Towards Molecularly Imprinted Polypyrrole-Based Sensor for the Detection of Methylene Blue
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Instrumentation
2.2. Pre-Treatment of the Working Electrode
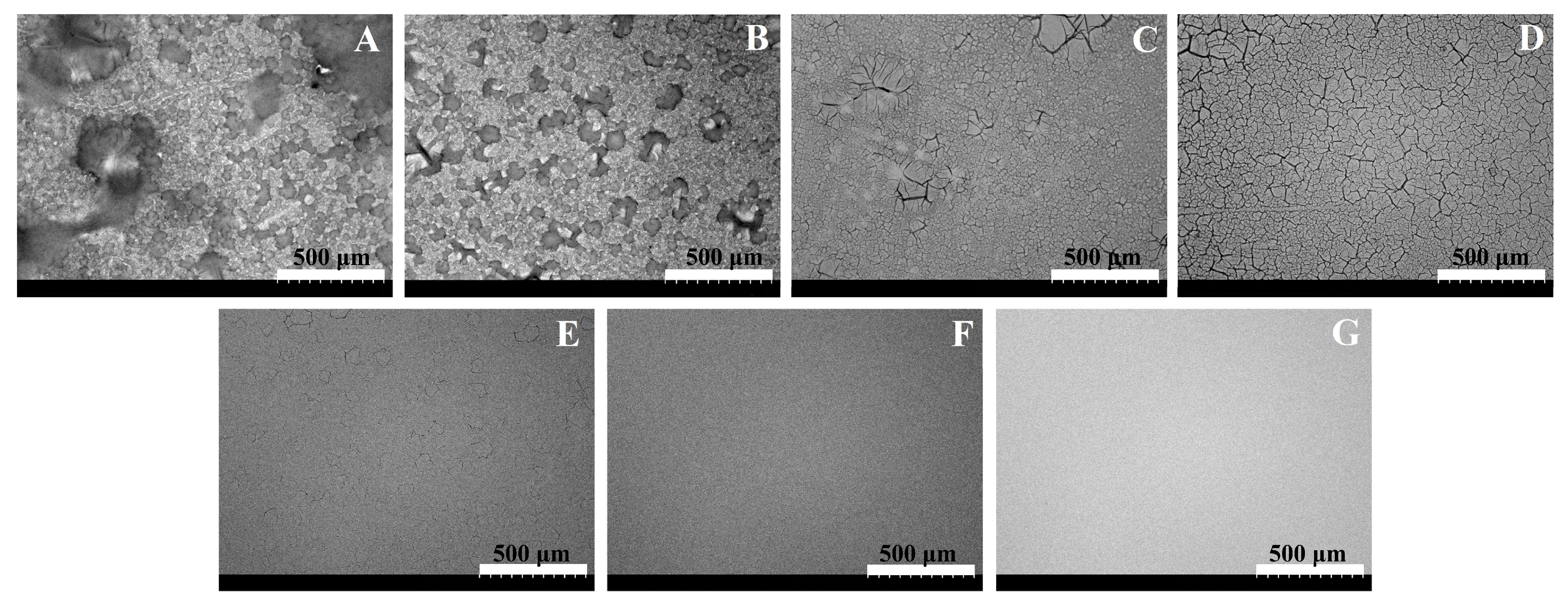
2.3. The Electrochemical Deposition of Ppy Layers
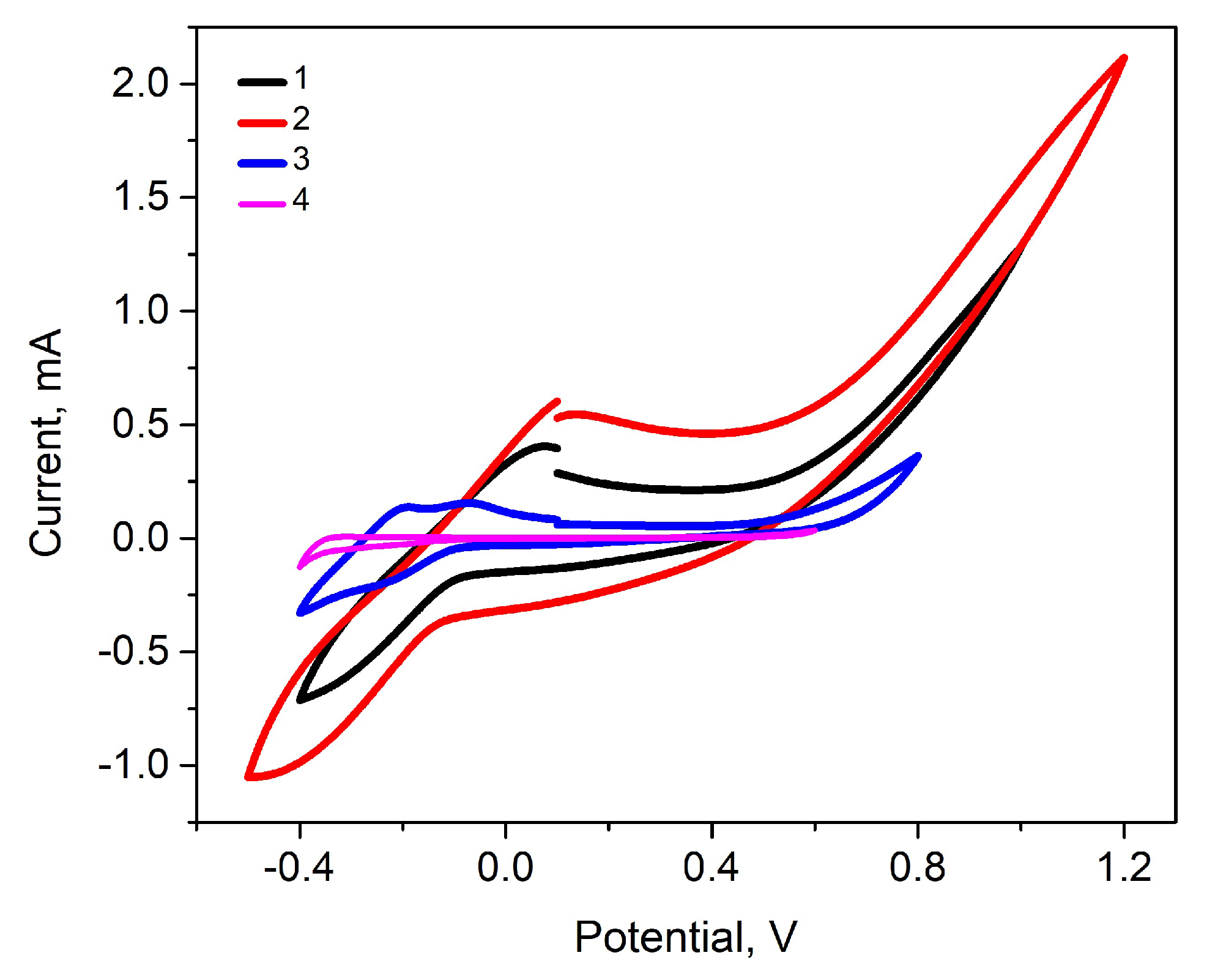
2.4. Evaluation of Ppy Layers
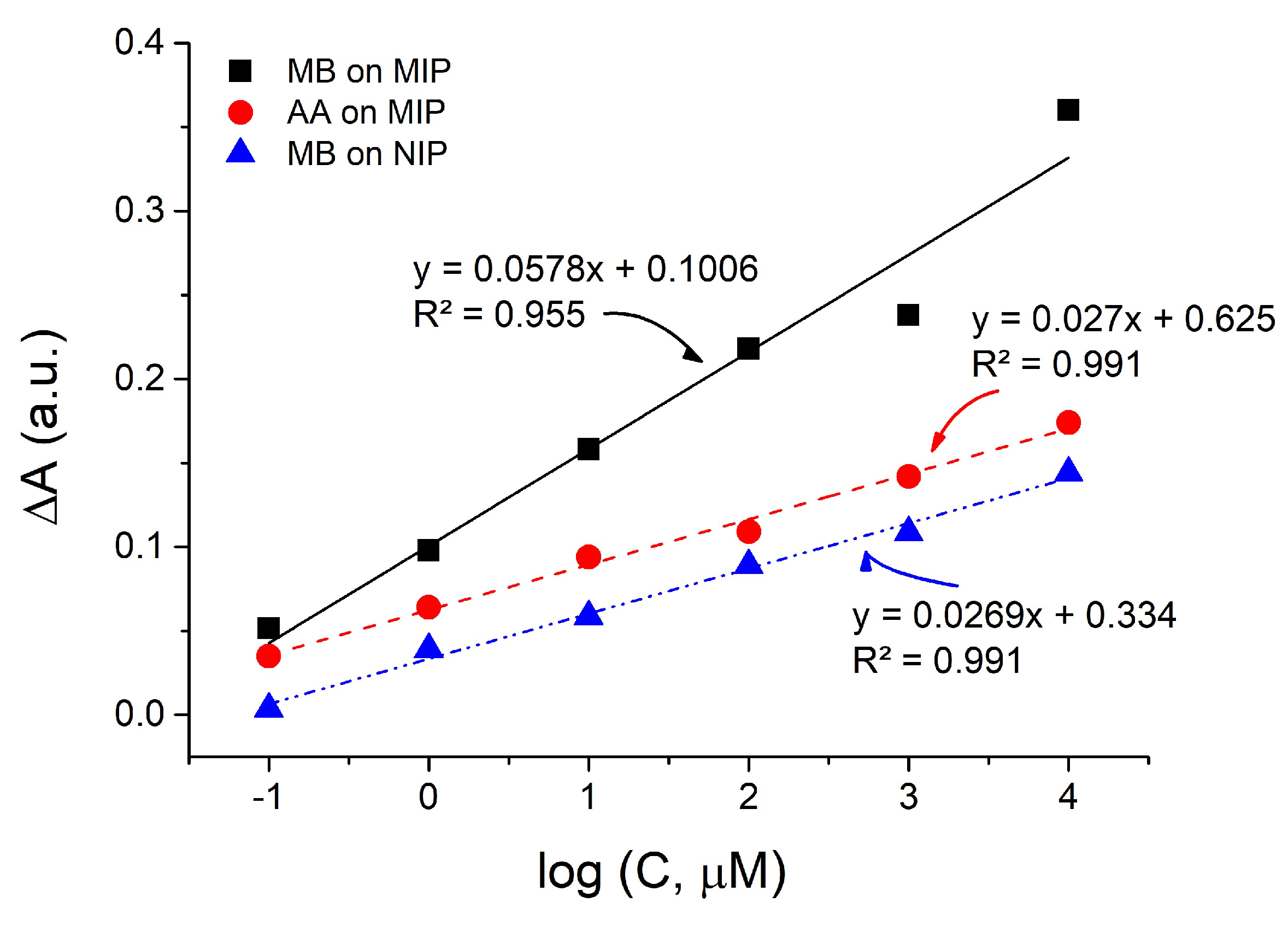
3. Results
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tretjakov, A.; Syritski, V.; Reut, J.; Boroznjak, R.; Öpik, A. Analytica Chimica Acta Molecularly Imprinted Polymer Film Interfaced with Surface Acoustic Wave Technology as a Sensing Platform for Label-Free Protein Detection. Anal. Chim. Acta 2016, 902, 182–188. [Google Scholar] [CrossRef]
- Kidakova, A.; Boroznjak, R.; Reut, J.; Öpik, A.; Saarma, M.; Syritski, V. Molecularly Imprinted Polymer-Based SAW Sensor for Label-Free Detection of Cerebral Dopamine Neurotrophic Factor Protein. Sens. Actuators B Chem. 2020, 308, 127708. [Google Scholar] [CrossRef]
- Lowdon, J.W.; Diliën, H.; Singla, P.; Peeters, M.; Cleij, T.J.; van Grinsven, B.; Eersels, K. MIPs for Commercial Application in Low-Cost Sensors and Assays—An Overview of the Current Status Quo. Sens. Actuators B Chem. 2020, 325, 128973. [Google Scholar] [CrossRef]
- Ratautaite, V.; Janssens, S.D.; Haenen, K.; Nesládek, M.; Ramanaviciene, A.; Baleviciute, I.; Ramanavicius, A. Molecularly Imprinted Polypyrrole Based Impedimetric Sensor for Theophylline Determination. Electrochim. Acta 2014, 130, 361–367. [Google Scholar] [CrossRef]
- Mazouz, Z.; Mokni, M.; Fourati, N.; Zerrouki, C.; Barbault, F.; Seydou, M.; Kalfat, R.; Yaakoubi, N.; Omezzine, A.; Bouslema, A.; et al. Biosensors and Bioelectronics Computational Approach and Electrochemical Measurements for Protein Detection with MIP-Based Sensor. Biosens. Bioelectron. 2020, 151, 111978. [Google Scholar] [CrossRef]
- Ansari, S.; Masoum, S. Molecularly Imprinted Polymers for Capturing and Sensing Proteins: Current Progress and Future Implications. TrAC—Trends Anal. Chem. 2019, 114, 29–47. [Google Scholar] [CrossRef]
- El-Sharif, H.F.; Stevenson, D.; Reddy, S.M. MIP-Based Protein Profiling: A Method for Interspecies Discrimination. Sens. Actuators B Chem. 2017, 241, 33–39. [Google Scholar] [CrossRef]
- Dabrowski, M.; Lach, P.; Cieplak, M.; Kutner, W. Nanostructured Molecularly Imprinted Polymers for Protein Chemosensing. Biosens. Bioelectron. 2018, 102, 17–26. [Google Scholar] [CrossRef]
- Shumyantseva, V.V.; Bulko, T.V.; Sigolaeva, L.V.; Kuzikov, A.V.; Archakov, A.I. Electrosynthesis and Binding Properties of Molecularly Imprinted Poly-o-Phenylenediamine for Selective Recognition and Direct Electrochemical Detection of Myoglobin. Biosens. Bioelectron. 2016, 86, 330–336. [Google Scholar] [CrossRef]
- Tlili, A.; Attia, G.; Khaoulani, S.; Mazouz, Z.; Zerrouki, C.; Yaakoubi, N.; Othmane, A.; Fourati, N. Contribution to the Understanding of the Interaction between a Polydopamine Molecular Imprint and a Protein Model: Ionic Strength and pH Effect Investigation. Sensors 2021, 21, 619. [Google Scholar] [CrossRef]
- Erdőssy, J.; Horváth, V.; Yarman, A.; Scheller, F.W.; Gyurcsányi, R.E. Electrosynthesized Molecularly Imprinted Polymers for Protein Recognition. TrAC—Trends Anal. Chem. 2016, 79, 179–190. [Google Scholar] [CrossRef]
- Piletsky, S.A.; Alcock, S.; Turner, A.P.F. Molecular Imprinting: At the Edge of the Third Millennium. Trends Biotechnol. 2001, 19, 9–12. [Google Scholar] [CrossRef]
- Vasapollo, G.; Sole, R.D.; Mergola, L.; Lazzoi, M.R.; Scardino, A.; Scorrano, S.; Mele, G. Molecularly Imprinted Polymers: Present and Future Prospective. Int. J. Mol. Sci. 2011, 12, 5908–5945. [Google Scholar] [CrossRef]
- Ding, S.; Lyu, Z.; Li, S.; Ruan, X.; Fei, M.; Zhou, Y.; Niu, X.; Zhu, W.; Du, D.; Lin, Y. Molecularly Imprinted Polypyrrole Nanotubes Based Electrochemical Sensor for Glyphosate Detection. Biosens. Bioelectron. 2021, 191, 113434. [Google Scholar] [CrossRef]
- Xing, X.; Liu, S.; Yu, J.; Lian, W.; Huang, J. Electrochemical Sensor Based on Molecularly Imprinted Film at Polypyrrole-Sulfonated Graphene/Hyaluronic Acid-Multiwalled Carbon Nanotubes Modified Electrode for Determination of Tryptamine. Biosens. Bioelectron. 2012, 31, 277–283. [Google Scholar] [CrossRef]
- Uzun, L.; Turner, A.P.F. Molecularly-Imprinted Polymer Sensors: Realising Their Potential. Biosens. Bioelectron. 2016, 76, 131–144. [Google Scholar] [CrossRef]
- Haupt, K. Molecularly Imprinted Polymers in Analytical Chemistry. Analyst 2001, 126, 747–756. [Google Scholar] [CrossRef]
- Ramanavicius, S.; Jagminas, A.; Ramanavicius, A. Advances in Molecularly Imprinted Polymers Based Affinity Sensors (Review). Polymers 2021, 13, 974. [Google Scholar] [CrossRef]
- El-Schich, Z.; Zhang, Y.; Feith, M.; Beyer, S.; Sternbæk, L.; Ohlsson, L.; Stollenwerk, M.; Wingren, A.G. Molecularly Imprinted Polymers in Biological Applications. Biotechniques 2020, 69, 407–420. [Google Scholar] [CrossRef]
- Turiel, E.; Esteban, A.M. Molecularly Imprinted Polymers; Elsevier Inc.: Amsterdam, The Netherlands, 2019; ISBN 9780128169063. [Google Scholar] [CrossRef]
- Kan, X.; Xing, Z.; Zhu, A.; Zhao, Z.; Xu, G.; Li, C.; Zhou, H. Molecularly Imprinted Polymers Based Electrochemical Sensor for Bovine Hemoglobin Recognition. Sens. Actuators B Chem. 2012, 168, 395–401. [Google Scholar] [CrossRef]
- Yola, M.L.; Atar, N. Development of Cardiac Troponin-I Biosensor Based on Boron Nitride Quantum Dots Including Molecularly Imprinted Polymer. Biosens. Bioelectron. 2019, 126, 418–424. [Google Scholar] [CrossRef] [PubMed]
- Silva, B.V.M.; Rodríguez, B.A.G.; Sales, G.F.; Sotomayor, M.D.P.T.; Dutra, R.F. An Ultrasensitive Human Cardiac Troponin T Graphene Screen-Printed Electrode Based on Electropolymerized-Molecularly Imprinted Conducting Polymer. Biosens. Bioelectron. 2016, 77, 978–985. [Google Scholar] [CrossRef] [PubMed]
- Ratautaite, V.; Boguzaite, R.; Mickeviciute, M.B.; Mikoliunaite, L.; Samukaite-bubniene, U.; Ramanavicius, A.; Ramanaviciene, A. Evaluation of Electrochromic Properties of Polypyrrole/Poly(Methylene Blue) Layer Doped by Polysaccharides. Sensors 2022, 22, 232. [Google Scholar] [CrossRef] [PubMed]
- Ion, R.M.; Scarlat, F.; Scarlat, F.; Niculescu, V.I.R. Methylene—Blue Modified Polypyrrole Film Electrode for Optoelectronic Applications. J. Optoelectron. Adv. Mater. 2003, 5, 109–115. [Google Scholar]
- Kaplan, I.H.; Daǧci, K.; Alanyalioǧlu, M. Nucleation and Growth Mechanism of Electropolymerization of Methylene Blue: The Effect of Preparation Potential on Poly(Methylene Blue) Structure. Electroanalysis 2010, 22, 2694–2701. [Google Scholar] [CrossRef]
- Erdem, A.; Kerman, K.; Meric, B.; Akarca, U.S.; Ozsoz, M. Novel Hybridization Indicator Methylene Blue for the Electrochemical Detection of Short DNA Sequences Related to the Hepatitis B Virus. Anal. Chim. Acta 2000, 422, 139–149. [Google Scholar] [CrossRef]
- Pfaffen, V.; Ortiz, P.I.; Córdoba de Torresi, S.I.; Torresi, R.M. On the pH Dependence of Electroactivity of Poly(Methylene Blue) Films. Electrochim. Acta 2010, 55, 1766–1771. [Google Scholar] [CrossRef]
- Brett, C.M.A.; Inzelt, G.; Kertesz, V. Poly(Methylene Blue) Modified Electrode Sensor for Haemoglobin. Anal. Chim. Acta 1999, 385, 119–123. [Google Scholar] [CrossRef]
- Karyakin, A.A.; Strakhova, A.K.; Karyakina, E.E.; Varfolomeyev, S.D.; Yatslmirsky, A.K. The Electrochemical Polymerization of Methylene Blue and Bioelectrochemical Activity of the Resulting Film. Synth. Met. 1993, 60, 289–292. [Google Scholar] [CrossRef]
- Clifton, J.; Leikin, J.B. Methylene Blue. Am. J. Ther. 2003, 10, 289–291. [Google Scholar] [CrossRef]
- Tonlé, I.K.; Ngameni, E.; Tcheumi, H.L.; Tchiéda, V.; Carteret, C.; Walcarius, A. Sorption of Methylene Blue on an Organoclay Bearing Thiol Groups and Application to Electrochemical Sensing of the Dye. Talanta 2008, 74, 489–497. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Huang, Y.; Wei, T.; Zhang, W.; Wang, W.; Lin, D.; Zhang, X.; Kumar, A.; Du, Q.; Xing, J. Amphiphilic and Biodegradable Methoxy Polyethylene Glycol-Block-(Polycaprolactone-Graft-Poly(2-(Dimethylamino)Ethyl Methacrylate)) as an Effective Gene Carrier. Biomaterials 2011, 32, 879–889. [Google Scholar] [CrossRef] [PubMed]
- Asman, S.; Yusof, N.A.; Abdullah, A.H.; Haron, M.J. Synthesis and Characterization of a Molecularly Imprinted Polymer for Methylene Blue. Asian J. Chem. 2011, 23, 4786–4794. [Google Scholar]
- Houas, A.; Lachheb, H.; Ksibi, M.; Elaloui, E.; Guillard, C.; Herrmann, J.M. Photocatalytic Degradation Pathway of Methylene Blue in Water. Appl. Catal. B 2001, 31, 145–157. [Google Scholar] [CrossRef]
- Liu, B.; Cang, H.; Cui, L.; Zhang, H. Electrochemical Polymerization of Methylene Blue on Glassy Carbon Electrode. Int. J. Electrochem. Sci. 2017, 12, 9907–9913. [Google Scholar] [CrossRef]
- Mokhtari, Z.; Khajehsharifi, H.; Hashemnia, S.; Solati, Z.; Azimpanah, R.; Shahrokhian, S. Evaluation of Molecular Imprinted Polymerized Methylene Blue/Aptamer as a Novel Hybrid Receptor for Cardiac Troponin I (CTnI) Detection at Glassy Carbon Electrodes Modified with New Biosynthesized ZnONPs. Sens. Actuators B Chem. 2020, 320, 128316. [Google Scholar] [CrossRef]
- El Fazdoune, M.; Bahend, K.; Ben Jadi, S.; Oubella, M.; García-García, F.J.; Bazzaoui, E.A.; Asserghine, A.; Bazzaoui, M. Different Electrochemical Techniques for the Electrosynthesis of Poly Methylene Blue in Sodium Saccharin Aqueous Medium. J. Solid. State Electrochem. 2023, 27, 667–678. [Google Scholar] [CrossRef]
- Sedelnikova, A.; Poletaeva, Y.; Golyshev, V.; Chubarov, A.; Dmitrienko, E. Preparation of Magnetic Molecularly Imprinted Polymer for Methylene Blue Capture. Magnetochemistry 2023, 9, 196. [Google Scholar] [CrossRef]
- Sun, W.; Wang, Y.; Zhang, Y.; Ju, X.; Li, G.; Sun, Z. Poly(Methylene Blue) Functionalized Graphene Modified Carbon Ionic Liquid Electrode for the Electrochemical Detection of Dopamine. Anal. Chim. Acta 2012, 751, 59–65. [Google Scholar] [CrossRef]
- Barsan, M.M.; Pinto, E.M.; Brett, C.M.A. Methylene Blue and Neutral Red Electropolymerisation on AuQCM and on Modified AuQCM Electrodes: An Electrochemical and Gravimetric Study. Phys. Chem. Chem. Phys. 2011, 13, 5462–5471. [Google Scholar] [CrossRef]
- Phonklam, K.; Wannapob, R.; Sriwimol, W.; Thavarungkul, P.; Phairatana, T. A Novel Molecularly Imprinted Polymer PMB/MWCNTs Sensor for Highly-Sensitive Cardiac Troponin T Detection. Sens. Actuators B Chem. 2020, 308, 127630. [Google Scholar] [CrossRef]
- Kim, S.; Jang, L.K.; Park, H.S.; Lee, J.Y. Electrochemical Deposition of Conductive and Adhesive Polypyrrole-Dopamine Films. Sci. Rep. 2016, 6, 30475. [Google Scholar] [CrossRef] [PubMed]
- Samukaite-Bubniene, U.; Valiūnienė, A.; Bucinskas, V.; Genys, P.; Ratautaite, V.; Ramanaviciene, A.; Aksun, E.; Tereshchenko, A.; Zeybek, B.; Ramanavicius, A. Towards Supercapacitors: Cyclic Voltammetry and Fast Fourier Transform Electrochemical Impedance Spectroscopy Based Evaluation of Polypyrrole Electrochemically Deposited on the Pencil Graphite Electrode. Colloids Surf. A Physicochem. Eng. Asp. 2021, 610, 125750. [Google Scholar] [CrossRef]
- Hu, Y.; Xing, H.; Li, G.; Wu, M. Magnetic Imprinted Polymer-Based Quartz Crystal Microbalance Sensor for Sensitive Label-Free Detection of Methylene Blue in Groundwater. Sensors 2020, 20, 5506. [Google Scholar] [CrossRef] [PubMed]
- Soysal, M.; Muti, M.; Esen, C.; Gençdaǧ, K.; Aslan, A.; Erdem, A.; Karagözler, A.E. A Novel and Selective Methylene Blue Imprinted Polymer Modified Carbon Paste Electrode. Electroanalysis 2013, 25, 1278–1285. [Google Scholar] [CrossRef]
- Wang, N.; Xiao, S.J.; Su, C.W. Preparation of Molecularly Imprinted Polymer for Methylene Blue and Study on Its Molecular Recognition Mechanism. Colloid. Polym. Sci. 2016, 294, 1305–1314. [Google Scholar] [CrossRef]
- Santhoshkumar, S.; Murugan, E. Rationally Designed SERS AgNPs/GO/g-CN Nanohybrids to Detect Methylene Blue and Hg2+ Ions in Aqueous Solution. Appl. Surf. Sci. 2021, 553, 149544. [Google Scholar] [CrossRef]
- Hayat, M.; Shah, A.; Nisar, J.; Shah, I.; Haleem, A.; Ashiq, M.N. A Novel Electrochemical Sensing Platform for the Sensitive Detection and Degradation Monitoring of Methylene Blue. Catalysts 2022, 12, 306. [Google Scholar] [CrossRef]
- Zhao, D.; Liu, X.; Wei, C.; Qu, Y.; Xiao, X.; Cheng, H. One-Step Synthesis of Red-Emitting Carbon Dots: Via a Solvothermal Method and Its Application in the Detection of Methylene Blue. RSC Adv. 2019, 9, 29533–29540. [Google Scholar] [CrossRef]
- Sadrolhosseini, A.R.; Ghasemi, E.; Pirkarimi, A.; Hamidi, S.M.; Taheri Ghahrizjani, R. Highly Sensitive Surface Plasmon Resonance Sensor for Detection of Methylene Blue and Methylene Orange Dyes Using NiCo-Layered Double Hydroxide. Opt. Commun. 2023, 529, 129057. [Google Scholar] [CrossRef]



| Sensing Platform | Evaluation | LOD | Ref. |
|---|---|---|---|
| QCM/Fe3O4NPs/MIP/Ppy | QCM-D | 1.4 µg/L | [45] |
| Carbon paste/MIP/PMAA | DPV | 36.4 µM | [46] |
| MIP/PAA | UV-Vis spectroscopy | - | [47] |
| AgNPs/GO/g-CN | Raman spectroscopy | 0.001 nM | [48] |
| GCE/NH2-fMWCNTs | SWV | 0.21 nM | [49] |
| Red-emitting CDs | Fluorescence spectroscopy | 10 nM | [50] |
| Au-glass/NiCo-layered double hydroxide | Surface plasmon resonance | 0.005 ppm | [51] |
| ITO-glass/MIP/Ppy | Potential pulse chronoamperometry, cyclic voltammetry, UV-Vis spectroscopy | - | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boguzaite, R.; Pilvenyte, G.; Ratautaite, V.; Brazys, E.; Ramanaviciene, A.; Ramanavicius, A. Towards Molecularly Imprinted Polypyrrole-Based Sensor for the Detection of Methylene Blue. Chemosensors 2023, 11, 549. https://doi.org/10.3390/chemosensors11110549
Boguzaite R, Pilvenyte G, Ratautaite V, Brazys E, Ramanaviciene A, Ramanavicius A. Towards Molecularly Imprinted Polypyrrole-Based Sensor for the Detection of Methylene Blue. Chemosensors. 2023; 11(11):549. https://doi.org/10.3390/chemosensors11110549
Chicago/Turabian StyleBoguzaite, Raimonda, Greta Pilvenyte, Vilma Ratautaite, Ernestas Brazys, Almira Ramanaviciene, and Arunas Ramanavicius. 2023. "Towards Molecularly Imprinted Polypyrrole-Based Sensor for the Detection of Methylene Blue" Chemosensors 11, no. 11: 549. https://doi.org/10.3390/chemosensors11110549







