Electrochemical Immunosensor for Detection of IgY in Food and Food Supplements
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Instrumentation
2.2. Samples Preparation
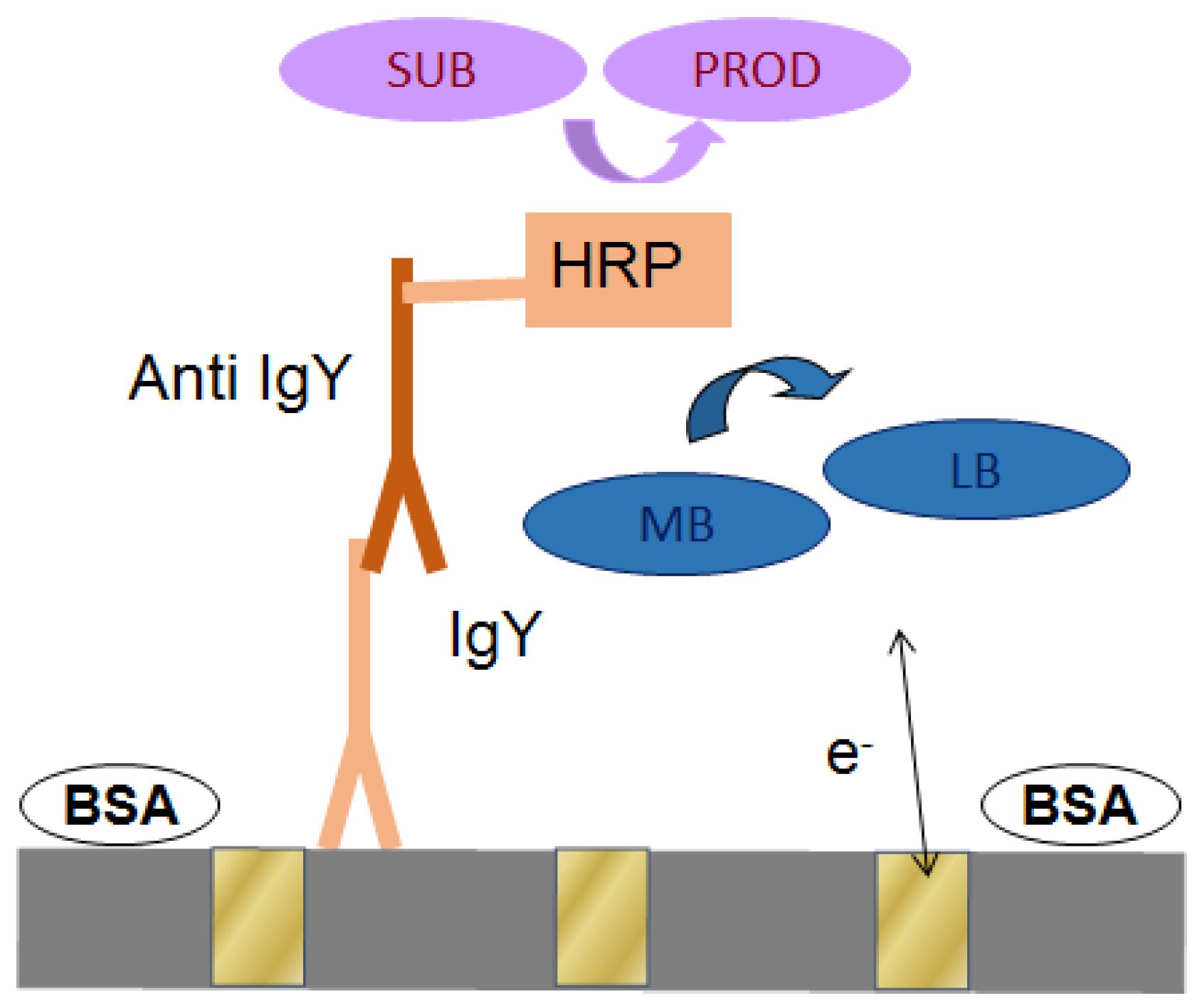
2.3. Operation of the Immunosensor
2.4. Capture of IgY on NEEs
- Incubation of 5 µL of the sample solution (in acetate buffer) for 30 min;
- Four times rinsing of the NEE with 1 mL of 0.01 M PBS, the electrode is then dried by a gentle stream of air;
- Immersion of the electrode in 1 mL of blocking solution (1% BSA in PBS) on orbital shaker plates for 30 min;
- Four times rinsing of the electrode with 1 mL of PBS;
- Incubation of 5 µL of 0.1 mg/mL of the secondary antibody Anti-IgY-HRP for 30 min;
- Four times rinsing the electrode with 1 mL of PBS.
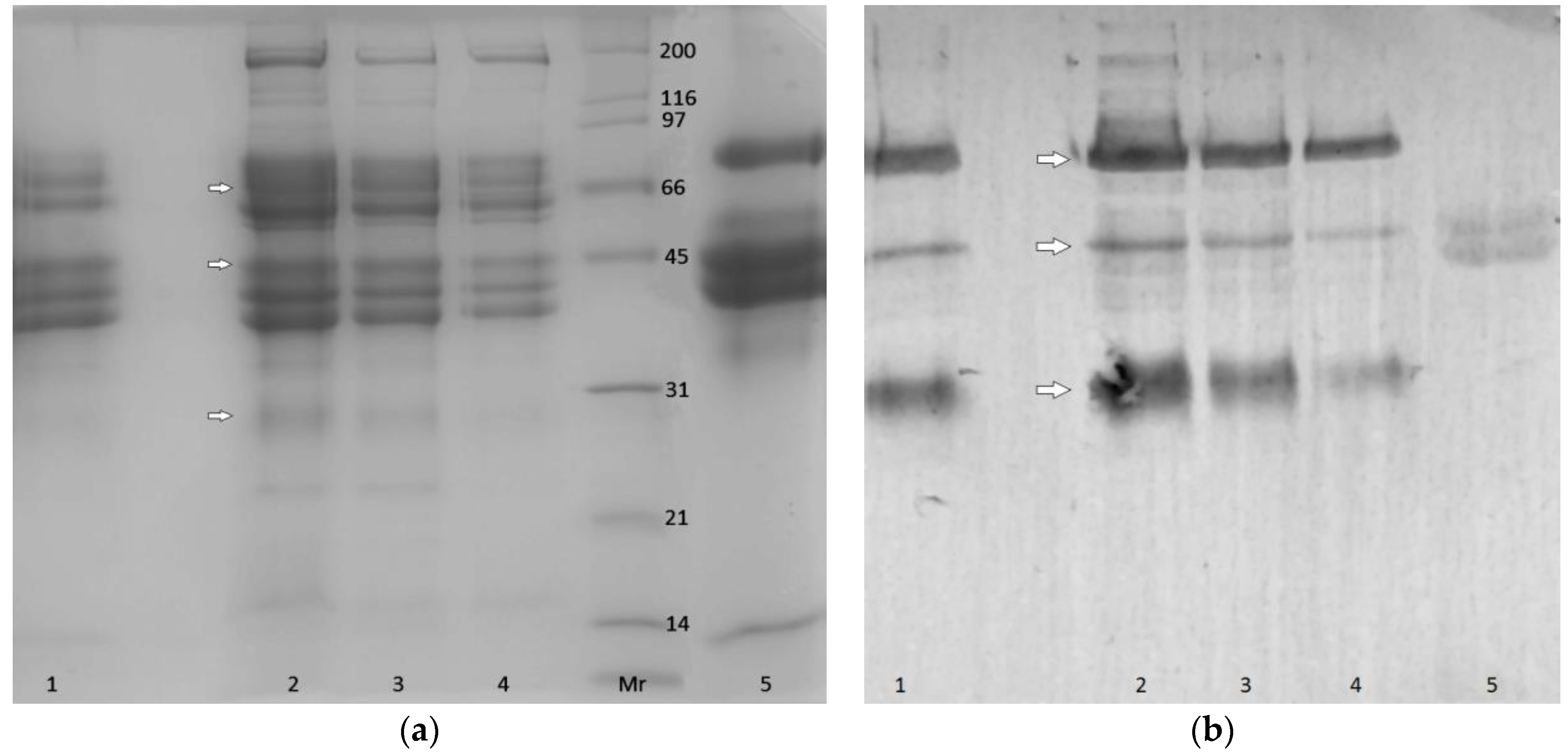
2.5. SDS-PAGE and Western Blot Analysis
3. Results and Discussion
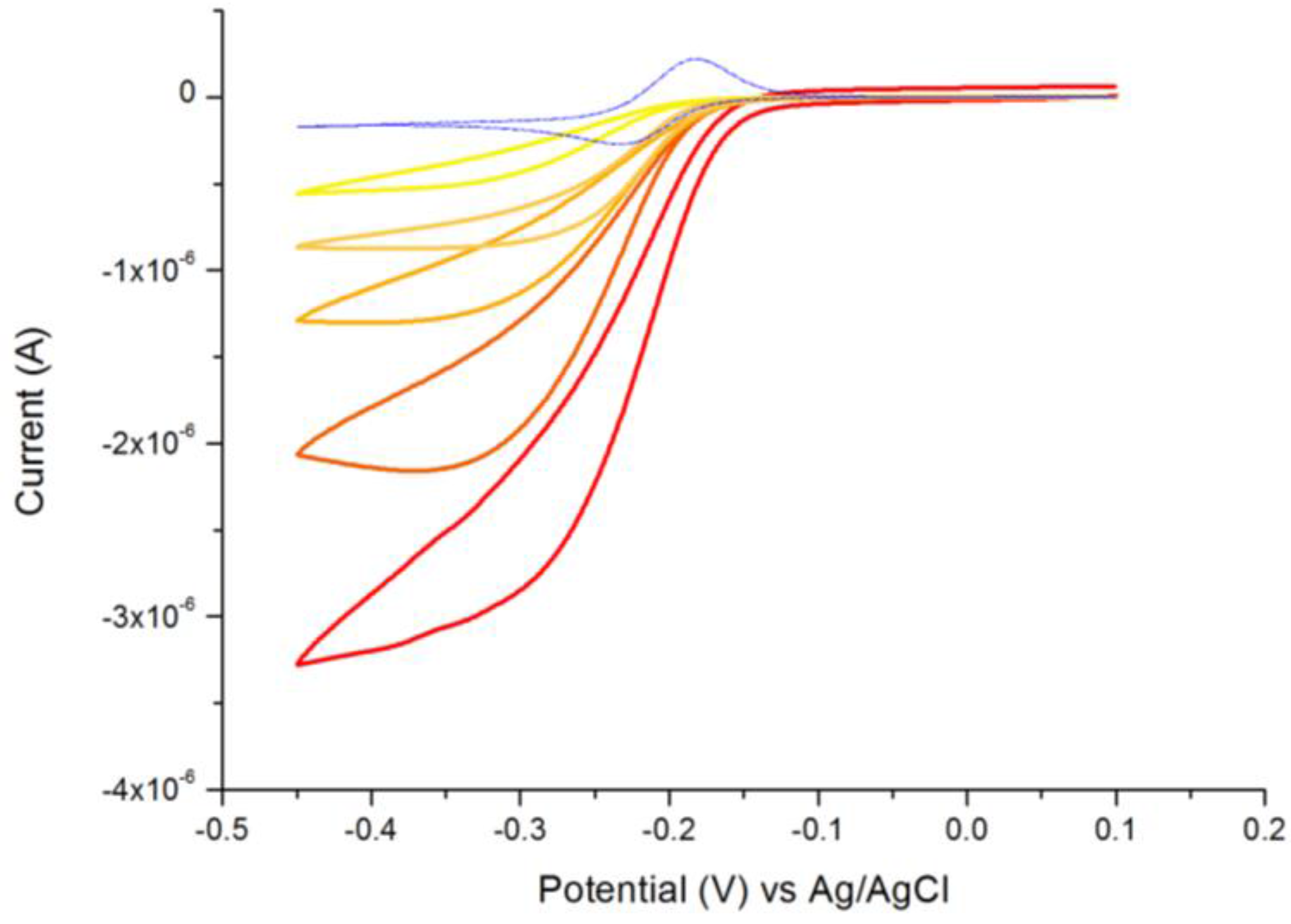
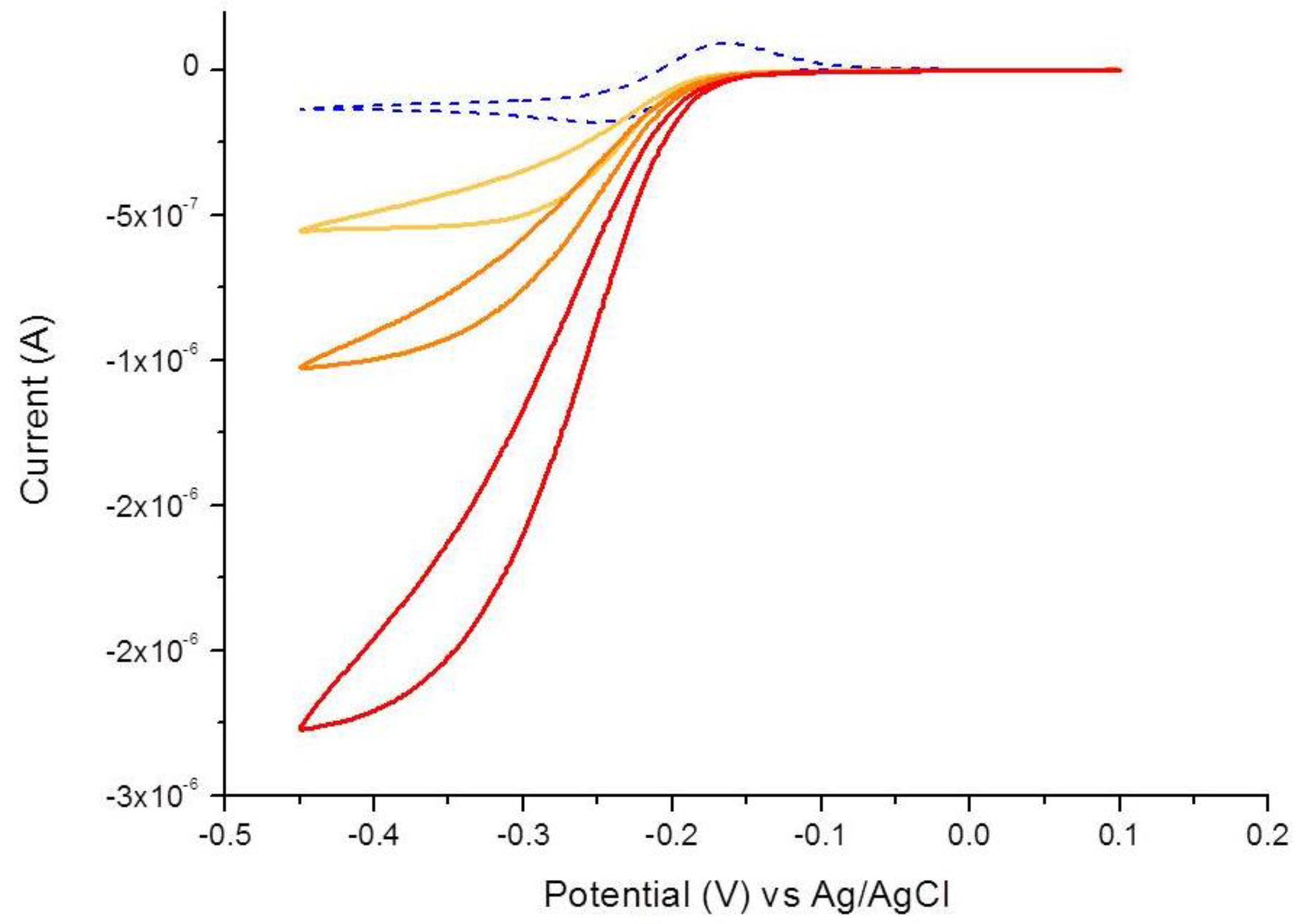
3.1. Characterization of the Immunosensor
- The CV shape moves from the peak shaped to a sigmoidal pattern, with overall current increasing with IgY amount;
- The anodic peak disappears.
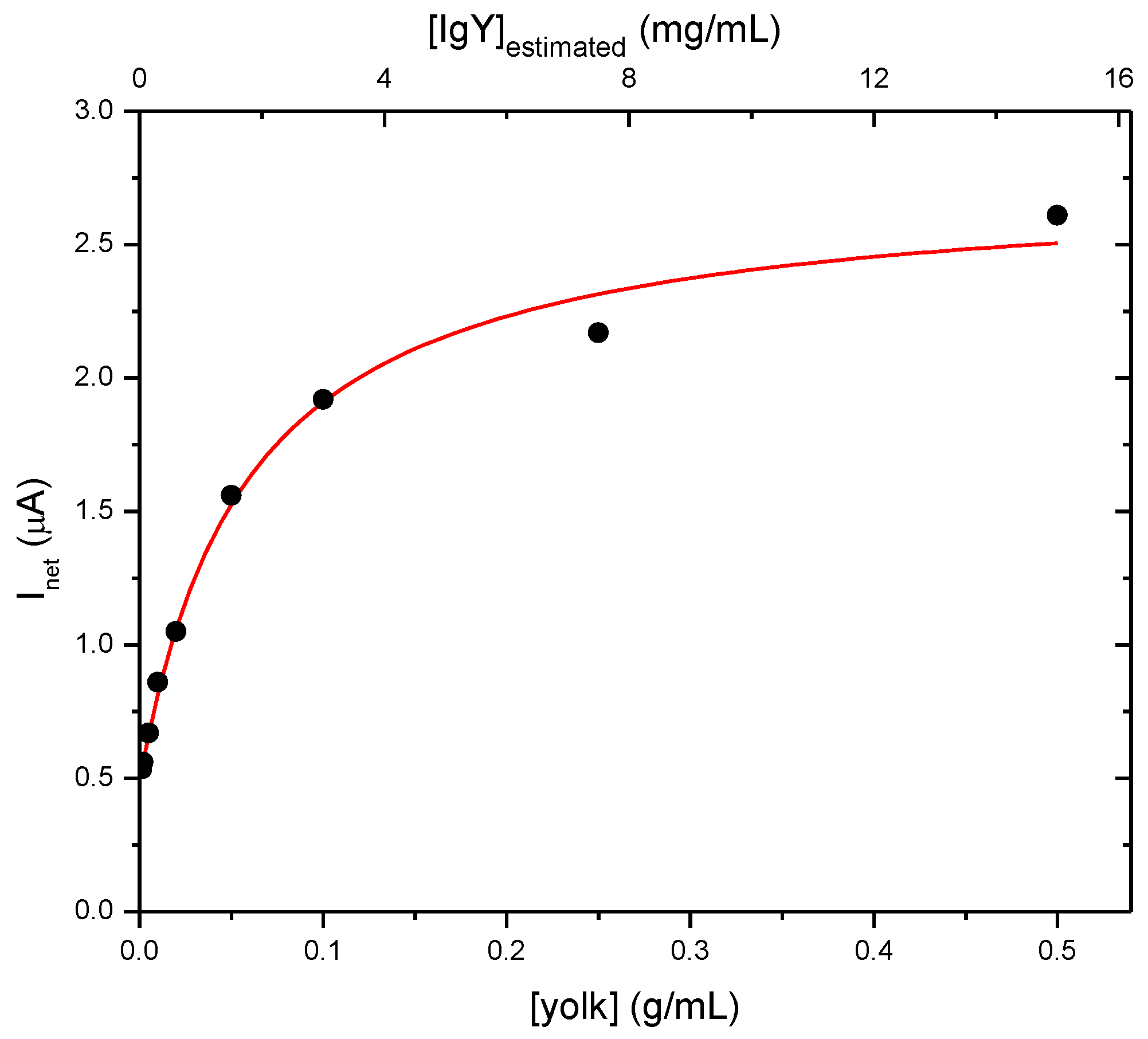
3.2. Determination of IgY in Egg
3.3. Determination of IgY in Food Integrators
3.4. SDS-PAGE and WB Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Conflicts of Interest
References
- Ulrichs, T.; Drotleff, A.M.; Ternes, W. Determination of heat-induced changes in the protein secondary structure of reconstituted livetins (water-soluble proteins from hen’s egg yolk) by FTIR. Food Chem. 2015, 172, 909–920. [Google Scholar] [CrossRef] [PubMed]
- Rahman, S.; Nguyen, S.V.; Icatlo, F.C., Jr.; Umeda, K.; Kodama, Y. Oral passive IgY-based immunotherapeutics. Hum. Vaccines Immunother. 2013, 9, 1039–1048. [Google Scholar] [CrossRef] [PubMed]
- Santos, F.N.; Brum, B.C.; Cruz, P.B.; Molinaro, C.M.; Silva, V.L.; Chaves, S.A.M. Production and Characterization of IgY against Canine IgG: Prospect of a New Tool for the Immunodiagnostic of Canine Diseases. Braz. Arch. Biol. Technol. 2014, 57, 523–531. [Google Scholar] [CrossRef]
- Spillner, E.; Braren, I.; Greunke, K.; Seismann, H.; Blank, S.; du Plessis, D. Avian IgY antibodies and their recombinant equivalents in research, diagnostics and therapy. Biologicals 2012, 40, 313–322. [Google Scholar] [CrossRef] [PubMed]
- Kovacs-Nolan, J.; Mine, Y. Egg yolk Antibodies for Passive Immunity. Annu. Rev. Food Sci. Technol. 2012, 3, 163–182. [Google Scholar] [CrossRef] [PubMed]
- Murai, A.; Kakiuchi, M.; Hmano, T.; Kobayashi, M.; Tsudzuki, M.; Nakano, M.; Matsuda, Y.; Horio, F. An ELISA for quantifying quail IgY and characterizing maternal IgY transfer to egg yolk in several quail strains. Vet. Immunol. Immunop. 2016, 175, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Fassbinder-Orth, C.A.; Wilcoxen, T.E.; Tran, T.; Boughton, R.K.; Fai, J.M.; Hofmeister, E.K.; Grindstaff, J.L.; Owen, G.C. Immunoglobulin detection in wild birds: Effectiveness of three secondary anti-avian IgY antibodies in direct ELISAs in 41 avian species. Methods Ecol. Evol. 2016, 7, 1174–1181. [Google Scholar] [CrossRef] [PubMed]
- Potenza, M.; Sabatino, G.; Giambi, F.; Rosi, L.; Papini, A.M.; Dei, L. Analysis of egg-based model wall painting by use of an innovative combined dot-ELISA and UPLC-based approach. Anal. Bioanal. Chem. 2013, 405, 691–701. [Google Scholar] [CrossRef] [PubMed]
- Scarano, S.; Carretti, E.; Dei, L.; Baglioni, P.; Minunni, M. Coupling non invasive and fast sampling of proteins from work of art surfaces to surface pasmon resonance biosensing: Differential and simultaneous detection of egg components for cultural heritage diagnosis and conservation. Biosens. Bioelectron. 2016, 85, 83–89. [Google Scholar] [CrossRef] [PubMed]
- Bottari, F.; Oliveri, P.; Ugo, P. Electrochemical immunosensor based on ensemble of nanoelectrodes for immunoglobulin IgY detection: Application to identify hen’s egg yolk in tempera paintings. Biosens. Bioelectron. 2014, 52, 403–410. [Google Scholar] [CrossRef] [PubMed]
- Wilson, M.S.; Nie, W. Electrochemical Multianalyte Immunoassays Using an Array-Based Sensor. Anal. Chem. 2006, 78, 2507–2513. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Fu, X.; Huang, X.; Wu, F.; Ma, M.; Cai, Z. A rapid triple-mode fluorescence switch assay for immunoglobulin detection by using quantum dots-gold nanoparticles nanocomposites. Sens. Actuators B 2016, 231, 779–786. [Google Scholar] [CrossRef]
- Wang, Q.; Wu, S.; Ma, M.; Cai, Z. Determination of Egg Yolk Immunoglobulin by Resonance Light Scattering of Affinity-Labeled Au Nanoparticles. Food Anal. Methods 2016, 9, 2052–2059. [Google Scholar] [CrossRef]
- Mucelli, S.P.; Zamuner, M.; Tormen, M.; Stanta, G.; Ugo, P. Nanoelectrode ensembles as recognition platform for electrochemical immunosensors. Biosens. Bioelectron. 2008, 23, 1900–1903. [Google Scholar] [CrossRef] [PubMed]
- Ongaro, M.; Ugo, P. Bioelectroanalysis with nanoelectrode ensembles and arrays. Anal. Bioanal. Chem. 2013, 405, 3715–3729. [Google Scholar] [CrossRef] [PubMed]
- Menon, V.P.; Martin, C.R. Fabrication and evaluation of nanoelectrode ensembles. Anal. Chem. 1995, 67, 1920–1928. [Google Scholar] [CrossRef]
- De Leo, M.; Pereira, F.C.; Moretto, L.M.; Scopece, P.; Polizzi, S.; Ugo, P. Towards a better understanding of gold electroless deposition in track-etched templates. Chem. Mater. 2007, 19, 5955–5964. [Google Scholar] [CrossRef]
- Gee, S.C.; Bate, I.M.; Thomas, T.M.; Rylatt, D.B. The purification of IgY from chicken egg yolk by preparative electrophoresis. Protein Expr. Purif. 2003, 30, 151–155. [Google Scholar] [CrossRef]
- Chang, H.M.; Lu, T.C.; Chen, C.C.; Tu, Y.Y.; Hwang, J.Y. Isolation of Immunoglobulin from Egg Yolk by Anionic Polysaccharides. J. Agric. Food Chem. 2000, 48, 995–999. [Google Scholar] [CrossRef] [PubMed]
- Sugita-Konishi, Y.; Shibata, K.; Yun, S.S.; Hara-Kudo, Y.; Yamaguchi, K.; Kumagai, S. Immune Functions of Immunoglobulin Y Isolated from Egg Yolk of Hens Immunized with Various Infectious Bacteria. Biosci. Biotechnol. Biochem. 1996, 60, 886–888. [Google Scholar] [CrossRef] [PubMed]
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef] [PubMed]
- Hodek, P.; Trefil, P.; Simunek, J.; Hudecek, J.; Stiborova, M. Optimized Protocol of Chicken Antibody (IgY) PurificationProviding Electrophoretically Homogenous Preparations. Int. J. Electrochem. Sci. 2013, 8, 113–124. [Google Scholar]
- Borhani, K.; Mobarez, A.M.; Khabiri, A.R.; Behmanesh, M.; Khoramabadi, N. Production of specific IgY Helicobacter pylori recombinant OipA protein and assessment of its inhibitory effects towards attachment of H. pylori to AGS cell line. Clin. Exp. Vaccine Res. 2015, 4, 177–183. [Google Scholar] [CrossRef] [PubMed]
- Pauly, D.; Chacana, P.A.; Calzado, E.G.; Brembs, B.; Schade, R. IgY Technology: Extraction of Chicken Antibodies from Egg Yolk by Polyethylene Glycol (PEG) Precipitation. J. Vis. Exp. 2011. [Google Scholar] [CrossRef] [PubMed]


| Sample | (Egg Yolk) g/mL | |
|---|---|---|
| Egg white | // | |
| Egg Yolk | 1 | 0.002 |
| 2 | 0.005 | |
| 3 | 0.01 | |
| 4 | 0.02 | |
| 5 | 0.05 | |
| 6 | 0.1 | |
| 7 | 0.25 | |
| 8 | 0.5 | |
| Sample | Powder (g) | Acetate Buffer pH: 5 (mL) | (Egg Yolk) g/mL | Protein Content * (%) |
|---|---|---|---|---|
| Egg White Powder | 1.5 | 25 | 0 | 79 |
| Egg Yolk Powder | 1.5 | 25 | 0.06 | 35 |
| Egg Yolk Powder | 1.5 | 250 | 0.006 | 35 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gaetani, C.; Ambrosi, E.; Ugo, P.; Moretto, L.M. Electrochemical Immunosensor for Detection of IgY in Food and Food Supplements. Chemosensors 2017, 5, 10. https://doi.org/10.3390/chemosensors5010010
Gaetani C, Ambrosi E, Ugo P, Moretto LM. Electrochemical Immunosensor for Detection of IgY in Food and Food Supplements. Chemosensors. 2017; 5(1):10. https://doi.org/10.3390/chemosensors5010010
Chicago/Turabian StyleGaetani, Chiara, Emmanuele Ambrosi, Paolo Ugo, and Ligia M. Moretto. 2017. "Electrochemical Immunosensor for Detection of IgY in Food and Food Supplements" Chemosensors 5, no. 1: 10. https://doi.org/10.3390/chemosensors5010010
APA StyleGaetani, C., Ambrosi, E., Ugo, P., & Moretto, L. M. (2017). Electrochemical Immunosensor for Detection of IgY in Food and Food Supplements. Chemosensors, 5(1), 10. https://doi.org/10.3390/chemosensors5010010








