Gas Sensing Studies of an n-n Hetero-Junction Array Based on SnO2 and ZnO Composites
Abstract
:1. Introduction
2. Experimental Section
| Sensor Device | Metal Oxide Powder(s) and Mass (g) | Metal Oxide Powder(s) and Moles (mol) | Mass of ESL 400 (g) |
|---|---|---|---|
| *100 wt% SnO2 | SnO2−2.10 | SnO2−0.014 | 1.61 |
| 90 wt% SnO2 – 10 wt% ZnO | SnO2−1.83 ZnO−0.21 | SnO2−0.012 ZnO−0.003 | 1.24 |
| 70 wt% SnO2 – 30 wt% ZnO | SnO2−1.42 ZnO−0.62 | SnO2−0.009 ZnO−0.008 | 0.74 |
| 50 wt% SnO2 – 50 wt% ZnO | SnO2−1.51 ZnO−1.52 | SnO2−0.010 ZnO−0.019 | 1.08 |
| 30 wt% SnO2 – 70 wt% ZnO | SnO2−0.60 ZnO−1.41 | SnO2−0.004 ZnO−0.017 | 0.85 |
| 10 wt% SnO2 – 90 wt% ZnO | SnO2−0.21 ZnO−1.80 | SnO2−0.001 ZnO−0.022 | 1.06 |
| *100 wt% ZnO | ZnO−2.00 | ZnO−0.025 | 1.18 |
2.1. Materials Characterisation
2.2. Gas Sensing Characterisation
3. Results and Discussion
3.1. Scanning Electron Microscopy

3.2. X-Ray Diffraction

3.3. Raman Spectroscopy

3.4. X-Ray Photoelectron Spectroscopy

3.5. Gas Sensing Characterisation
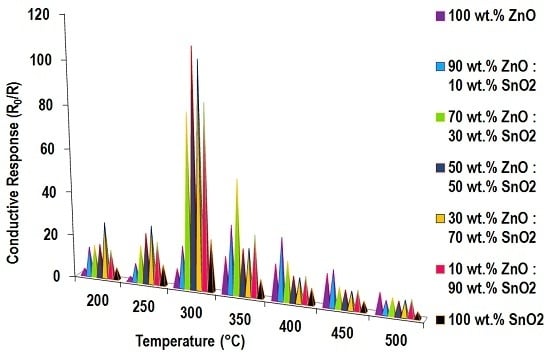
3.5.1. Ethanol Sensing


3.5.2. NO2 Sensing


3.5.3. Cross Sensitivity Testing
NH3 Sensing

Acetone Sensing

CO Sensing

4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Capone, S.; Forleo, A.; Francioso, L.; Rella, R.; Siciliano, P.; Spadavecchia, J.; Presicce, D.S.; Taurino, A.M. Solid state gas sensors: State of the art and future activities. J. Optoelectron. Adv. Mater. 2003, 5, 1335–1348. [Google Scholar] [CrossRef]
- Wang, C.X.; Yin, L.W.; Zhang, L.Y.; Xiang, D.; Gao, R. Metal Oxide Gas Sensors: Sensitivity and Influencing Factors. Sensors 2010, 10, 2088–2106. [Google Scholar] [CrossRef] [PubMed]
- Trakhtenberg, L.I.; Gerasimov, G.N.; Gromov, V.F.; Brelysheva, T.V.; Ilegbusi, O.J. Gas Semiconducting Sensors Based on Metal Oxide Nanocomposites. J. Mater. Sci. Res. 2012, 1, 56–68. [Google Scholar] [CrossRef]
- Costello, B.P.J.d.; Ewen, R.J.; Guernion, N.; Ratcliffe, N.M. Highly sensitive mixed oxide sensors for the detection of ethanol. Sens. Actuators B: Chem. 2002, 87, 207–210. [Google Scholar] [CrossRef]
- Tang, H.; Yan, M.; Zhang, H.; Li, S.; Ma, X.; Wang, M.; Yang, D. A selective NH3 gas sensor based on Fe2O3–ZnO nanocomposites at room temperature. Sens. Actuators B: Chem. 2006, 114, 910–915. [Google Scholar] [CrossRef]
- Bai, S.; Li, D.; Han, D.; Luo, R.; Chen, A.; Chung, C.L. Preparation, characterization of WO3–SnO2 nanocomposites and their sensing properties for NO2. Sens. Actuators B: Chem. 2010, 150, 749–755. [Google Scholar] [CrossRef]
- Ivanovskaya, M.; Kotsikau, D.; Faglia, G.; Nelli, P. Influence of chemical composition and structural factors of Fe2O3/In2O3 sensors on their selectivity and sensitivity to ethanol. Sens. Actuators B: Chem. 2003, 96, 498–503. [Google Scholar] [CrossRef]
- Sun, C.; Maduraiveeran, G.; Dutta, P. Nitric oxide sensors using combination of p- and n-type semiconducting oxides and its application for detecting NO in human breath. Sens. Actuators B: Chem. 2013, 186, 117–125. [Google Scholar] [CrossRef]
- Costello, B.P.J.d.; Ewen, R.J.; Jones, P.R.H.; Ratcliffe, N.M.; Wat, R.K.M. A study of the catalytic and vapour-sensing properties of zinc oxide and tin dioxide in relation to 1-butanol and dimethyldisulphide. Sens. Actuators B: Chem. 1999, 61, 199–207. [Google Scholar] [CrossRef]
- Kaciulis, S.; Pandolfi, L.; Viticoli, S.; Sberveglieri, G.; Zampiceni, E.; Wlodarski, W.; Galatsis, K.; Li, Y.X. Investigation of thin films of mixed oxides for gas-sensing applications. Surf. Interface Anal. 2002, 34, 672–676. [Google Scholar] [CrossRef]
- Barreca, D.; Carraro, G.; Comini, E.; Gasparotto, A.; Maccato, C.; Sada, C.; Sberveglieri, G.; Tondello, E. Novel Synthesis and Gas Sensing Performances of CuO–TiO2 Nanocomposites Functionalized with Au Nanoparticles. J. Phys. Chem. C 2011, 115, 10510–10517. [Google Scholar] [CrossRef]
- Zakrzewska, K. Mixed oxides as gas sensors. Thin Solid Films 2001, 391, 229–238. [Google Scholar] [CrossRef]
- Yamazoe, N.; Tamaki, J.; Miura, N. Role of hetero-junctions in oxide semiconductor gas sensors. Mater. Sci. Eng. B 1996, 41, 178–181. [Google Scholar] [CrossRef]
- Costello, B.P.D.; Ewen, R.J.; Ratcliffe, N.M.; Sivanand, P. Thick film organic vapour sensors based on binary mixtures of metal oxides. Sens. Actuators B: Chem. 2003, 92, 159–166. [Google Scholar] [CrossRef]
- Chen, Y.; Xiao, G.; Wang, T.; Zhang, F.; Ma, Y.; Gao, P.; Zhu, C.; Zhang, E.; Xu, Z.; Li, Q. Synthesis and enhanced gas sensing properties of crystalline CeO2/TiO2 core/shell nanorods. Sens. Actuators B: Chem. 2011, 156, 867–874. [Google Scholar] [CrossRef]
- Eranna, G.; Joshi, B.C.; Runthala, D.P.; Gupta, R.P. Oxide materials for development of integrated gas sensors—A comprehensive review. Crit. Rev. Solid State Mater. Sci. 2004, 29, 111–188. [Google Scholar] [CrossRef]
- Wager, J.F. Transparent electronics: Schottky barrier and heterojunction considerations. Thin Solid Films 2008, 516, 1755–1764. [Google Scholar] [CrossRef]
- Yamazoe, N.; Shimanoe, K. Proposal of contact potential promoted oxide semiconductor gas sensor. Sens. Actuators B: Chem. 2013, 187, 162–167. [Google Scholar] [CrossRef]
- Jayalakshmi, M.; Balasubramanian, K. Hydrothermal Synthesis of CuO-SnO2 and CuO-SnO2-Fe2O3 Mixed Oxides and Electrochemical Characterization in Neutral Electrolyte. Int. J. Electrochem. Sci. 2009, 4, 571–581. [Google Scholar]
- Zeng, W.; Liu, T.; Wang, Z. Enhanced gas sensing properties by SnO2 nanosphere functionalized TiO2 nanobelts. J. Mater. Chem. 2012, 22, 3544–3548. [Google Scholar] [CrossRef]
- Yamazoe, N.; Shimanoe, K. Basic approach to the transducer function of oxide semiconductor gas sensors. Sens. Actuators B: Chem. 2011, 160, 1352–1362. [Google Scholar] [CrossRef]
- Binions, R.; Afonja, O.A.; Dungey, S.; Lewis, D.W.; Parkin, I.P.; Williams, D.E. Discrimination Effects in Metal Oxide Semiconductor Gas Sensors. IEEE Sens. J. 2011, 11, 1145–1151. [Google Scholar] [CrossRef]
- Korotcenkov, G. The role of morphology and crystallographic structure of metal oxides in response of conductometric-type gas sensors. Mater. Sci. Eng. R. 2008, 61, 1–39. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, J.; Guo, X.; Wu, S.; Wang, S. Enhanced sensor response of Ni-doped SnO2 hollow spheres. Sens. Actuators B: Chem. 2011, 152, 162–167. [Google Scholar] [CrossRef]
- Lupan, O.; Chow, L.; Chai, G.; Schulte, A.; Park, S.; Heinrich, H. A rapid hydrothermal synthesis of rutile SnO2 nanowires. Mater. Sci. Eng. B 2009, 157, 101–104. [Google Scholar] [CrossRef]
- Sun, S.H.; Meng, G.W.; Zhang, G.X.; Gao, T.; Geng, B.Y.; Zhang, L.D.; Zuo, J. Raman scattering study of rutile SnO2 nanobelts synthesized by thermal evaporation of Sn powders. Chem. Phys. Lett. 2003, 376, 103–107. [Google Scholar] [CrossRef]
- Sin, N.D.M.; Kamel, M.F.; Alip, R.I.; Mohamad, Z.; Rusop, M. The Electrical Characteristics of Aluminium Doped Zinc Oxide Thin Film for Humidity Sensor Applications. Adv. Mater. Sci. Eng. 2011, 2011, 1–5. [Google Scholar] [CrossRef]
- Anderson, J.; Chris, G.V.d.W. Fundamentals of zinc oxide as a semiconductor. Rep. Prog. Phys. 2009, 72, 126501. [Google Scholar]
- Zhang, Y.; Zhu, F.; Zhang, J.; Xia, L. Converting Layered Zinc Acetate Nanobelts to One-dimensional Structured ZnO Nanoparticle Aggregates and their Photocatalytic Activity. Nanoscale Res. Lett. 2008, 3, 201–204. [Google Scholar] [CrossRef]
- Huang, Y.; Liu, M.; Li, Z.; Zeng, Y.; Liu, S. Raman spectroscopy study of ZnO-based ceramic films fabricated by novel sol–gel process. Mater. Sci. Eng., B 2003, 97, 111–116. [Google Scholar] [CrossRef]
- Loudon, R. The Raman effect in crystals. Adv. Phys. 2001, 50, 813–864. [Google Scholar] [CrossRef]
- Choopun, S.; Hongsith, N.; Mangkorntong, P.; Mangkorntong, N. Zinc oxide nanobelts by RF sputtering for ethanol sensor. Phys. E: Low-dimensional Syst. Nanostruct. 2007, 39, 53–56. [Google Scholar] [CrossRef]
- LaSurface.com. Database. Available from: http://www.lasurface.com/database/elementxps.php. (accessed on 10 November 2015).
- Dementjev, A.P.; de Graaf, A.; van de Sanden, M.C.M.; Maslakov, K.I.; Naumkin, A.V.; Serov, A.A. X-Ray photoelectron spectroscopy reference data for identification of the C3N4 phase in carbon–nitrogen films. Diamond Relat. Mater. 2000, 9, 1904–1907. [Google Scholar] [CrossRef]
- Boyd, K.J.; Marton, D.; Todorov, S.S.; Al-Bayati, A.H.; Kulik, J.; Zuhr, R.A.; Rabalais, J.W. Formation of C–N thin films by ion beam deposition. J. Vac. Sci. Technol. A 1995, 13, 2110–2122. [Google Scholar] [CrossRef]
- Akgul, F.A.; Gumus, C.; Er, A.O.; Farha, A.H.; Akgul, G.; Ufuktepe, Y.; Liu, Z. Structural and electronic properties of SnO2. J. Alloys Compd. 2013, 579, 50–56. [Google Scholar] [CrossRef]
- Biesinger, M.C.; Lau, L.W.M.; Gerson, A.R.; Smart, R.S.C. Resolving surface chemical states in XPS analysis of first row transition metals, oxides and hydroxides: Sc, Ti, V, Cu and Zn. Appl. Surf. Sci. 2010, 257, 887–898. [Google Scholar] [CrossRef]
- Kim, K.; Cho, P.; Kim, S.; Lee, J.; Kang, C.; Kim, J.; Yoon, S. The selective detection of C2H5OH using SnO2–ZnO thin film gas sensors prepared by combinatorial solution deposition. Sens. Actuators B: Chem. 2007, 123, 318–324. [Google Scholar] [CrossRef]
- Yu, J.H.; Choi, G.M. Electrical and CO gas sensing properties of ZnO–SnO2 composites. Sens. Actuators B: Chem. 1998, 52, 251–256. [Google Scholar]
- Song, X.; Wang, Z.; Liu, Y.; Wang, C.; Li, L. A highly sensitive ethanol sensor based on mesoporous ZnO–SnO2 nanofibers. Nanotechnology 2009, 20, 075501. [Google Scholar] [CrossRef] [PubMed]
- Zeng, W.; Liu, T.; Wang, Z. Enhanced gas sensing properties by SnO2 nanosphere functionalized TiO2 nanobelts. J. Mater. Chem. 2012, 22, 3544–3548. [Google Scholar] [CrossRef]
- Halawy, S.A.; Mohamed, M.A. The effect of different ZnO precursors on the catalytic decomposition of ethanol. J. Mol. Catal. A: Chem. 1995, 98, L63–L68. [Google Scholar] [CrossRef]
- Cheong, H.; Lee, M. Sensing characteristics and surface reaction mechanism of alcohol sensors based on doped SnO2. J. Ceram. Process. Res. 2006, 7, 183–191. [Google Scholar]
- Xu, J.; Han, J.; Zhang, Y.; Sun, Y.a.; Xie, B. Studies on alcohol sensing mechanism of ZnO based gas sensors. Sens. Actuators B: Chem. 2008, 132, 334–339. [Google Scholar] [CrossRef]
- Tricoli, A.; Righettoni, M.; Teleki, A. Semiconductor Gas Sensors: Dry Synthesis and Application. Angew. Chem. Int. Ed. 2010, 49, 7632–7659. [Google Scholar] [CrossRef] [PubMed]
- Khadayate, R.S.; Waghulde, R.B.; Wankhede, M.G.; Sali, J.V.; Patil, P.P. Ethanol vapour sensing properties of screen printed WO3 thick films. Bull. Mater. Sci. 2007, 30, 129–133. [Google Scholar] [CrossRef]
- Song, X.; Liu, L. Characterization of electrospun ZnO–SnO2 nanofibers for ethanol sensor. Sens. Actuators A 2009, 154, 175–179. [Google Scholar] [CrossRef]
- Park, J.; Moon, J.; Lee, S.; Kim, S.H.; Chu, H.Y.; Zyung, T. SnO2–ZnO hybrid nanofibers-based highly sensitive nitrogen dioxides sensor. Sens. Actuators B: Chem. 2010, 145, 592–595. [Google Scholar] [CrossRef]
- Xu, H.; Chen, X.; Zhang, J.; Wang, J.; Cao, B.; Cui, D. NO2 gas sensing with SnO2–ZnO/PANI composite thick film fabricated from porous nanosolid. Sens. Actuators B: Chem. 2013, 176, 166–173. [Google Scholar] [CrossRef]
- Jiménez, I.; Vila, A.M.; Calveras, A.C.; Morante, J.R. Gas-sensing properties of catalytically modified WO3 with copper and vanadium for NH3 detection. IEEE Sens. J. 2005, 5, 385–391. [Google Scholar] [CrossRef]
- Jiménez, I.; Centeno, M.A.; Scotti, R.; Morazzoni, F.; Cornet, A.; Morante, J.R. NH3 Interaction with Catalytically Modified Nano-WO3 Powders for Gas Sensing Applications. J. Electrochem. Soc. 2003, 150. [Google Scholar] [CrossRef]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Naik, A.; Parkin, I.; Binions, R. Gas Sensing Studies of an n-n Hetero-Junction Array Based on SnO2 and ZnO Composites. Chemosensors 2016, 4, 3. https://doi.org/10.3390/chemosensors4010003
Naik A, Parkin I, Binions R. Gas Sensing Studies of an n-n Hetero-Junction Array Based on SnO2 and ZnO Composites. Chemosensors. 2016; 4(1):3. https://doi.org/10.3390/chemosensors4010003
Chicago/Turabian StyleNaik, Anupriya, Ivan Parkin, and Russell Binions. 2016. "Gas Sensing Studies of an n-n Hetero-Junction Array Based on SnO2 and ZnO Composites" Chemosensors 4, no. 1: 3. https://doi.org/10.3390/chemosensors4010003
APA StyleNaik, A., Parkin, I., & Binions, R. (2016). Gas Sensing Studies of an n-n Hetero-Junction Array Based on SnO2 and ZnO Composites. Chemosensors, 4(1), 3. https://doi.org/10.3390/chemosensors4010003