Carbon Electrode Modified with Molecularly Imprinted Polymers for the Development of Electrochemical Sensor: Application to Pharmacy, Food Safety, Environmental Monitoring, and Biomedical Analysis
Abstract
:1. Introduction
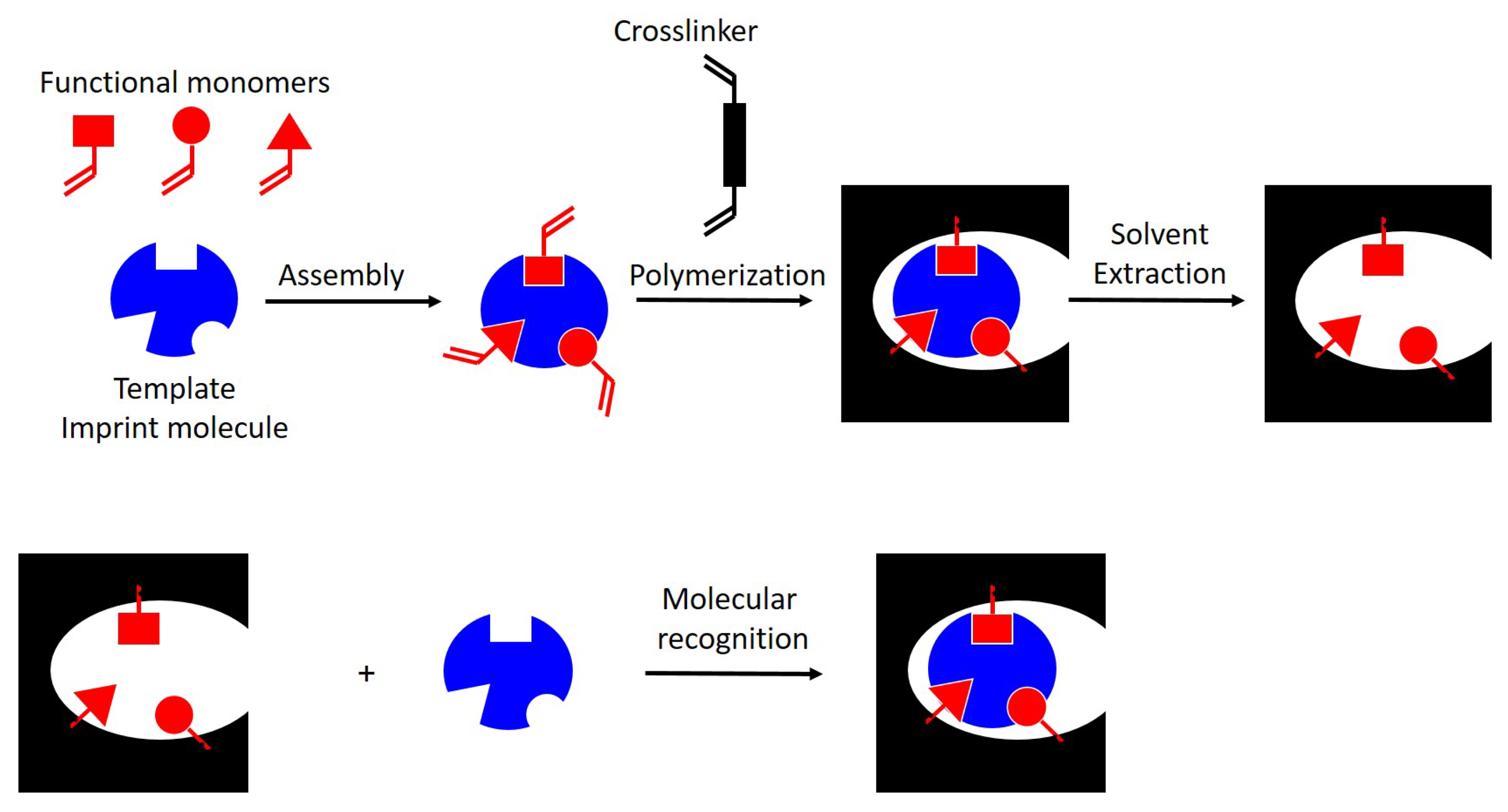
2. Molecularly Imprinted Polymers
3. Main Targets and Template
| Analyte | Analyte Family | Matrix * | Electrode | MIP Deposition Details | Deposition Method | Detection Method | Selectivity | LOD | Reference |
|---|---|---|---|---|---|---|---|---|---|
| Cytokine interleukin-1β | Protein | Human serum solution | SPCE | o-PD under-layer than electropolymerization with C2R | CV electropolymerization | EIS | Other proteins | 0.23 ng/L | [23] |
| Atorvastatin | Drug | Water | SPCE | Electropolymerization with 4-ABA | CV electropolymerization | DPV | Other statins | 0.56 µg/L | [26] |
| Genistein | Allergen marker | Food products | SPCE | Electropolymerization with o-PD | CV electropolymerization | DPV | Isoflavones and flavones | 100 µg/kg | [30] |
| Paracetamol | Drug | Plasma | SPCE | Covalent attachment of nanoMIPs after deposition of APTES | Chemical grafting | DPV | Caffeine, procainamide, or ethyl 4-aminobenzoate | 7.56 mg/L | [27] |
| Isoproturon | Herbicide | Groundwater sample | GCE | Electropolymerization with pyrrole | CV electropolymerization | SWV | Carbendazim, diuron, and carbamazepine | 2.2 µg/L | [33] |
| Tryptophan | AA, precursor of neurotransmitters | Human serum | MWCNTs | Drop-coating of an imprinted chitosan film | Acid polymerization | LSV | Ascorbic acid, dopamine, uric acid, and tyrosine | 204 ng/L | [35] |
| RDX | Explosive | Water | GCE | Drop-coating of MIP/MWCNTs mixture. MIP was prepared MAA as FM | Drop casting | DPV | TNT and HMX | 4.4 ng/L | [36] |
| Profenofos | Insecticide | Vegetable samples | CNTs | Grafting of SiO2 and vinyl end groups on the carboxylated CNTs, then thermal polymerization (grafting) of MIP | Thermal polymerization | CV | Carbofuran, chlopyrifos, hydroquinone, caffeine, phenol, MgSO4 and NaCl | 747 ng/L | [31] |
| Sunset Yellow | Synthetic dye | Candy, jelly powder, juice powder, and beverage | MWCNTs | Electropolymerization with acrylamide | CV electropolymerization | DPV | Tartrazine, erythrosine, indigo carmine, glucose, sucrose, and ascorbic acid | 2.26 µg/L | [37] |
| Sulfanilamide | Antibiotic | Buffer aqueous solution | GCE | Synthesis of MIP by precipitation polymerization in the presence of GO. Drop-coating of MIP/GO in a chitosan matrix | Drop casting | SWV | not studied | 10 mg/L | [38] |
| Creatinine | Disease marker | Buffer aqueous solution | GCE | Electropolymerization with aniline and methacrylic acid as bifunctional monomers in the presence of Ni nanoparticles | CV electropolymerization | DPV | Tyrosine, uric acid, dopamine, creatine, and ascorbic acid | 22.6 ng/L | [24] |
| HIV-p24 | Virus | Human serum | MWCNTs modified GCE | Grafting of HIV-P24 protein on a drop casted chitosan layer. Polymerization of the MIP using AAM as FM | RT polymerization | DPV | CEA, HCG, AFP, and BSA | 83 µg/L | [34] |
| Bisphenol A | Endocrine disruptor | Mineralized water and fresh milk | GCE | Functionalization of GO with APTES. Template immobilization. Grafting of EGDMA onto the APTES coated GO. MIP thermal polymerization. GO/APTES-MIP was immobilized on a GCE using chitosan | Drop casting | DPV | Estradiol, ethinyl estradiol, and phenol | 685 ng/L | [39] |
| Paracetamol | Drug | Pharmaceutical formulation | GCE | Nanocomposite: Oxidation of MWCNTs, then functionalization with VTMS, then MIP thermal polymerization using MAA as FM. Drop coating of the nanocomposite | Drop casting | SWV | Acetaminophen, hydroquinone, catechol, 3,4-Dihydroxy-L-phenylalanine, ascorbic acid, and uric acid | 166 µg/L | [40] |
| TBHQ, BHA | Synthetic antioxidant | Soybean oil, margarine, mayonnaise, and biodiesel | GCE | Nanocomposite: Oxidation of MWCNTs, then functionalization with VTMS, then MIP thermal polymerization using MAA as FM. Drop coating of the nanocomposite | Drop casting | DPV | L-ascorbic acid, epinephrine hydrochloride, butylated hydroxyanisole, catechol, dopamine hydrochloride, hydroquinone, acetaminophen, and propyl gallate | 90.1 µg/L for BHA and 141.3 µg/L for TBHQ | [41] |
| Chloramphenicol | Antibiotic | Milk and honey | CKM-3 and P-r-GO modified GCE | Synthesis of a MWCNTs@MIP thermal polymerization using 3-hexadecyl-1-vinylimidazoliumchloride as functional monomer. Coating of the MWCNTs@MIP on the modified GCE | Drop casting | DPV | Glucose, ascorbic acid, uric acid, and glutamic acid | 32.3 ng/L | [28] |
| Ganciclovir | Antiviral drug | Human serum | MWCNTs modified GCE | Electropolymerization with 2,2’-dithiodianiline as FM in the presence of Au nanoparticles | CV electropolymerization | DPV | Valganciclovir, aciclovir, valaciclovir, guanine, deoxyguanosine, aniline, and cysteine | 383 ng/L | [42] |
| Metronidazole | Antibiotic | Fish meat and pharmaceutical tablets | MWCNTs modified GCE | Electropolymerization with dopamine | CV electropolymerization | CV | Ronidazole, 4-nitroimidazole, 1,2-dimethylimidazole and dimetridazole | 49 ng/L | [43] |
| Methidathion | Insecticide | Waste water | SPCE | Thermal polymerization of bulk MIP using MBAA as FM. Drop casting of MIP@sol-gel/PEG on the surface of SPCE | Drop casting | EIS | Malathion, fenthion, parathion, and chlorfenvinphos | 5.14 µg/L | [32] |
| Metronidazole | Antibiotic, antiprotozoal | Milk and honey | MGCE | Synthesis of a sol-gel and magnetic MIP using APTES as functional monomer (Fe3O4@SiO2-MIP). Coating of Fe3O4@SiO2-MIP on MGCE | Attachment using magnetic force | DPSV | Ronidazole and dimetridazole | 2.74 µg/L | [29] |
| Sucrose | Table sugar | Sugar beet juices | MWCNTs modified GCE | Electropolymerization with o-phenylenediamine | CV electropolymerization | DPV | Raffinose, kestose, glucose, and fructose | 1 mg/L | [44] |
| Cholesterol | Disease marker | Hydro-alcoholic solution | CCE | Thermal polymerization MWCNT@MIP, graphite powder, and silicon alkoxide | Packing | LSV | Cholic acid and deoxycholic acid | 386 ng/L | [25] |
4. The Rational Selection of the Functional Monomer
5. Carbon-Based Electrodes
6. Functionalization Methods of the Carbon-Based Electrode with MIPs
6.1. Electropolymerization
6.2. Drop Casting
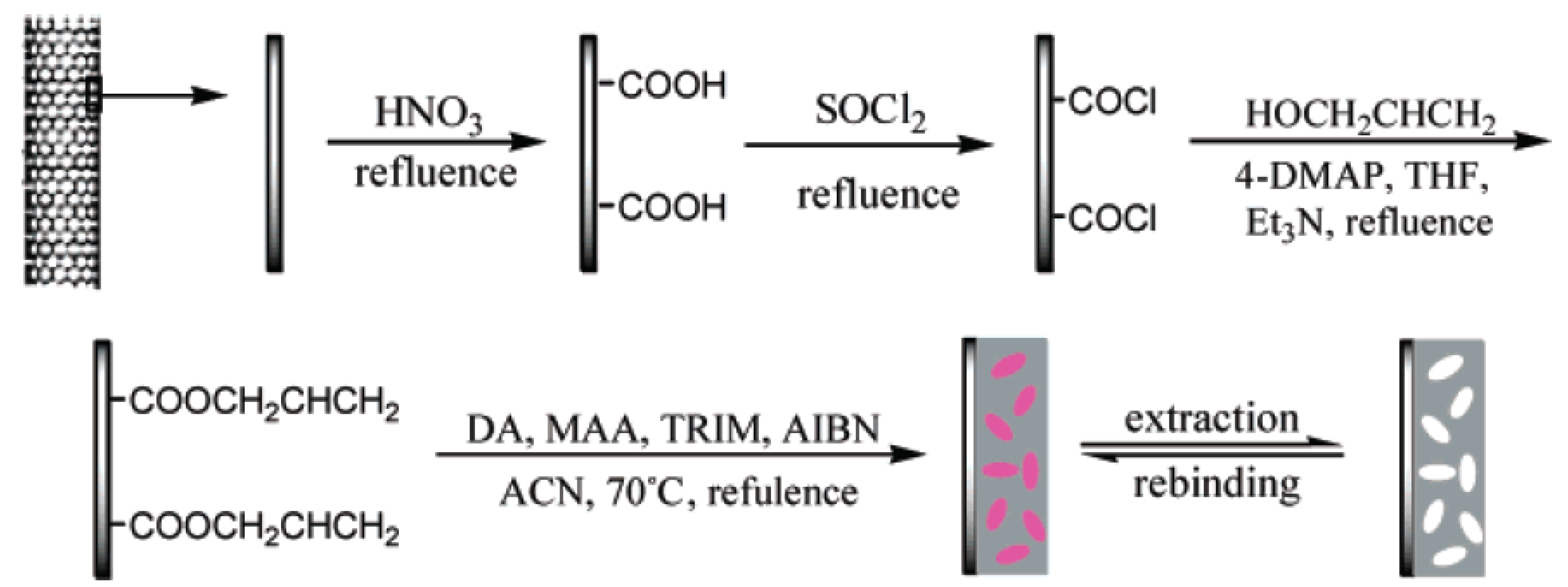
6.3. Chemical Grafting
6.4. Other Functionalization Methods
6.5. Comparison of Functionalization Methods
7. Electrochemical Detection Methods
8. Screen-Printed Carbon Electrode
9. Conclusions and Future Directions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| 4-ABA | 4-aminobenzoic acid |
| AA | Amino Acid |
| AAM | Acrylamide |
| AFP | Alpha fetal protein |
| APTES | 3-Aminopropyltriethoxysilane |
| BHA | Butylated hydroxyanisole |
| BSA | Bovine serum albumin |
| C2R | Chromotrope 2R |
| CA | Chronoamperometry |
| CCE | Ceramic carbon electrode |
| CEA | Carcinoembryonic antigen |
| CKM-3 | Mesoporous carbon |
| CNTs | Carbon nanotube |
| CV | Cyclic voltammetry |
| DMF | N,N-Dimethylformamide |
| DPSV | Differential pulse stripping voltammetry |
| EGDMA | Ethylene glycol dimethacrylate |
| EIS | Electrochemical impedance spectroscopy |
| FcMMA | Ferrocenyl-methylmethacrylate |
| FM | Functional monomer |
| GCE | Glassy carbon electrode |
| GO | Graphene oxide |
| HCG | Human chorionic gonadotropin |
| HIV | Human immune deficiency virus |
| HMX | 1,3,5,7-tetranitro-1,3,5,7-tetrazocane |
| LSV | Linear sweep voltammetry |
| MAA | Methacrylic acid |
| MBAA | N-methylenebisacrylamide |
| MDMA | 3,4-methylenedioxymethamphetamine |
| MGCE | Magnetic glassy carbon electrode |
| MWCNT | Multi-walled carbon nanotube |
| o-PD | O-phenylenediamine |
| PEG | Poly ethylene glycol |
| PETN | Pentaerythritol tetranitrate |
| P-r-GO | 3-dimensional porous graphene |
| RDX | Trinitroperhydro-1,3,5-triazine |
| RT | Room temperature |
| SPCE | Screen printed carbon electrode |
| SWCNT | Single-walled carbon nanotube |
| SWV | Square wave voltammetry |
| TBHQ | Tert-butylhydroquinone |
| TNT | 2,4,6-trinitrotoluene |
| TRIM | Trimethylolpropane trimethacrylate |
| VTMS | Vinyltrimethoxysilane |
References
- Nekrasov, N.; Jaric, S.; Kireev, D.; Emelianov, A.V.; Orlov, A.V.; Gadjanski, I.; Nikitin, P.I.; Akinwande, D.; Bobrinetskiy, I. Real-Time Detection of Ochratoxin A in Wine through Insight of Aptamer Conformation in Conjunction with Graphene Field-Effect Transistor. Biosens. Bioelectron. 2022, 200, 113890. [Google Scholar] [CrossRef]
- Yang, T.; Huang, H.; Zhu, F.; Lin, Q.; Zhang, L.; Liu, J. Recent Progresses in Nanobiosensing for Food Safety Analysis. Sensors 2016, 16, 1118. [Google Scholar] [CrossRef] [PubMed]
- Adumitrăchioaie, A.; Tertiș, M.; Cernat, A.; Săndulescu, R.; Cristea, C. Electrochemical Methods Based on Molecularly Imprinted Polymers for Drug Detection. A Review. Int. J. Electrochem. Sci. 2018, 13, 2556–2576. [Google Scholar] [CrossRef]
- Rebollar-Pérez, G.; Campos-Terán, J.; Ornelas-Soto, N.; Méndez-Albores, A.; Torres, E. Biosensors Based on Oxidative Enzymes for Detection of Environmental Pollutants. Biocatalysis 2016, 1, 118–129. [Google Scholar] [CrossRef]
- Staden, R.-I.S. Perspective—Challenges in Biomedical Analysis: From Classical Sensors to Stochastic Sensors. ECS Sens. Plus 2022, 1, 011603. [Google Scholar] [CrossRef]
- Leva-Bueno, J.; Peyman, S.A.; Millner, P.A. A Review on Impedimetric Immunosensors for Pathogen and Biomarker Detection. Med. Microbiol. Immunol. 2020, 209, 343–362. [Google Scholar] [CrossRef] [PubMed]
- Kharajinezhadian, R.; Javad Chaichi, M.; Nazari, O.; Mansour Lakouraj, M.; Hasantabar, V. Fraud Monitoring Using a New Disposable Photoluminescence Sensor in Milk. Microchem. J. 2023, 189, 108437. [Google Scholar] [CrossRef]
- Apak, R.; Üzer, A.; Sağlam, Ş.; Arman, A. Selective Electrochemical Detection of Explosives with Nanomaterial Based Electrodes. Electroanalysis 2023, 35, e202200175. [Google Scholar] [CrossRef]
- Karimi-Maleh, H.; Karimi, F.; Alizadeh, M.; Sanati, A.L. Electrochemical Sensors, a Bright Future in the Fabrication of Portable Kits in Analytical Systems. Chem. Rec. 2020, 20, 682–692. [Google Scholar] [CrossRef]
- Adarakatti, P.S.; Kempahanumakkagari, S.K. Modified Electrodes for Sensing. In Electrochemistry; Banks, C., McIntosh, S., Eds.; Royal Society of Chemistry: Cambridge, UK, 2018; Volume 15, pp. 58–95. ISBN 978-1-78801-373-4. [Google Scholar]
- Ayerdurai, V.; Cieplak, M.; Kutner, W. Molecularly Imprinted Polymer-Based Electrochemical Sensors for Food Contaminants Determination. TrAC Trends Anal. Chem. 2023, 158, 116830. [Google Scholar] [CrossRef]
- Karimi-Maleh, H.; Beitollahi, H.; Senthil Kumar, P.; Tajik, S.; Mohammadzadeh Jahani, P.; Karimi, F.; Karaman, C.; Vasseghian, Y.; Baghayeri, M.; Rouhi, J.; et al. Recent Advances in Carbon Nanomaterials-Based Electrochemical Sensors for Food Azo Dyes Detection. Food Chem. Toxicol. 2022, 164, 112961. [Google Scholar] [CrossRef] [PubMed]
- Kumar Singh, A.; Agrahari, S.; Kumar Gautam, R.; Tiwari, I. Fabrication of a Novel Screen-Printed Carbon Electrode Based Disposable Sensor for Sensitive Determination of an Endocrine Disruptor BPSIP in Environmental and Biological Matrices. Microchem. J. 2023, 193, 109031. [Google Scholar] [CrossRef]
- Nicholls, I.A.; Andersson, H.S.; Charlton, C.; Henschel, H.; Karlsson, B.C.G.; Karlsson, J.G.; O’Mahony, J.; Rosengren, A.M.; Rosengren, K.J.; Wikman, S. Theoretical and Computational Strategies for Rational Molecularly Imprinted Polymer Design. Biosens. Bioelectron. 2009, 25, 543–552. [Google Scholar] [CrossRef]
- Haupt, K. Molecularly Imprinted Polymers in Analytical Chemistry. Analyst 2001, 126, 747–756. [Google Scholar] [CrossRef] [PubMed]
- Cormack, P.A.G.; Elorza, A.Z. Molecularly Imprinted Polymers: Synthesis and Characterisation. J. Chromatogr. B Anal. Technol. Biomed Life Sci. 2004, 804, 173–182. [Google Scholar] [CrossRef] [PubMed]
- Mijangos, I.; Navarro-Villoslada, F.; Guerreiro, A.; Piletska, E.; Chianella, I.; Karim, K.; Turner, A.; Piletsky, S. Influence of Initiator and Different Polymerisation Conditions on Performance of Molecularly Imprinted Polymers. Biosens. Bioelectron. 2006, 22, 381–387. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.; Row, K.H. Characteristic and Synthetic Approach of Molecularly Imprinted Polymer. Int. J. Mol. Sci. 2006, 7, 155–178. [Google Scholar] [CrossRef]
- Séverin, I.; Lionti, K.; Dahbi, L.; Loriot, C.; Toury, B.; Chagnon, M.-C. In Vitro Toxicity Assessment of Extracts Derived from Sol–Gel Coatings on Polycarbonate Intended to Be Used in Food Contact Applications. Food Chem. Toxicol. 2016, 93, 51–57. [Google Scholar] [CrossRef]
- Bitar, M.; Lafarge, C.; Sok, N.; Cayot, P.; Bou-Maroun, E. Molecularly Imprinted Sol-Gel Polymers for the Analysis of Iprodione Fungicide in Wine: Synthesis in Green Solvent. Food Chem. 2019, 293, 226–232. [Google Scholar] [CrossRef]
- Mamo, S.K.; Gonzalez-Rodriguez, J. Development of a Molecularly Imprinted Polymer-Based Sensor for the Electrochemical Determination of Triacetone Triperoxide (TATP). Sensors 2014, 14, 23269–23282. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; Du, X.; Sun, M.; Zhang, Y.; Li, Y.; Wang, X.; Wang, Y.; Du, H.; Yin, H.; Rao, H. Novel Dual-Template Molecular Imprinted Electrochemical Sensor for Simultaneous Detection of CA and TPH Based on Peanut Twin-like NiFe2O4/CoFe2O4/NCDs Nanospheres: Fabrication, Application and DFT Theoretical Study. Biosens. Bioelectron. 2021, 190, 113408. [Google Scholar] [CrossRef] [PubMed]
- Choi, D.Y.; Yang, J.C.; Hong, S.W.; Park, J. Molecularly Imprinted Polymer-Based Electrochemical Impedimetric Sensors on Screen-Printed Carbon Electrodes for the Detection of Trace Cytokine IL-1β. Biosens. Bioelectron. 2022, 204, 114073. [Google Scholar] [CrossRef] [PubMed]
- Rao, H.; Lu, Z.; Ge, H.; Liu, X.; Chen, B.; Zou, P.; Wang, X.; He, H.; Zeng, X.; Wang, Y. Electrochemical Creatinine Sensor Based on a Glassy Carbon Electrode Modified with a Molecularly Imprinted Polymer and a Ni@polyaniline Nanocomposite. Microchim. Acta 2017, 184, 261–269. [Google Scholar] [CrossRef]
- Tong, Y.; Li, H.; Guan, H.; Zhao, J.; Majeed, S.; Anjum, S.; Liang, F.; Xu, G. Electrochemical Cholesterol Sensor Based on Carbon Nanotube@molecularly Imprinted Polymer Modified Ceramic Carbon Electrode. Biosens. Bioelectron. 2013, 47, 553–558. [Google Scholar] [CrossRef]
- Rebelo, P.; Pacheco, J.G.; Voroshylova, I.V.; Melo, A.; Cordeiro, M.N.D.S.; Delerue-Matos, C. A Simple Electrochemical Detection of Atorvastatin Based on Disposable Screen-Printed Carbon Electrodes Modified by Molecularly Imprinted Polymer: Experiment and Simulation. Anal. Chim. Acta 2022, 1194, 339410. [Google Scholar] [CrossRef]
- Alanazi, K.; Garcia Cruz, A.; Di Masi, S.; Voorhaar, A.; Ahmad, O.S.; Cowen, T.; Piletska, E.; Langford, N.; Coats, T.J.; Sims, M.R.; et al. Disposable Paracetamol Sensor Based on Electroactive Molecularly Imprinted Polymer Nanoparticles for Plasma Monitoring. Sens. Actuators B Chem. 2021, 329, 129128. [Google Scholar] [CrossRef]
- Yang, G.; Zhao, F. Electrochemical Sensor for Chloramphenicol Based on Novel Multiwalled Carbon Nanotubes@molecularly Imprinted Polymer. Biosens. Bioelectron. 2015, 64, 416–422. [Google Scholar] [CrossRef]
- Chen, D.; Deng, J.; Liang, J.; Xie, J.; Hu, C.; Huang, K. A Core–Shell Molecularly Imprinted Polymer Grafted onto a Magnetic Glassy Carbon Electrode as a Selective Sensor for the Determination of Metronidazole. Sens. Actuators B Chem. 2013, 183, 594–600. [Google Scholar] [CrossRef]
- Sundhoro, M.; Agnihotra, S.R.; Amberger, B.; Augustus, K.; Khan, N.D.; Barnes, A.; BelBruno, J.; Mendecki, L. An Electrochemical Molecularly Imprinted Polymer Sensor for Rapid and Selective Food Allergen Detection. Food Chem. 2021, 344, 128648. [Google Scholar] [CrossRef] [PubMed]
- Amatatongchai, M.; Sroysee, W.; Sodkrathok, P.; Kesangam, N.; Chairam, S.; Jarujamrus, P. Novel Three-Dimensional Molecularly Imprinted Polymer-Coated Carbon Nanotubes (3D-CNTs@MIP) for Selective Detection of Profenofos in Food. Anal. Chim. Acta 2019, 1076, 64–72. [Google Scholar] [CrossRef]
- Bakas, I.; Hayat, A.; Piletsky, S.; Piletska, E.; Chehimi, M.M.; Noguer, T.; Rouillon, R. Electrochemical Impedimetric Sensor Based on Molecularly Imprinted Polymers/Sol–Gel Chemistry for Methidathion Organophosphorous Insecticide Recognition. Talanta 2014, 130, 294–298. [Google Scholar] [CrossRef]
- Sadriu, I.; Bouden, S.; Nicolle, J.; Podvorica, F.I.; Bertagna, V.; Berho, C.; Amalric, L.; Vautrin-Ul, C. Molecularly Imprinted Polymer Modified Glassy Carbon Electrodes for the Electrochemical Analysis of Isoproturon in Water. Talanta 2020, 207, 120222. [Google Scholar] [CrossRef]
- Ma, Y.; Shen, X.-L.; Zeng, Q.; Wang, H.-S.; Wang, L.-S. A Multi-Walled Carbon Nanotubes Based Molecularly Imprinted Polymers Electrochemical Sensor for the Sensitive Determination of HIV-P24. Talanta 2017, 164, 121–127. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Deng, P.; Tian, Y.; Ding, Z.; Li, G.; Liu, J.; Zuberi, Z.; He, Q. Rapid Recognition and Determination of Tryptophan by Carbon Nanotubes and Molecularly Imprinted Polymer-Modified Glassy Carbon Electrode. Bioelectrochemistry 2020, 131, 107393. [Google Scholar] [CrossRef] [PubMed]
- Alizadeh, T.; Atashi, F.; Ganjali, M.R. Molecularly Imprinted Polymer Nano-Sphere/Multi-Walled Carbon Nanotube Coated Glassy Carbon Electrode as an Ultra-Sensitive Voltammetric Sensor for Picomolar Level Determination of RDX. Talanta 2019, 194, 415–421. [Google Scholar] [CrossRef]
- Arvand, M.; Zamani, M.; Sayyar Ardaki, M. Rapid Electrochemical Synthesis of Molecularly Imprinted Polymers on Functionalized Multi-Walled Carbon Nanotubes for Selective Recognition of Sunset Yellow in Food Samples. Sens. Actuators B Chem. 2017, 243, 927–939. [Google Scholar] [CrossRef]
- Wei, X.; Xu, X.; Qi, W.; Wu, Y.; Wang, L. Molecularly Imprinted Polymer/Graphene Oxide Modified Glassy Carbon Electrode for Selective Detection of Sulfanilamide. Prog. Nat. Sci. Mater. Int. 2017, 27, 374–379. [Google Scholar] [CrossRef]
- Dadkhah, S.; Ziaei, E.; Mehdinia, A.; Baradaran Kayyal, T.; Jabbari, A. A Glassy Carbon Electrode Modified with Amino-Functionalized Graphene Oxide and Molecularly Imprinted Polymer for Electrochemical Sensing of Bisphenol A. Microchim. Acta 2016, 183, 1933–1941. [Google Scholar] [CrossRef]
- dos Santos Moretti, E.; de Fátima Giarola, J.; Kuceki, M.; Prete, M.C.; Pereira, A.C.; Tarley, C.R.T. A Nanocomposite Based on Multi-Walled Carbon Nanotubes Grafted by Molecularly Imprinted Poly(Methacrylic Acid–Hemin) as a Peroxidase-like Catalyst for Biomimetic Sensing of Acetaminophen. RSC Adv. 2016, 6, 28751–28760. [Google Scholar] [CrossRef]
- dos Santos Moretti, E.; de Oliveira, F.M.; Scheel, G.L.; DalĺAntônia, L.H.; Borsato, D.; Kubota, L.T.; Segatelli, M.G.; Tarley, C.R.T. Synthesis of Surface Molecularly Imprinted Poly(Methacrylic Acid-Hemin) on Carbon Nanotubes for the Voltammetric Simultaneous Determination of Antioxidants from Lipid Matrices and Biodiesel. Electrochim. Acta 2016, 212, 322–332. [Google Scholar] [CrossRef]
- Gholivand, M.B.; Karimian, N. Fabrication of a Highly Selective and Sensitive Voltammetric Ganciclovir Sensor Based on Electropolymerized Molecularly Imprinted Polymer and Gold Nanoparticles on Multiwall Carbon Nanotubes/Glassy Carbon Electrode. Sens. Actuators B Chem. 2015, 215, 471–479. [Google Scholar] [CrossRef]
- Yuan, L.; Jiang, L.; Hui, T.; Jie, L.; Bingbin, X.; Feng, Y.; Yingchun, L. Fabrication of Highly Sensitive and Selective Electrochemical Sensor by Using Optimized Molecularly Imprinted Polymers on Multi-Walled Carbon Nanotubes for Metronidazole Measurement. Sens. Actuators B Chem. 2015, 206, 647–652. [Google Scholar] [CrossRef]
- Shekarchizadeh, H.; Ensafi, A.A.; Kadivar, M. Selective Determination of Sucrose Based on Electropolymerized Molecularly Imprinted Polymer Modified Multiwall Carbon Nanotubes/Glassy Carbon Electrode. Mater. Sci. Eng. C 2013, 33, 3553–3561. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Li, G.; Zhang, M. A Novel Sensor Based on Bifunctional Monomer Molecularly Imprinted Film at Graphene Modified Glassy Carbon Electrode for Detecting Traces of Moxifloxacin. RSC Adv. 2016, 6, 32915–32921. [Google Scholar] [CrossRef]
- Dechtrirat, D.; Sookcharoenpinyo, B.; Prajongtat, P.; Sriprachuabwong, C.; Sanguankiat, A.; Tuantranont, A.; Hannongbua, S. An Electrochemical MIP Sensor for Selective Detection of Salbutamol Based on a Graphene/PEDOT:PSS Modified Screen Printed Carbon Electrode. RSC Adv. 2017, 8, 206–212. [Google Scholar] [CrossRef]
- Dong, C.; Li, X.; Guo, Z.; Qi, J. Development of a Model for the Rational Design of Molecular Imprinted Polymer: Computational Approach for Combined Molecular Dynamics/Quantum Mechanics Calculations. Anal. Chim. Acta 2009, 647, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Xu, Z.; Wang, D.; Yang, Y.; Duan, Y.; Ma, L.; Lin, T.; Liu, H. A Review on Molecularly Imprinted Polymers Preparation by Computational Simulation-Aided Methods. Polymers 2021, 13, 2657. [Google Scholar] [CrossRef] [PubMed]
- Xie, L.; Xiao, N.; Li, L.; Xie, X.; Li, Y. An Investigation of the Intermolecular Interactions and Recognition Properties of Molecular Imprinted Polymers for Deltamethrin through Computational Strategies. Polymers 2019, 11, 1872. [Google Scholar] [CrossRef]
- Terracina, J.J.; Bergkvist, M.; Sharfstein, S.T. Computational Investigation of Stoichiometric Effects, Binding Site Heterogeneities, and Selectivities of Molecularly Imprinted Polymers. J. Mol. Model. 2016, 22, 139. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Liu, J.; Tang, S.; Jin, R. Theoretical Research of Molecular Imprinted Polymers Formed from Formaldehyde and Methacrylic Acid. J. Mol. Model. 2020, 26, 88. [Google Scholar] [CrossRef]
- Bitar, M.; Bou-Maroun, E.; Lerbret, A.; Ouaini, N.; Cayot, P. Binding Characteristics of Molecularly Imprinted Polymers Based on Fungicides in Hydroalcoholic Media. J. Sep. Sci. 2015, 38, 3607–3614. [Google Scholar] [CrossRef]
- He, Q.; Liang, J.-J.; Chen, L.-X.; Chen, S.-L.; Zheng, H.-L.; Liu, H.-X.; Zhang, H.-J. Removal of the Environmental Pollutant Carbamazepine Using Molecular Imprinted Adsorbents: Molecular Simulation, Adsorption Properties, and Mechanisms. Water Res. 2020, 168, 115164. [Google Scholar] [CrossRef]
- Ao, J.; Gu, J.; Yuan, T.; Li, D.; Ma, Y.; Shen, Z. Applying Molecular Modelling and Experimental Studies to Develop Molecularly Imprinted Polymer for Domoic Acid Enrichment from Both Seawater and Shellfish. Chemosphere 2018, 199, 98–106. [Google Scholar] [CrossRef] [PubMed]
- Xiong, H.; Zhao, Y.; Liu, P.; Zhang, X.; Wang, S. Electrochemical Properties and the Determination of Nicotine at a Multi-Walled Carbon Nanotubes Modified Glassy Carbon Electrode. Microchim. Acta 2010, 168, 31–36. [Google Scholar] [CrossRef]
- Smart, A.; Crew, A.; Pemberton, R.; Hughes, G.; Doran, O.; Hart, J.P. Screen-Printed Carbon Based Biosensors and Their Applications in Agri-Food Safety. TrAC Trends Anal. Chem. 2020, 127, 115898. [Google Scholar] [CrossRef]
- Crapnell, R.D.; Dempsey-Hibbert, N.C.; Peeters, M.; Tridente, A.; Banks, C.E. Molecularly Imprinted Polymer Based Electrochemical Biosensors: Overcoming the Challenges of Detecting Vital Biomarkers and Speeding up Diagnosis. Talanta Open 2020, 2, 100018. [Google Scholar] [CrossRef]
- Wang, Q.; Paim, L.L.; Zhang, X.; Wang, S.; Stradiotto, N.R. An Electrochemical Sensor for Reducing Sugars Based on a Glassy Carbon Electrode Modified with Electropolymerized Molecularly Imprinted Poly-o-Phenylenediamine Film. Electroanalysis 2014, 26, 1612–1622. [Google Scholar] [CrossRef]
- Rayanasukha, Y.; Pratontep, S.; Porntheeraphat, S.; Bunjongpru, W.; Nukeaw, J. Non-Enzymatic Urea Sensor Using Molecularly Imprinted Polymers Surface Modified Based-on Ion-Sensitive Field Effect Transistor (ISFET). Surf. Coat. Technol. 2016, 306, 147–150. [Google Scholar] [CrossRef]
- Karrat, A.; Lamaoui, A.; Amine, A.; Palacios-Santander, J.M.; Cubillana-Aguilera, L. Applications of Chitosan in Molecularly and Ion Imprinted Polymers. Chem. Afr. 2020, 3, 513–533. [Google Scholar] [CrossRef]
- Kan, X.; Zhao, Y.; Geng, Z.; Wang, Z.; Zhu, J.-J. Composites of Multiwalled Carbon Nanotubes and Molecularly Imprinted Polymers for Dopamine Recognition. J. Phys. Chem. C 2008, 112, 4849–4854. [Google Scholar] [CrossRef]
- Dourandish, Z.; Beitollahi, H. Electrochemical Sensing of Isoproterenol Using Graphite Screen-Printed Electrode Modified with Graphene Quantum Dots. Anal. Bioanal. Electrochem. 2018, 10, 192–202. [Google Scholar]
- Sharma, A.; Arya, S.; Chauhan, D.; Solanki, P.R.; Khajuria, S.; Khosla, A. Synthesis of Au–SnO2 Nanoparticles for Electrochemical Determination of Vitamin B12. J. Mater. Res. Technol. 2020, 9, 14321–14337. [Google Scholar] [CrossRef]
- Rai, V.; Deng, J.; Toh, C.-S. Electrochemical Nanoporous Alumina Membrane-Based Label-Free DNA Biosensor for the Detection of Legionella Sp. Talanta 2012, 98, 112–117. [Google Scholar] [CrossRef] [PubMed]
- Magar, H.S.; Hassan, R.Y.A.; Mulchandani, A. Electrochemical Impedance Spectroscopy (EIS): Principles, Construction, and Biosensing Applications. Sensors 2021, 21, 6578. [Google Scholar] [CrossRef] [PubMed]
- Lisdat, F.; Schäfer, D. The Use of Electrochemical Impedance Spectroscopy for Biosensing. Anal. Bioanal. Chem. 2008, 391, 1555–1567. [Google Scholar] [CrossRef] [PubMed]
- ErtuğruL, H.D.; Uygun, Z.O.; ErtuğruL, H.D.; Uygun, Z.O. Impedimetric Biosensors for Label-Free and Enzymless Detection. In State of the Art in Biosensors—General Aspects; IntechOpen: London, UK, 2013; ISBN 978-953-51-1004-0. [Google Scholar]
- Laschuk, N.O.; Easton, E.B.; Zenkina, O.V. Reducing the Resistance for the Use of Electrochemical Impedance Spectroscopy Analysis in Materials Chemistry. RSC Adv. 2021, 11, 27925–27936. [Google Scholar] [CrossRef] [PubMed]
- Skalová, Š.; Vyskočil, V.; Barek, J.; Navrátil, T. Model Biological Membranes and Possibilities of Application of Electrochemical Impedance Spectroscopy for Their Characterization. Electroanalysis 2018, 30, 207–219. [Google Scholar] [CrossRef]
- Martins, J.C.; Neto, J.C.D.M.; Passos, R.R.; Pocrifka, L.A. Electrochemical Behavior of Polyaniline: A Study by Electrochemical Impedance Spectroscopy (EIS) in Low-Frequency. Solid State Ion. 2020, 346, 115198. [Google Scholar] [CrossRef]
- Washe, A.P.; Lozano-Sánchez, P.; Bejarano-Nosas, D.; Katakis, I. Facile and Versatile Approaches to Enhancing Electrochemical Performance of Screen Printed Electrodes. Electrochim. Acta 2013, 91, 166–172. [Google Scholar] [CrossRef]
- Mazzaracchio, V.; Tomei, M.R.; Cacciotti, I.; Chiodoni, A.; Novara, C.; Castellino, M.; Scordo, G.; Amine, A.; Moscone, D.; Arduini, F. Inside the Different Types of Carbon Black as Nanomodifiers for Screen-Printed Electrodes. Electrochim. Acta 2019, 317, 673–683. [Google Scholar] [CrossRef]
- Niu, X.; Chen, C.; Zhao, H.; Tang, J.; Li, Y.; Lan, M. Porous Screen-Printed Carbon Electrode. Electrochem. Commun. 2012, 22, 170–173. [Google Scholar] [CrossRef]
- Araújo, D.A.G.; Camargo, J.R.; Pradela-Filho, L.A.; Lima, A.P.; Muñoz, R.A.A.; Takeuchi, R.M.; Janegitz, B.C.; Santos, A.L. A Lab-Made Screen-Printed Electrode as a Platform to Study the Effect of the Size and Functionalization of Carbon Nanotubes on the Voltammetric Determination of Caffeic Acid. Microchem. J. 2020, 158, 105297. [Google Scholar] [CrossRef]
- Pellicer, C.; Gomez-Caballero, A.; Unceta, N.; Goicolea, M.A.; Barrio, R.J. Using a Portable Device Based on a Screen-Printed Sensor Modified with a Molecularly Imprinted Polymer for the Determination of the Insecticide Fenitrothion in Forest Samples. Anal. Methods 2010, 2, 1280–1285. [Google Scholar] [CrossRef]
- Kumar, D.; Prasad, B.B. Multiwalled Carbon Nanotubes Embedded Molecularly Imprinted Polymer-Modified Screen Printed Carbon Electrode for the Quantitative Analysis of C-Reactive Protein. Sens. Actuators B Chem. 2012, 171–172, 1141–1150. [Google Scholar] [CrossRef]
- Radi, A.-E.; El-Naggar, A.-E.; Nassef, H.M. Molecularly Imprinted Polymer Based Electrochemical Sensor for the Determination of the Anthelmintic Drug Oxfendazole. J. Electroanal. Chem. 2014, 729, 135–141. [Google Scholar] [CrossRef]
- Lopes, F.; Pacheco, J.G.; Rebelo, P.; Delerue-Matos, C. Molecularly Imprinted Electrochemical Sensor Prepared on a Screen Printed Carbon Electrode for Naloxone Detection. Sens. Actuators B Chem. 2017, 243, 745–752. [Google Scholar] [CrossRef]
- Ekomo, V.M.; Branger, C.; Bikanga, R.; Florea, A.-M.; Istamboulie, G.; Calas-Blanchard, C.; Noguer, T.; Sarbu, A.; Brisset, H. Detection of Bisphenol A in Aqueous Medium by Screen Printed Carbon Electrodes Incorporating Electrochemical Molecularly Imprinted Polymers. Biosens. Bioelectron. 2018, 112, 156–161. [Google Scholar] [CrossRef]
- Pacheco, J.G.; Rebelo, P.; Cagide, F.; Gonçalves, L.M.; Borges, F.; Rodrigues, J.A.; Delerue-Matos, C. Electrochemical Sensing of the Thyroid Hormone Thyronamine (T0AM) via Molecular Imprinted Polymers (MIPs). Talanta 2019, 194, 689–696. [Google Scholar] [CrossRef]
- Couto, R.A.S.; Costa, S.S.; Mounssef, B.; Pacheco, J.G.; Fernandes, E.; Carvalho, F.; Rodrigues, C.M.P.; Delerue-Matos, C.; Braga, A.A.C.; Moreira Gonçalves, L.; et al. Electrochemical Sensing of Ecstasy with Electropolymerized Molecularly Imprinted Poly(o-Phenylenediamine) Polymer on the Surface of Disposable Screen-Printed Carbon Electrodes. Sens. Actuators B Chem. 2019, 290, 378–386. [Google Scholar] [CrossRef]
- Moro, G.; Bottari, F.; Sleegers, N.; Florea, A.; Cowen, T.; Moretto, L.M.; Piletsky, S.; De Wael, K. Conductive Imprinted Polymers for the Direct Electrochemical Detection of β-Lactam Antibiotics: The Case of Cefquinome. Sens. Actuators B Chem. 2019, 297, 126786. [Google Scholar] [CrossRef]
- Roushani, M.; Jalilian, Z.; Nezhadali, A. Screen Printed Carbon Electrode Sensor with Thiol Graphene Quantum Dots and Gold Nanoparticles for Voltammetric Determination of Solatol. Heliyon 2019, 5, e01984. [Google Scholar] [CrossRef]
- Khosrokhavar, R.; Motaharian, A.; Milani Hosseini, M.R.; Mohammadsadegh, S. Screen-Printed Carbon Electrode (SPCE) Modified by Molecularly Imprinted Polymer (MIP) Nanoparticles and Graphene Nanosheets for Determination of Sertraline Antidepressant Drug. Microchem. J. 2020, 159, 105348. [Google Scholar] [CrossRef]
- Rebelo, P.; Pacheco, J.G.; Cordeiro, M.N.D.S.; Melo, A.; Delerue-Matos, C. Azithromycin Electrochemical Detection Using a Molecularly Imprinted Polymer Prepared on a Disposable Screen-Printed Electrode. Anal. Methods 2020, 12, 1486–1494. [Google Scholar] [CrossRef]
- Abo-Elmagd, I.F.; Mahmoud, A.M.; Al-Ghobashy, M.A.; Nebsen, M.; El Sayed, N.S.; Nofal, S.; Soror, S.H.; Todd, R.; Elgebaly, S.A. Impedimetric Sensors for Cyclocreatine Phosphate Determination in Plasma Based on Electropolymerized Poly(o-Phenylenediamine) Molecularly Imprinted Polymers. ACS Omega 2021, 6, 31282–31291. [Google Scholar] [CrossRef] [PubMed]
- Ben Hassine, A.; Raouafi, N.; Moreira, F.T.C. Novel Electrochemical Molecularly Imprinted Polymer-Based Biosensor for Tau Protein Detection. Chemosensors 2021, 9, 238. [Google Scholar] [CrossRef]
- Jesadabundit, W.; Jampasa, S.; Patarakul, K.; Siangproh, W.; Chailapakul, O. Enzyme-Free Impedimetric Biosensor-Based Molecularly Imprinted Polymer for Selective Determination of L-Hydroxyproline. Biosens. Bioelectron. 2021, 191, 113387. [Google Scholar] [CrossRef] [PubMed]
- Leepheng, P.; Limthin, D.; Onlaor, K.; Tunhoo, B.; Thiwawong, T.; Suramitr, S.; Phromyothin, D. Selective Electrochemical Determination Based on Magnetic Molecularly Imprinted Polymers for Albumin Detection. Jpn. J. Appl. Phys. 2022, 61, SD1009. [Google Scholar] [CrossRef]
- Seguro, I.; Rebelo, P.; Pacheco, J.G.; Delerue-Matos, C. Electropolymerized, Molecularly Imprinted Polymer on a Screen-Printed Electrode—A Simple, Fast, and Disposable Voltammetric Sensor for Trazodone. Sensors 2022, 22, 2819. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Catanante, G.; Huang, X.; Marty, J.-L.; Wang, H.; Zhang, Q.; Li, P. Screen-Printed Electrochemical Immunosensor Based on a Novel Nanobody for Analyzing Aflatoxin M1 in Milk. Food Chem. 2022, 383, 132598. [Google Scholar] [CrossRef]
- Zidarič, T.; Majer, D.; Maver, T.; Finšgar, M.; Maver, U. The Development of an Electropolymerized, Molecularly Imprinted Polymer (MIP) Sensor for Insulin Determination Using Single-Drop Analysis. Analyst 2023, 148, 1102–1115. [Google Scholar] [CrossRef] [PubMed]


| Functionalization Method | Advantages | Drawbacks |
|---|---|---|
| Electropolymerization | Easy, simple Soft polymerization in comparison with UV Control of the layer thickness Covalent attachment of the monomer Suitable for large scale production | Limited to aqueous medium |
| Drop casting | Fast Does not require specific equipment Freedom in the choice of MIP formulation | Weak interactions between the MIP and the electrode The MIP synthesis is time consuming Not suitable for large scale production Use of immobilization matrix: PEG, chitosan |
| Chemical grafting | Covalent attachment of MIP | Time consuming Not suitable for large scale production Layer thickness difficult to control |
| Analyte | Matrix | MIP Deposition Method | Detection Method | LOD | Reference |
|---|---|---|---|---|---|
| Fenitrothion insecticide | Forest sample (leaves) | EP | SWV | 222 µg/L | [75] |
| C-reactive protein | Blood serum | SC | DPV | 0.04 mg/L | [76] |
| Oxfendazole drug | Milk | EP | DPV, SWV | 8.0 µg/kg | [77] |
| Methidathion insecticide | Waste water | DC | EIS | 5.1 µg/L | [32] |
| Naloxone drug | Urine/human serum | EP | DPV | 65 µg/L | [78] |
| Salbutamol drug | Swine meat feed samples | EP | DPV | 23.9 ng/L | [46] |
| Bisphenol A Plastic component | water/acetonitrile (99/1) | SC | DPV then CV | 13.7 ng/L | [79] |
| Thyroid hormone Thyronamine | PBS buffer | EP | SWV | 19 µg/L | [80] |
| MDMA ecstasy | Human blood serum and urine | EP | SWV | 0.15 mg/L | [81] |
| Cefquinome antibiotic | Phosphate buffer | EP | SWV | 26.4 µg/L | [82] |
| Solatol drug | Tablet and human blood serum | DC | CV | 9.53 µg/L | [83] |
| Sertraline drug | Human serum | DC | DPV | 609 ng/L | [84] |
| Azithromycin antibiotic | Environmental water | EP | DPV | 59.9 µg/L | [85] |
| Cyclocreatine drug | Plasma sample | EP | EIS | 55.1 ng/L | [86] |
| Tau protein, biomarker of Alzheimer’s disease | Serum sample | EP | EIS | 1.1 ng/L | [87] |
| Genistein allergen | Food products | EP | DPV | 100 µg/kg | [30] |
| L-hydroxyproline, biomarker of bone disease | Human serum | EP | EIS | 0.13 mg/L | [88] |
| Paracetamol drug | Plasma | CG | DPV | 7.56 mg/L | [27] |
| Atorvastatin drug | Water | EP | DPV | 0.56 µg/L | [26] |
| Cytokine interleukin 1-β protein | Human serum | EP | EIS | 0.23 ng/L | [23] |
| Albumin allergen | PBS buffer | DC | CV, Am | 180 mg/L | [89] |
| Trazodone drug | Tap water samples Human serum | EP | DPV | 595 µg/L | [90] |
| Aflatoxin M1 | Milk | CG | Chronoamperometry | 0.09 µg/L | [91] |
| Insulin hormone | Pharmaceutical sample | EP | SWV | 11.0 µg/L | [92] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bou-Maroun, E. Carbon Electrode Modified with Molecularly Imprinted Polymers for the Development of Electrochemical Sensor: Application to Pharmacy, Food Safety, Environmental Monitoring, and Biomedical Analysis. Chemosensors 2023, 11, 548. https://doi.org/10.3390/chemosensors11110548
Bou-Maroun E. Carbon Electrode Modified with Molecularly Imprinted Polymers for the Development of Electrochemical Sensor: Application to Pharmacy, Food Safety, Environmental Monitoring, and Biomedical Analysis. Chemosensors. 2023; 11(11):548. https://doi.org/10.3390/chemosensors11110548
Chicago/Turabian StyleBou-Maroun, Elias. 2023. "Carbon Electrode Modified with Molecularly Imprinted Polymers for the Development of Electrochemical Sensor: Application to Pharmacy, Food Safety, Environmental Monitoring, and Biomedical Analysis" Chemosensors 11, no. 11: 548. https://doi.org/10.3390/chemosensors11110548





