Sens. Actuators B Chem. 2017, 251, 601–608. [Google Scholar] [CrossRef]
- Xie, Y.; Gao, F.; Tu, X.; Ma, X.; Xu, Q.; Dai, R.; Huang, X.; Yu, Y.; Lu, L. Facile Synthesis of MXene/Electrochemically Reduced Graphene Oxide Composites and Their Application for Electrochemical Sensing of Carbendazim. J. Electrochem. Soc. 2019, 166, B1673–B1680. [Google Scholar] [CrossRef]
- Wang, H.; Li, H.; Huang, Y.; Xiong, M.; Wang, F.; Li, C. A label-free electrochemical biosensor for highly sensitive detection of gliotoxin based on DNA nanostructure/MXene nanocomplexes. Biosens. Bioelectron. 2019, 142, 111531. [Google Scholar] [CrossRef]
- Nah, J.S.; Barman, S.C.; Zahed, M.A.; Sharifuzzaman, M.; Yoon, H.; Park, C.; Yoon, S.; Zhang, S.; Park, J.Y. A wearable microfluidics-integrated impedimetric immunosensor based on Ti3C2T MXene incorporated laser-burned graphene for noninvasive sweat cortisol detection. Sens. Actuators B Chem. 2021, 329, 129206. [Google Scholar] [CrossRef]
- Ma, X.; Tu, X.; Gao, F.; Xie, Y.; Huang, X.; Fernandez, C.; Qu, F.; Liu, G.; Lu, L.; Yu, Y. Hierarchical porous MXene/amino carbon nanotubes-based molecular imprinting sensor for highly sensitive and selective sensing of fisetin. Sens. Actuators B Chem. 2020, 309, 127815. [Google Scholar] [CrossRef]
- Mohammadniaei, M.; Koyappayil, A.; Sun, Y.; Min, J.; Lee, M.H. Gold nanoparticle/MXene for multiple and sensitive detection of oncomiRs based on synergetic signal amplification. Biosens. Bioelectron. 2020, 159, 112208. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Pan, B.; Tang, S.; Wang, W.; Hou, H.; Xie, B.; Liang, A.; Luo, A. A Label-Free Molecularly Imprinted Electrochemical Sensor Based on MXene Nanosheets Modified by Gold Nanoparticles for Sensitive and Selective Detection of Homocysteine. J. Electrochem. Soc. 2022, 169, 087503. [Google Scholar] [CrossRef]
- Li, K.; Liang, M.; Wang, H.; Wang, X.; Huang, Y.; Coelho, J.; Pinilla, S.; Zhang, Y.; Qi, F.; Nicolosi, V.; et al. 3D MXene Architectures for Efficient Energy Storage and Conversion. Adv. Funct. Mater. 2020, 30, 2000842. [Google Scholar] [CrossRef]
- Wen, X.-H.; Zhao, X.-F.; Peng, B.-F.; Yuan, K.-P.; Li, X.-X.; Zhu, L.-Y.; Lu, H.-L. Facile preparation of an electrochemical aptasensor based on Au NPs/graphene sponge for detection of homocysteine. Appl. Surf. Sci. 2021, 556, 149735. [Google Scholar] [CrossRef]
- Idili, A.; Parolo, C.; Ortega, G.; Plaxco, K.W. Calibration-Free Measurement of Phenylalanine Levels in the Blood Using an Electrochemical Aptamer-Based Sensor Suitable for Point-of-Care Applications. ACS Sens. 2019, 4, 3227–3233. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Jin, H.; Sun, Y.; Jiang, X.; Gui, R. Mn-Doping-induced hierarchical petal growth of a flower-like 3D MOF assembled with black phosphorous nanosheets as an electrochemical aptasensor of human stress-induced phosphoprotein 1. Nanoscale 2020, 12, 14538–14548. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Li, Z.; Zhou, X.; Yang, G.; Yang, J.; Wang, H.; Wang, M.; Liang, C.; Wen, Y.; Lu, Y. Hybridization performance of DNA/mercaptohexanol mixed monolayers on electrodeposited nanoAu and rough Au surfaces. J. Electroanal. Chem. 2015, 757, 203–209. [Google Scholar] [CrossRef]
- Lawrence, N.S.; Deo, R.P.; Wang, J. Detection of homocysteine at carbon nanotube paste electrodes. Talanta 2004, 63, 443–449. [Google Scholar] [CrossRef]
- Beitollahi, H.; Zaimbashi, R.; Mahani, M.T.; Tajik, S. A label-free aptasensor for highly sensitive detection of homocysteine based on gold nanoparticles. Bioelectrochemistry 2020, 134, 107497. [Google Scholar] [CrossRef]
- Neufeld, G.; Cohen, T.; Gengrinovitch, S.; Poltorak, Z. Vascular endothelial growth factor (VEGF) and its receptors. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 1999, 13, 9–22. [Google Scholar] [CrossRef]
- Sullivan, L.A.; Brekken, R.A. The VEGF family in cancer and antibody-based strategies for their inhibition. MAbs 2010, 2, 165–175. [Google Scholar] [CrossRef]
- Aly, M.H.; Arafat, M.A.; Hussein, O.A.; Elsaid, H.H.; Abdel-Hammed, A.R. WITHDRAWN: Study of Angiopoietin-2 and vascular endothelial growth factor as markers of diabetic nephropathy onset in egyptians diabetic patients with non-albuminuric state. Diabetes Metab. Syndr. Clin. Res. Rev. 2019, 13, 1623–1627. [Google Scholar] [CrossRef] [PubMed]
- Bozal-Palabiyik, B.; Lettieri, M.; Uslu, B.; Marrazza, G. Electrochemical Detection of Vascular Endothelial Growth Factor by Molecularly Imprinted Polymer. Electroanalysis 2019, 31, 1458–1464. [Google Scholar] [CrossRef]
- Cheng, J.L.; Liu, X.P.; Chen, J.S.; Mao, C.J.; Jin, B.K. Highly sensitive electrochemiluminescence biosensor for VEGF(165) detection based on a g-C(3)N(4)/PDDA/CdSe nanocomposite. Anal. Bioanal. Chem. 2020, 412, 3073–3081. [Google Scholar] [CrossRef] [PubMed]
- Harris, R.C. The Role of the Epidermal Growth Factor Receptor in Diabetic Kidney Disease. Cells 2022, 11, 3416. [Google Scholar] [CrossRef]
- Li, N.; Nan, C.; Mei, X.; Sun, Y.; Feng, H.; Li, Y. Electrochemical sensor based on dual-template molecularly imprinted polymer and nanoporous gold leaf modified electrode for simultaneous determination of dopamine and uric acid. Mikrochim. Acta. 2020, 187, 496. [Google Scholar] [CrossRef]
- Johari-Ahar, M.; Karami, P.; Ghanei, M.; Afkhami, A.; Bagheri, H. Development of a molecularly imprinted polymer tailored on disposable screen-printed electrodes for dual detection of EGFR and VEGF using nano-liposomal amplification strategy. Biosens. Bioelectron. 2018, 107, 26–33. [Google Scholar] [CrossRef]
- Wang, J.-Y.; Xiao, L.; Wang, J.-Y. Posttranscriptional regulation of intestinal epithelial integrity by noncoding RNAs. Wiley Interdiscip. Rev. RNA 2017, 8, e1399. [Google Scholar] [CrossRef]
- Wang, J.; Chen, J.; Sen, S. MicroRNA as Biomarkers and Diagnostics. J. Cell Physiol. 2016, 231, 25–30. [Google Scholar] [CrossRef]
- Treiber, T.; Treiber, N.; Meister, G. Regulation of microRNA biogenesis and its crosstalk with other cellular pathways. Nat. Rev. Mol. Cell Biol. 2019, 20, 5–20. [Google Scholar] [CrossRef] [PubMed]
- Trionfini, P.; Benigni, A.; Remuzzi, G. MicroRNAs in kidney physiology and disease. Nat. Rev. Nephrol. 2015, 11, 23–33. [Google Scholar] [CrossRef] [PubMed]
- Parrizas, M.; Mundet, X.; Castano, C.; Canivell, S.; Cos, X.; Brugnara, L.; Giraldez-Garcia, C.; Regidor, E.; Mata-Cases, M.; Franch-Nadal, J.; et al. miR-10b and miR-223-3p in serum microvesicles signal progression from prediabetes to type 2 diabetes. J. Endocrinol. Investig. 2020, 43, 451–459. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Zhao, L.; Liang, D.; Zhang, X.; Song, W. An CuInS2 photocathode for the sensitive photoelectrochemical determination of microRNA-21 based on DNA-protein interaction and exonuclease III assisted target recycling amplification. Mikrochim. Acta. 2019, 186, 692. [Google Scholar] [CrossRef]
- Ma, Z.Y.; Ruan, Y.F.; Xu, F.; Zhao, W.W.; Xu, J.J.; Chen, H.Y. Protein Binding Bends the Gold Nanoparticle Capped DNA Sequence: Toward Novel Energy-Transfer-Based Photoelectrochemical Protein Detection. Anal. Chem. 2016, 88, 3864–3871. [Google Scholar] [CrossRef]
- Takuma, N.; Mihoko, H.; Masafumi, K.; Seiki, I. Increased urinary excretions of immunoglobulin g, ceruloplasmin, and transferrin predict development of microalbuminuria in patients with type 2 diabetes. Diabetes Care 2006, 29, 142–144. [Google Scholar]
- Narita, T.; Fujita, H.; Koshimura, J.; Meguro, H.; Kitazato, H.; Shimotomai, T.; Kagaya, E.; Suzuki, K.; Murata, M.; Usami, A.; et al. Glycemic control reverses increases in urinary excretions of immunoglobulin G and ceruloplasmin in type 2 diabetic patients with normoalbuminuria. Horm. Metab. Res. Horm. Und Stoffwechselforschung Horm. Metab. 2001, 33, 370–378. [Google Scholar] [CrossRef]
- Yamazaki, M.; Ito, S.; Usami, A.; Tani, N.; Hanyu, O.; Nakagawa, O.; Nakamura, H.; Shibata, A. Urinary excretion rate of ceruloplasmin in non-insulin-dependent diabetic patients with different stages of nephropathy. Eur. J. Endocrinol. 1995, 132, 681–687. [Google Scholar] [CrossRef]
- Haghshenas, E.; Madrakian, T.; Afkhami, A.; Saify Nabiabad, H. An electrochemical ceruloplasmin aptasensor using a glassy carbon electrode modified by diazonium-functionalized multiwalled carbon nanotubes. J. Iran. Chem. Soc. 2018, 16, 593–602. [Google Scholar] [CrossRef]
- Ocana, C.; Hayat, A.; Mishra, R.K.; Vasilescu, A.; Del Valle, M.; Marty, J.L. Label free aptasensor for Lysozyme detection: A comparison of the analytical performance of two aptamers. Bioelectrochemistry 2015, 105, 72–77. [Google Scholar] [CrossRef]
- Nancy, K.; Lipton, A. Platelets as a source of fibroblast growth-promoting activity. Exp. Cell Res. 1974, 87, 297–301. [Google Scholar]
- Hongquan, Z.; Xing-Fang, L.; Chris, L.X. Differentiation and detection of PDGF isomers and their receptors by tunable aptamer capillary electrophoresis. Anal. Chem. 2009, 81, 7795–7800. [Google Scholar]
- Pierce, G.F.; Tarpley, J.E.; Tseng, J.; Bready, J.; Chang, D.; Kenney, W.C.; Rudolph, R.; Robson, M.C.; Berg, J.V.; Reid, P. Detection of platelet-derived growth factor (PDGF)-AA in actively healing human wounds treated with recombinant PDGF-BB and absence of PDGF in chronic nonhealing wounds. J. Clin. Investig. 1995, 96, 1336–1350. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.J.; Cao, J.T.; Shi, G.F.; Huang, K.J.; Liu, Y.M.; Ren, S.W. A luminol electrochemiluminescence aptasensor based on glucose oxidase modified gold nanoparticles for measurement of platelet-derived growth factor BB. Talanta 2015, 132, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Huang, K.J.; Liu, Y.J.; Zhang, J.Z.; Cao, J.T.; Liu, Y.M. Aptamer/Au nanoparticles/cobalt sulfide nanosheets biosensor for 17beta-estradiol detection using a guanine-rich complementary DNA sequence for signal amplification. Biosens. Bioelectron. 2015, 67, 184–191. [Google Scholar] [CrossRef]
- Ge, Z.; Gu, H.; Li, Q.; Fan, C. Concept and Development of Framework Nucleic Acids. J. Am. Chem. Soc. 2018, 140, 17808–17819. [Google Scholar] [CrossRef]
- Campuzano, S.; Yáñez-Sedeño, P.; Pingarrón, J.M. Tailoring Sensitivity in Electrochemical Nucleic Acid Hybridization Biosensing: Role of Surface Chemistry and Labeling Strategies. ChemElectroChem 2019, 6, 60–72. [Google Scholar] [CrossRef]
- Ge, Z.; Fu, J.; Liu, M.; Jiang, S.; Andreoni, A.; Zuo, X.; Liu, Y.; Yan, H.; Fan, C. Constructing Submonolayer DNA Origami Scaffold on Gold Electrode for Wiring of Redox Enzymatic Cascade Pathways. ACS Appl. Mater. Interfaces 2019, 11, 13881–13887. [Google Scholar] [CrossRef]
- Lu, N.; Pei, H.; Ge, Z.; Simmons, C.R.; Yan, H.; Fan, C. Charge transport within a three-dimensional DNA nanostructure framework. J. Am. Chem. Soc. 2012, 134, 13148–13151. [Google Scholar] [CrossRef]
- Chen, X.; Zhou, G.; Song, P.; Wang, J.; Gao, J.; Lu, J.; Fan, C.; Zuo, X. Ultrasensitive electrochemical detection of prostate-specific antigen by using antibodies anchored on a DNA nanostructural scaffold. Anal. Chem. 2014, 86, 7337–7342. [Google Scholar] [CrossRef] [PubMed]
- Huang, K.J.; Liu, Y.J.; Zhai, Q.F. Ultrasensitive biosensing platform based on layered vanadium disulfide-graphene composites coupling with tetrahedron-structured DNA probes and exonuclease III assisted signal amplification. J. Mater. Chem. B 2015, 3, 8180–8187. [Google Scholar] [CrossRef] [PubMed]
- Ge, Z.; Lin, M.; Wang, P.; Pei, H.; Yan, J.; Shi, J.; Huang, Q.; He, D.; Fan, C.; Zuo, X. Hybridization Chain Reaction Amplification of MicroRNA Detection with a Tetrahedral DNA Nanostructure-Based Electrochemical Biosensor. Anal. Chem. 2014, 86, 2124–2130. [Google Scholar] [CrossRef] [PubMed]
- Abi, A.; Lin, M.; Pei, H.; Fan, C.; Ferapontova, E.E.; Zuo, X. Electrochemical switching with 3D DNA tetrahedral nanostructures self-assembled at gold electrodes. ACS Appl. Mater. Interfaces 2014, 6, 8928–8931. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tian, Y.; Gao, L.; Abdussalam, A.; Xu, G. Research Progress on Bionic Recognition and Biosensors for the Detection of Biomarkers of Diabetic Nephropathy. Chemosensors 2023, 11, 510. https://doi.org/10.3390/chemosensors11100510
Tian Y, Gao L, Abdussalam A, Xu G. Research Progress on Bionic Recognition and Biosensors for the Detection of Biomarkers of Diabetic Nephropathy. Chemosensors. 2023; 11(10):510. https://doi.org/10.3390/chemosensors11100510
Chicago/Turabian StyleTian, Ye, Lili Gao, Abubakar Abdussalam, and Guobao Xu. 2023. "Research Progress on Bionic Recognition and Biosensors for the Detection of Biomarkers of Diabetic Nephropathy" Chemosensors 11, no. 10: 510. https://doi.org/10.3390/chemosensors11100510





