Label-Free Biosensors Based onto Monolithically Integrated onto Silicon Optical Transducers
Abstract
1. Introduction
2. Evanescent Field Optics
3. Integrated Optical Sensors
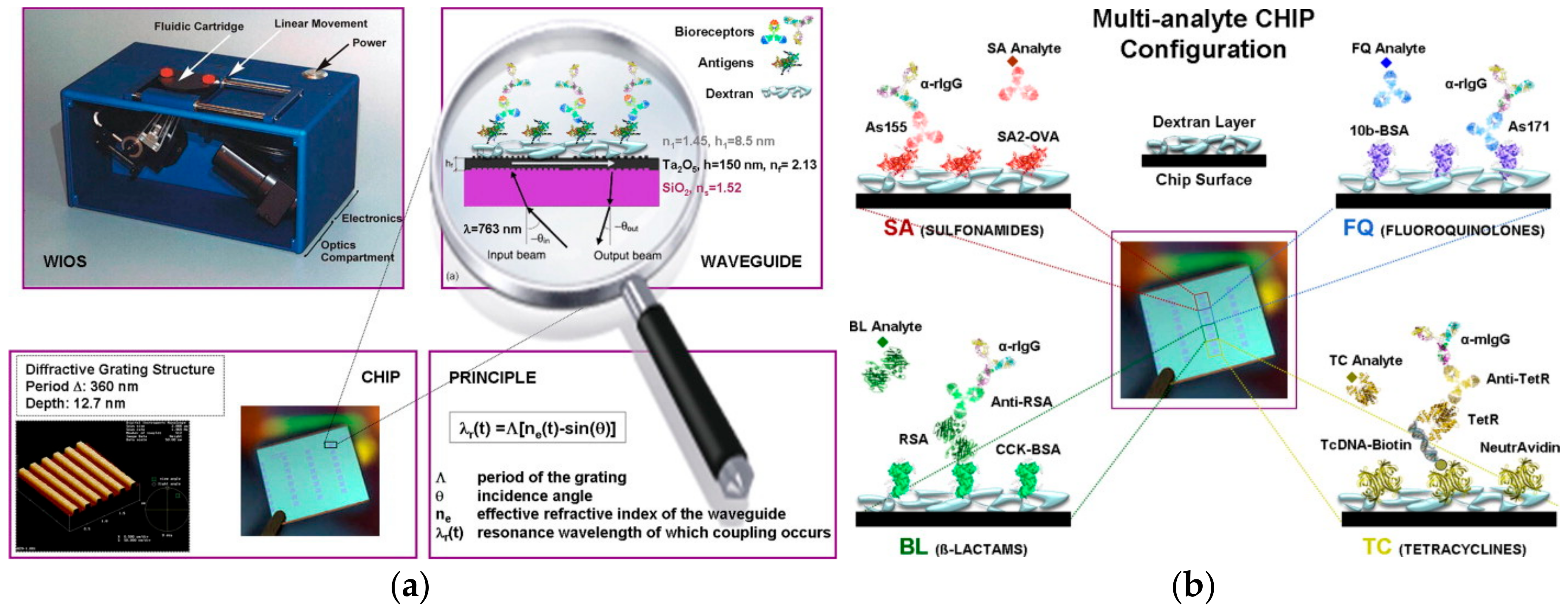
3.1. Grating-Coupled Waveguide Sensors
3.2. Microring Resonators
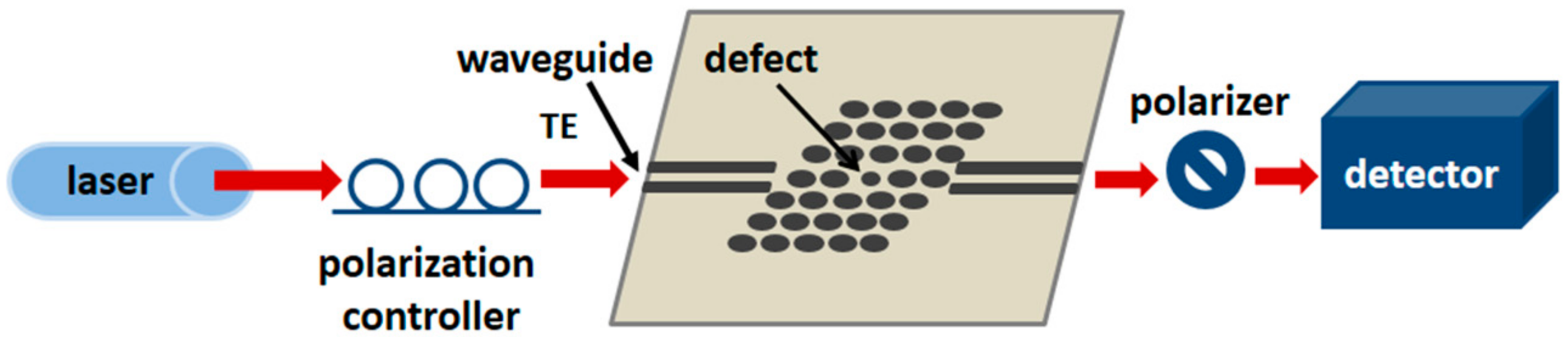
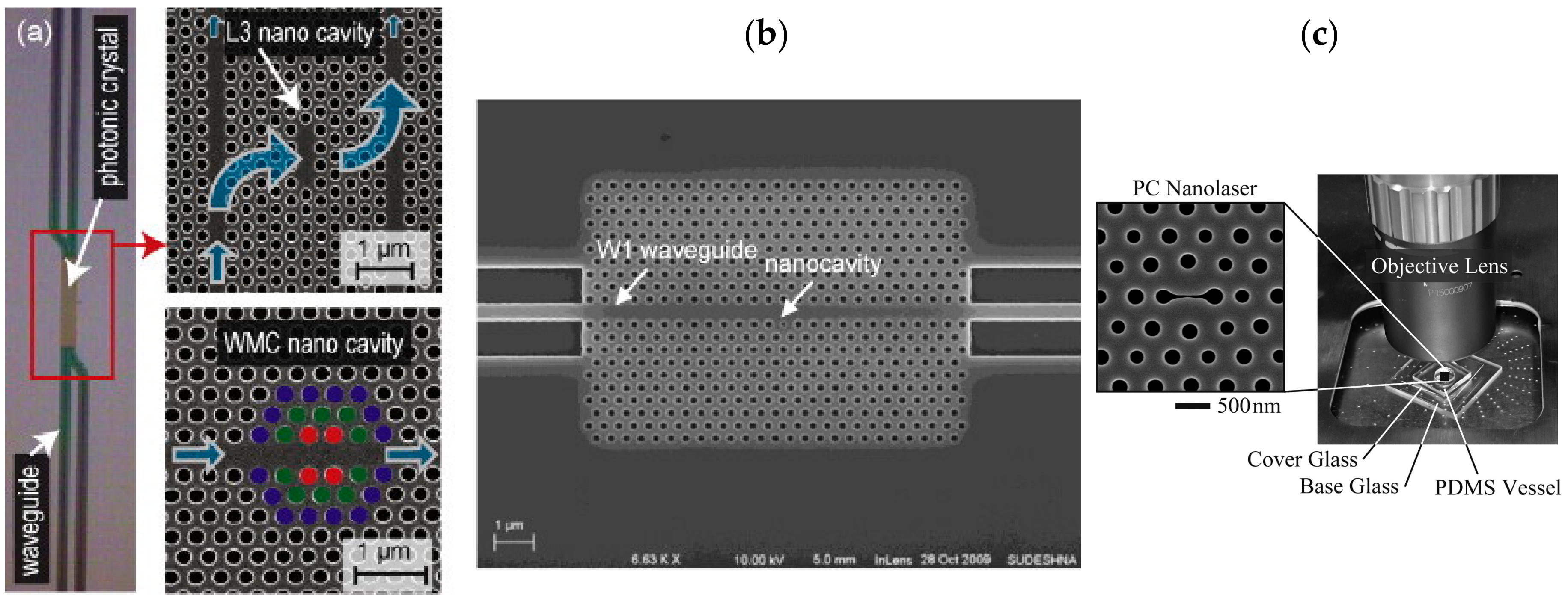
3.3. Photonic Crystal Waveguides
3.4. Integrated Interferometers
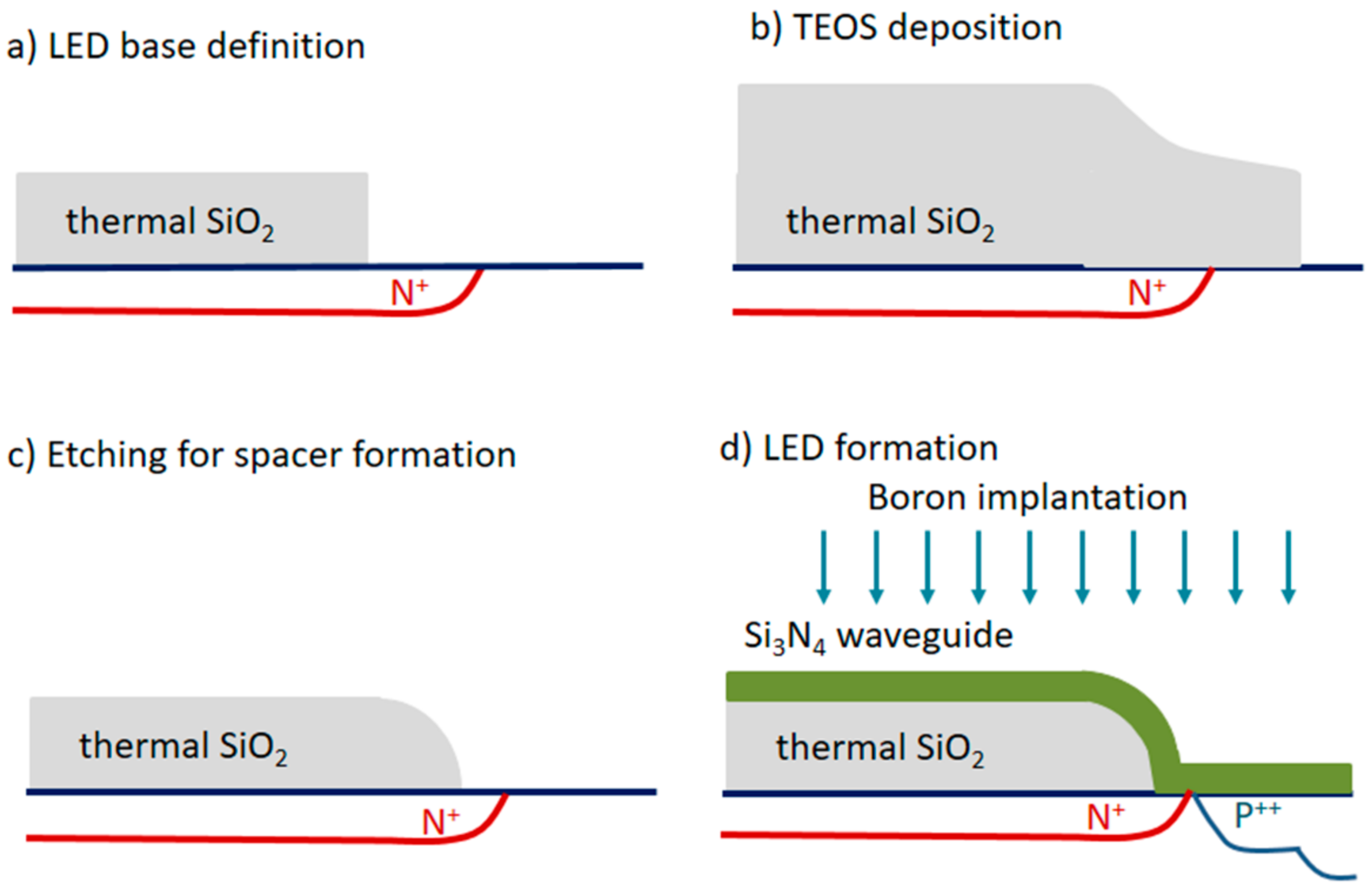
4. Monolithically Integrated Broad-Band Optoelectronic Transducers
5. Conclusions and Outlook
Author Contributions
Acknowledgement
Conflicts of Interest
References
- Clark, L., Jr.; Lyons, C. Electrode systems for continuous monitoring in cardiovascular surgery. Ann. N. Y. Acad. Sci. 1962, 102, 29–45. [Google Scholar] [CrossRef] [PubMed]
- Anthony, P.F.; Turner, A.P.F. Biosensors: Sense and sensibility. Chem. Soc. Rev. 2013, 42, 3184–3196. [Google Scholar]
- Walt, D.R. Optical methods for single molecule detection and analysis. Anal. Chem. 2013, 85, 1258–1263. [Google Scholar] [CrossRef] [PubMed]
- Estevez, M.-C.; Alvarez, M.; Lechuga, L.M. Integrated optical devices for lab-on-a-chip biosensing applications. Laser Photonics Rev. 2011, 6, 463–487. [Google Scholar] [CrossRef]
- Vashist, S.K.; Luppa, P.B.; Yeo, L.Y.; Ozcan, A.; Luong, J.H.T. Emerging technologies for next-generation point-of-care testing. Trend Biotechnol. 2015, 33, 692–705. [Google Scholar] [CrossRef] [PubMed]
- Lopez, G.A.; Estevez, M.-C.; Solera, M.; Lechuga, L.M. Recent advances in nanoplasmonic biosensors: Applications and lab-on-a-chip integration. Nanophotonic 2017, 6, 123–136. [Google Scholar] [CrossRef]
- Makarona, E.; Petrou, P.; Kakabakos, S.; Misiakos, K.; Raptis, I. Point-of-Need bioanalytics based on planar optical interferometry. Biotechnol. Adv. 2016, 34, 209–233. [Google Scholar] [CrossRef] [PubMed]
- Homola, J. Surface plasmon resonance sensors for detection of chemical and biological species. Chem. Rev. 2008, 108, 462–493. [Google Scholar] [CrossRef] [PubMed]
- Piliarik, M.; Sipova, H.; Kvasnicka, P.; Galler, N.; Krenn, J.R.; Homola, J. High-resolution biosensor based on localized surface plasmons. Opt. Express 2012, 20, 672–680. [Google Scholar] [CrossRef] [PubMed]
- Sipova, H.; Vrba, D.; Homola, J. Analytical value of detecting an individual molecular binding event: The case of the surface plasmon resonance biosensor. Anal. Chem. 2012, 84, 30–33. [Google Scholar] [CrossRef] [PubMed]
- Svedendahl, M.; Chen, S.; Dmitriev, A.; Kall, M. Refractometric sensing using propagating versus localized surface plasmons: A direct comparison. Nano Lett. 2009, 9, 4428–4433. [Google Scholar] [CrossRef] [PubMed]
- Estevez, M.C.; Otte, M.A.; Sepulveda, B.; Lechuga, L.M. Trends and challenges of refractometric nanoplasmonic biosensors: A review. Anal. Chim. Acta 2014, 806, 55–73. [Google Scholar] [CrossRef] [PubMed]
- Otte, M.A.; Sepulveda, B.; Ni, W.H.; Juste, J.P.; Liz-Marzan, L.M.; Lechuga, L.M. Identification of the optimal spectral region for plasmonic and nanoplasmonic sensing. ACS Nano 2010, 4, 349–357. [Google Scholar] [CrossRef] [PubMed]
- Yockell-Lelievre, H.; Bukar, N.; McKeating, K.S.; Arnaud, M.; Cosin, P.; Guo, Y.; Dupret-Carruel, J.; Mougin, B.; Masson, J.F. Plasmonic sensors for the competitive detection of testosterone. Analyst 2015, 140, 5105–5111. [Google Scholar] [CrossRef] [PubMed]
- Soler, M.; Estevez, M.C.; Alvarez, M.; Otte, M.A.; Sepulveda, B.; Lechuga, L.M. Direct detection of protein biomarkers in human fluids using site-specific antibody immobilization strategies. Sensors 2014, 14, 2239–2258. [Google Scholar] [CrossRef] [PubMed]
- Mazzotta, F.; Johnson, T.W.; Dahlin, A.B.; Shaver, J.; Oh, S.-H.; Höök, F. Influence of the evanescent field decay length on the sensitivity of plasmonic nanodisks and nanoholes. ACS Photonics 2015, 2, 256–262. [Google Scholar] [CrossRef]
- Brolo, A.G.; Gordon, R.; Leathem, B.; Kavanagh, K.L. Surface plasmon sensor based on the enhanced light transmission through arrays of nanoholes in gold films. Langmuir 2004, 20, 4813–4815. [Google Scholar] [CrossRef] [PubMed]
- Stewart, M.E.; Mack, N.H.; Malyarchuk, V.; Soares, J.A.; Lee, T.W.; Gray, S.K.; Nuzzo, R.G.; Rogers, J.A. Quantitative multispectral biosensing and 1D imaging using quasi-3D plasmonic crystals. Proc. Natl. Acad. Sci. USA 2006, 103, 17143–17148. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Lindquist, N.C.; Wittenberg, N.J.; Jordan, L.R.; Oh, S.H. Real-time full-spectral imaging and affinity measurements from 50 microfluidic channels using nanohole surface plasmon resonance. Lab Chip 2012, 12, 3882–3890. [Google Scholar] [CrossRef] [PubMed]
- Eftekhari, F.; Escobedo, C.; Ferreira, J.; Duan, X.; Girotto, E.M.; Brolo, A.G.; Gordon, R.; Sinton, D. Nanoholes as nanochannels: Flow-through plasmonic sensing. Anal. Chem. 2009, 81, 4308–4311. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Wittenberg, N.J.; Oh, S.H. Nanopore-induced spontaneous concentration for optofluidic sensing and particle assembly. Anal. Chem. 2012, 85, 971–977. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Cherukulappurath, S.; Johnson, T.W.; Oh, S.H. Millimeter-sized suspended plasmonic nanohole arrays for surface-tension-driven flow-through SERS. Chem. Mater. 2014, 26, 6523–6530. [Google Scholar] [CrossRef] [PubMed]
- Yanik, A.A.; Huang, M.; Kamohara, O.; Artar, A.; Geisbert, T.W.; Connor, J.H.; Altug, H. An optofluidic nanoplasmonic biosensor for direct detection of live viruses from biological media. Nano Lett. 2010, 10, 4962–4969. [Google Scholar] [CrossRef] [PubMed]
- Voros, J.; Ramsden, J.J.; Csucs, G.; Szendro, I.; De Paul, S.M.; Textor, M.; Spencer, N.D. Optical grating coupler biosensors. Biomaterials 2002, 23, 3699–3710. [Google Scholar] [CrossRef]
- Nellen, P.M.; Tiefenthaler, K.; Lukosz, W. Integrated optical input grating couplers as biochemical sensors. Sens. Actuators 1988, 15, 285–295. [Google Scholar] [CrossRef]
- Nellen, P.M.; Lukosz, W. Integrated optical input grating couplers as chemo- and immunosensors. Sens. Actuators B: Chem. 1990, 1, 592–595. [Google Scholar] [CrossRef]
- Lukosz, W.; Clerc, D.; Nellen, P.M.; Stamm, C.; Weiss, P. Output grating couplers on planar optical waveguides as direct immunosensors. Biosens. Bioelectron. 1991, 6, 227–232. [Google Scholar] [CrossRef]
- Lukosz, W.; Nellen, P.M.; Stamm, C.; Weis, P. Output grating couplers on planar waveguides as integrated optical chemical sensors. Sens. Actuators B: Chem. 1990, 1, 585–588. [Google Scholar] [CrossRef]
- Brandenburg, A.; Polzius, R.; Bier, F.; Bitewski, U.; Wagner, E. Direct observation of affinity reactions by reflected-mode operation of integrated optical grating coupler. Sens. Actuators B: Chem. 1996, 30, 55–59. [Google Scholar] [CrossRef]
- Piehler, J.; Brandenburg, A.; Brecht, A.; Wagner, E.; Gauglitz, G. Characterization of grating couplers for affinity-based pesticide sensing. Appl. Opt. 1997, 36, 6554–6562. [Google Scholar] [CrossRef] [PubMed]
- Bier, F.F.; Kleinjung, F.; Scheller, F.W. Real-time measurement of nucleic-acid hybridization using evanescent-wave sensors: Steps towards the genosensor. Sens. Actuators B: Chem. 1997, 38, 78–82. [Google Scholar] [CrossRef]
- Grego, S.; McDaniel, J.R.; Stoner, B.R. Wavelength interrogation of grating-based optical biosensors in the input coupler configuration. Sens. Actuators B: Chem. 2008, 131, 347–355. [Google Scholar] [CrossRef]
- Wiki, M.; Kunz, R.E. Wavelength-interrogated optical sensor for biochemical applications. Opt. Lett. 2000, 25, 463–465. [Google Scholar] [CrossRef] [PubMed]
- Cottier, K.; Wiki, M.; Voirin, G.; Gao, H.; Kunz, R.E. Label-free highly sensitive detection of (small) molecules by wavelength interrogation of integrated optical chips. Sens. Actuators B: Chem. 2003, 91, 241–251. [Google Scholar] [CrossRef]
- Adrian, J.; Pasche, S.; Diserens, J.M.; Sanchez-Baeza, F.; Gao, H.; Marco, M.P.; Voirin, G. Waveguide interrogated optical immunosensor (WIOS) for detection of sulfonamide antibiotics in milk. Biosens. Bioelectron. 2009, 24, 3340–3346. [Google Scholar] [CrossRef] [PubMed]
- Adrian, J.; Pasche, S.; Pinacho, D.G.; Font, H.; Diserens, J.M.; Sanchez-Baeza, J.; Granier, B.; Voirin, G.; Marco, M.P. Wavelength-interrogated optical biosensor for multi-analyte screening of sulfonamide, fluoroquinolone, β-lactam and tetracycline antibiotics in milk. TrAC Trends Anal. Chem. 2009, 28, 769–777. [Google Scholar] [CrossRef]
- Suarez, G.; Jin, Y.H.; Auerswald, J.; Berchtold, S.; Knapp, H.F.; Diserens, J.M.; Leterrier, Y.; Manson, J.A.E.; Voirin, G. Lab-on-a-chip for multiplexed biosensing of residual antibiotics in milk. Lab Chip 2009, 9, 1625–1630. [Google Scholar] [CrossRef] [PubMed]
- Pasche, S.; Giazzon, M.; Wenger, B.; Franc, G.; Ischer, R.; Oostingh, G.J.; Voirin, G. Monitoring of cellular immune responses with an optical biosensor: A new tool to assess nanoparticle toxicity. Procedia Chem. 2009, 1, 738–741. [Google Scholar] [CrossRef]
- Kehl, F.; Etlinger, G.; Gartmann, T.E.; Tscharner, N.S.R.U.; Heub, S.; Follonier, S. Introduction of an angle interrogated, MEMS-based, optical waveguide grating system for label-free biosensing. Sens. Actuators B: Chem. 2016, 226, 135–143. [Google Scholar] [CrossRef]
- OWL Sensors. Available online: http://www.owls-sensors.com/ (accessed on 2 November 2018).
- Chocarro-Ruiz, B.; Fernandez-Gavela, A.; Sonia Herranz, S.; Lechuga, L.M. Nanophotonic label-free biosensors for environmental monitoring. Curr. Opin. Biotechnol. 2017, 45, 175–183. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Eom, S.C.; Chang, J.S.; Huh, C.; Sung, G.Y.; Shin, J.H. Label-free optical biosensing using a horizontal air-slot SiNx microdisk resonator. Opt. Exp. 2010, 18, 20638–20644. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Eom, S.C.; Chang, J.S.; Huh, C.; Sung, G.Y.; Shin, J.H. A silicon nitride microdisk resonator with a 40-nm-thin horizontal air slot. Opt. Exp. 2010, 18, 11209–11215. [Google Scholar] [CrossRef] [PubMed]
- Schweinsberg, A.; Hocde, S.; Lepeshkin, N.N.; Boyd, R.W.; Chase, C.; Fajardo, J.E. An environmental sensor based on an integrated optical whispering gallery mode disk resonator. Sens. Actuators B: Chem. 2007, 123, 727–732. [Google Scholar] [CrossRef]
- DeVos, K.; Bartolozzi, I.; Schacht, E.; Bienstman, P.; Baets, R. Silicon-on-Insulator microring resonator for sensitive and label-free biosensing. Opt. Exp. 2007, 15, 7610–7615. [Google Scholar] [CrossRef]
- Luchansky, M.S.; Washburn, A.L.; Martin, T.A.; Iqbal, M.; Gunn, L.C.; Bailey, R.C. Characterization of the evanescent field profile and bound mass sensitivity of a label-free silicon photonic microring resonator biosensing platform. Biosens. Bioelectron. 2010, 26, 1283–1291. [Google Scholar] [CrossRef] [PubMed]
- Armani, A.M.; Kulkarni, R.P.; Fraser, S.E.; Flagan, R.C.; Vahala, K.J. Label-free, single-molecule detection with optical microcavities. Science 2007, 317, 783–786. [Google Scholar] [CrossRef] [PubMed]
- Armani, A.M.; Srinivasan, A.; Vahala, K.J. Soft lithographic fabrication of high Q polymer microcavity arrays. Nano Lett. 2007, 7, 1823–1826. [Google Scholar] [CrossRef] [PubMed]
- Hunt, H.K.; Soteropulos, C.; Armani, A.M. Bioconjugation strategies for microtoroidal optical resonators. Sensors 2010, 10, 9317–9336. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.M.; Choi, H.S.; Armani, A.M. Ultimate quality factor of silica microtoroid resonant cavities. Appl. Phys. Lett. 2010, 96, 153304. [Google Scholar] [CrossRef]
- Ramachandran, A.; Wang, S.; Clarke, J.; Ja, S.J.; Goad, D.; Wald, L.; Flood, E.M.; Knobbe, E.; Hryniewicz, J.V.; Chu, S.T.; et al. A universal biosensing platform based on optical micro-ring resonators. Biosens. Bioelectron. 2008, 23, 939–944. [Google Scholar] [CrossRef] [PubMed]
- Ksendzov, A.; Lin, Y. Integrated optics ring-resonator sensors for protein detection. Opt. Lett. 2005, 30, 3344–3346. [Google Scholar] [CrossRef] [PubMed]
- Chao, C.-Y.; Guo, L.J. Biochemical sensors based on polymer microrings with sharp asymmetrical resonance. Appl. Phys. Lett. 2003, 83, 1527. [Google Scholar] [CrossRef]
- DeVos, K.; Girones, J.; Popelka, S.; Schacht, E.; Baets, R.; Bienstman, P. SOI optical microring resonator with poly(ethylene glycol) polymer brush for label-free biosensor applications. Biosens. Bioelectron. 2009, 24, 2528–2533. [Google Scholar] [CrossRef] [PubMed]
- DeVos, K.; Girones, J.; Claes, T.; DeKoninck, Y.; Popelka, S.; Schacht, E.; Baets, R.; Bienstman, P. Multiplexed antibody detection with an array of silicon-on-insulator microring resonators. IEEE Photonics J. 2009, 1, 225–235. [Google Scholar] [CrossRef]
- Luchansky, M.S.; Bailey, R.C. Silicon photonic microring resonators for quantitative cytokine detection and T-cell secretion analysis. Anal. Chem. 2010, 82, 1975–1981. [Google Scholar] [CrossRef] [PubMed]
- Washburn, A.L.; Gunn, L.C.; Bailey, R.C. Label-free quantitation of a cancer biomarker in complex media using silicon photonic microring resonators. Anal. Chem. 2009, 81, 9499–9506. [Google Scholar] [CrossRef] [PubMed]
- Washburn, A.L.; Luchansky, M.S.; Bowman, A.L.; Bailey, R.C. Quantitative label-free detection of five protein biomarkers using multiplexed arrays of silicon photonic microring resonators. Anal. Chem. 2010, 82, 69–72. [Google Scholar] [CrossRef] [PubMed]
- Qavi, A.J.; Bailey, R.C. Multiplexed detection and label-free quantitation of microRNAs using arrays of silicon photonic microring resonators. Angew. Chem. Int. Ed. 2010, 49, 4608–4611. [Google Scholar] [CrossRef] [PubMed]
- Park, M.K.; Kee, J.S.; Quah, J.Y.; Vivian Netto, V.; Song, J.; Fang, Q.; La Fosse, E.M.; Lo, G.-Q. Label-free aptamer sensor based on silicon microring resonators. Sens. Actuators B: Chem. 2013, 176, 552–559. [Google Scholar] [CrossRef]
- Kim, K.W.; Song, J.; Kee, J.S.; Liu, Q.; Lo, G.-Q. Label-free biosensor based on an electrical tracing-assisted silicon microring resonator with a low-cost broad band source. Biosens. Bioelectron. 2013, 46, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Shin, Y.; Perera, A.P.; Tang, W.Y.; Fu, D.L.; Liu, Q.; Sheng, J.K.; Gu, Z.; Lee, T.Y.; Barkham, T.; Park, M.K. A rapid amplification/detection assay for analysis of Mycobacterium tuberculosis using an isothermal and silicon bio-photonic sensor complex. Biosens. Bioelectron. 2015, 68, 390–396. [Google Scholar] [CrossRef] [PubMed]
- Armani, D.K.; Kippenberg, T.J.; Spillane, S.M.; Vahala, K.J. Ultra-high-Q toroid microcavity on a chip. Nature 2003, 421, 925–928. [Google Scholar] [CrossRef] [PubMed]
- Armani, A.M.; Armani, D.K.; Min, B.; Vahala, K.J.; Spillane, S.M. Ultra-high-QQ microcavity operation in H2O and D2O. Appl. Phys. Lett. 2005, 87, 151118. [Google Scholar] [CrossRef]
- Knapper, K.A.; Heylman, K.D.; Horak, E.H.; Goldsmith, R.H. Chip-scale fabrication of high-Q all-glass toroidal microresonators for single-particle label-free imaging. Adv. Mater. 2016, 28, 2945–2950. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.S.; Zhang, X.M.; Armani, A.M. Hybrid silica-polymer ultra-high-Q microresonators. Opt. Lett. 2010, 35, 459–461. [Google Scholar] [CrossRef] [PubMed]
- Anderson, M.E.; O’Brien, E.C.; Grayek, E.N.; Hermansen, J.K.; Hunt, H.K. The detection of Helicobacter hepaticus using whispering-gallery mode microcavity optical sensors. Biosensors 2015, 5, 562–576. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, B.; Li, P.; Lin, B.; Pepper, J. Colorimetric resonant reflection as a direct biochemical assay technique. Sens. Actuators B: Chem. 2002, 81, 316–328. [Google Scholar] [CrossRef]
- Cunningham, B.; Lin, B.; Qiu, J.; Li, P.; Pepper, J.; Hugh, B. A plastic colorimetric resonant optical biosensor for multiparallel detection of label-free biochemical interactions. Sens. Actuators B: Chem. 2002, 85, 219–226. [Google Scholar] [CrossRef]
- Cunningham, B.; Qiu, J.; Li, P.; Lin, B. Enhancing the surface sensitivity of colorimetric resonant optical biosensors. Sens. Actuators B: Chem. 2002, 87, 365–370. [Google Scholar] [CrossRef]
- Block, I.D.; Chan, L.L.; Cunningham, B.T. Photonic crystal optical biosensor incorporating structured low-index porous dielectric. Sens. Actuators B: Chem. 2006, 120, 187–193. [Google Scholar] [CrossRef]
- Cunningham, B.T.; Li, P.; Schulz, S.; Lin, B.; Baird, C.; Gerstenmaier, J.; Genick, C.; Wang, F.; Fine, E.; Laing, L. Label-Free Assays on the BIND System. J. Biomol. Screen. 2004, 9, 481–489. [Google Scholar] [CrossRef] [PubMed]
- Choi, C.J.; Cunningham, B.T. A 96-well microplate incorporating a replica molded microfluidic network integrated with photonic crystal biosensors for high throughput kinetic biomolecular interaction analysis. Lab Chip 2007, 7, 550–556. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, B.T.; Laing, L. Microplate-based, label-free detection of biomolecular interactions: Applications in proteomics. Expert Rev. Proteom. 2006, 3, 271–281. [Google Scholar] [CrossRef] [PubMed]
- Srubio Systems. Available online: www.srubiosystems.com (accessed on 2 November 2018).
- Foresi, J.S.; Villeneuve, P.R.; Ferrera, J.; Thoen, E.R.; Steinmeyer, G.; Fan, S.; Joannopoulos, J.D.; Kimerling, L.C.; Smith, H.I.; Ippen, E.P. Photonic-bandgap microcavities in optical waveguides. Nature 1997, 390, 143–145. [Google Scholar] [CrossRef]
- Dorfner, D.; Zabel, T.; Hurlimann, T.; Hauke, N.; Frandsen, L.; Rant, U.; Abstreiter, G.; Finley, J. Photonic crystal nanostructures for optical biosensing applications. Biosens. Bioelectron. 2009, 24, 3688–3692. [Google Scholar] [CrossRef] [PubMed]
- Garcıa-Ruperez, J.; Toccafondo, V.; Banuls, M.J.; Castello, J.G.; Griol, A.; Peransi-Llopis, S.; Maquieira, A. Label-free antibody detection using band edge fringes in SOI planar photonic crystal waveguides in the slow-light regime. Opt. Exp. 2010, 18, 24276–24286. [Google Scholar] [CrossRef] [PubMed]
- Toccafondo, V.; Garcıa-Ruperez, J.; Banuls, M.J.; Griol, A.; Castello, J.G.; Peransi-Llopis, S.; Maquieira, A. Single-strand DNA detection using a planar photonic-crystal-waveguide-based sensor. Opt. Lett. 2010, 35, 3673–3675. [Google Scholar] [CrossRef] [PubMed]
- Mandal, S.; Erickson, D. Nanoscale optofluidic sensor arrays. Opt. Exp. 2008, 16, 1623–1631. [Google Scholar] [CrossRef]
- Mandal, S.; Goddard, J.M.; Erickson, D. A multiplexed optofluidic biomolecular sensor for low mass detection. Lab Chip 2009, 9, 2924–2932. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.; Fauchet, P.M. Two-dimensional silicon photonic crystal based biosensing platform for protein detection. Opt. Exp. 2007, 15, 4530–4535. [Google Scholar] [CrossRef]
- Pal, S.; Guillermain, E.; Sriram, R.; Miller, B.L.; Fauchet, P.M. Silicon photonic crystal nanocavity-coupled waveguides for error-corrected optical biosensing. Biosens. Bioelectron. 2011, 26, 4024–4031. [Google Scholar] [CrossRef] [PubMed]
- Zlatanovic, S.; Mirkarimi, L.W.; Sigalas, M.M.; Bynum, M.A.; Chow, E.; Robotti, K.M.; Burr, G.W.; Esener, S.; Grot, A. Photonic crystal microcavity sensor for ultracompact monitoring of reaction kinetics and protein concentration. Sens. Actuators B: Chem. 2009, 141, 13–19. [Google Scholar] [CrossRef]
- Zhang, B.; Morales, A.W.; Peterson, R.; Tang, L.; Ye, J.Y. Label-free detection of cardiac troponin I with a photonic crystal biosensor. Biosens. Bioelectron. 2014, 58, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, D.; Hachuda, S.; Watanabe, T.; Nishijima, Y.; Baba, T. Detection of endotoxin using a photonic crystal nanolaser. Appl. Phys. Lett. 2015, 106, 131112. [Google Scholar] [CrossRef]
- Mandal, S.; Serey, X.; Erickson, D. Nanomanipulation using silicon photonic crystal resonators. Nano Lett. 2010, 10, 99–104. [Google Scholar] [CrossRef] [PubMed]
- Erickson, D.; Serey, X.; Chen, Y.-F.; Mandal, S. Nanomanipulation using near field photonics. Lab Chip 2011, 11, 995–1009. [Google Scholar] [CrossRef] [PubMed]
- Kozma, P.; Kehl, F.; Ehrentreich-Förster, E.; Stamm, C.; Bier, F.F. Integrated planar optical waveguide interferometer biosensors: A comparative review. Biosens. Bioelectron. 2014, 58, 287–307. [Google Scholar] [CrossRef] [PubMed]
- Heideman, R.G.; Kooyman, R.P.H.; Greve, J. Development of an optical waveguide interferometric immunosensor. Sens. Actuators B: Chem. 1991, 4, 297–299. [Google Scholar] [CrossRef]
- Heideman, R.G.; Kooyman, R.P.H.; Greve, J. Performance of a highly sensitive optical waveguide Mach-Zehnder interferometer immunosensor. Sens. Actuators B: Chem. 1993, 10, 209–217. [Google Scholar] [CrossRef]
- Schipper, E.F.; Brugman, A.M.; Dominguez, C.; Lechuga, L.M.; Kooyman, R.P.H.; Greve, J. The realization of an integrated Mach-Zehnder waveguide immunosensor in silicon technology. Sens. Actuators B: Chem. 1997, 40, 147–153. [Google Scholar] [CrossRef]
- Brosinger, F.; Freimuth, H.; Lacher, M.; Ehrfeld, W.; Gedig, E.; Katerkamp, A.; Spener, F.; Cammann, K. A label-free affinity sensor with compensation of unspecific protein interaction by a highly sensitive integrated optical Mach-Zehnder interferometer on silicon. Sens. Actuators B: Chem. 1997, 44, 350–355. [Google Scholar] [CrossRef]
- Drapp, B.; Piehler, J.; Brecht, A.; Gauglitz, G.; Luff, B.J.; Wilkinson, J.S.; Ingenhoff, J. Integrated optical Mach-Zehnder interferometers as simazine immunoprobes. Sens. Actuators B: Chem. 1997, 38–39, 277–282. [Google Scholar] [CrossRef]
- Prieto, F.; Sepulveda, B.; Calle, A.; Llobera, A.; Dominguez, C.; Abad, A.; Montoya, A.; Lechuga, L.M. An integrated optical interferometric nanodevice based on silicon technology for biosensor applications. Nanotechnology 2003, 14, 907–912. [Google Scholar] [CrossRef]
- Prieto, F.; Sepulveda, B.; Calle, A.; Llobera, A.; Domınguez, C.; Lechuga, L.M. Integrated Mach-Zehnder interferometer based on ARROW structures for biosensor applications. Sens. Actuators B: Chem. 2003, 92, 151–158. [Google Scholar] [CrossRef]
- Sarkar, D.; Gunda, N.S.K.; Jamal, I.; Mitra, S.K. Optical biosensors with an integrated Mach-Zehnder Interferometer for detection of Listeria monocytogenes. Biomed. Microdevices 2014, 16, 509–520. [Google Scholar] [CrossRef] [PubMed]
- Chalyan, T.; Guider, R.; Pasquardini, L.; Zanetti, M.; Falke, F.; Schreuder, E.; Heideman, R.G.; Pederzolli, C.; Pavesi, L. Asymmetric Mach-Zehnder interferometer based biosensors for aflatoxin M1 detection. Biosensors 2016, 6, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Barrios, C.A. Optical slot-waveguide based biochemical sensors. Sensors 2009, 9, 4751–4765. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Tu, X.; Kim, K.W.; Kee, J.S.; Shin, Y.; Han, K.; Yoon, Y.-J.; Lo, G.-Q.; Park, M.K. Highly sensitive Mach–Zehnder interferometer biosensor based on silicon nitride slot waveguide. Sens. Actuators B: Chem. 2013, 188, 681–688. [Google Scholar] [CrossRef]
- Liu, Q.; Shin, Y.; Kee, J.S.; Kim, K.W.; Rafei, S.R.M.; Perera, A.P.; Tu, X.; Lo, G.-Q.; Ricci, E.; Colombel, M.; et al. Mach–Zehnder interferometer (MZI) point-of-care system for rapid multiplexed detection of microRNAs in human urine specimens. Biosens. Bioelectron. 2015, 71, 365–372. [Google Scholar] [CrossRef] [PubMed]
- Melnik, E.; Bruck, R.; Muellner, P.; Schlederer, T.; Hainberger, R.; Lammerhofer, M. Human IgG detection in serum on polymer based Mach-Zehnder interferometric biosensors. J. Biophotonics 2016, 9, 218–223. [Google Scholar] [CrossRef] [PubMed]
- Ymeti, A.; Greve, J.; Lambeck, P.V.; Wink, T.; van Hovell, S.W.F.M.; Beumer, T.A.M.; Wijn, R.R.; Heideman, R.G.; Subramaniam, V.; Kanger, J.S. Fast, Ultrasensitive virus detection using a Young Interferometer sensor. Nano Lett. 2007, 7, 394–397. [Google Scholar] [CrossRef] [PubMed]
- Brandenburg, A.; Krauter, R.; Kunzel, C.; Stefan, M.; Schulte, H. Interferometric sensor for detection of surface-bound bioreactions. Appl. Opt. 2000, 39, 6396–6405. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, K.; Schirmer, B.; Hoffmann, C.; Brandenburg, A.; Meyrueis, P. Interferometric biosensor based on planar optical waveguide sensor chips for label-free detection of surface bound bioreactions. Biosens. Bioelectron. 2007, 22, 2591–2597. [Google Scholar] [CrossRef] [PubMed]
- Brynda, E.; Houska, M.; Brandenburg, A.; Wikerstal, A. Optical biosensors for real-time measurement of analytes in blood plasma. Biosens. Bioelectron. 2002, 17, 665–675. [Google Scholar] [CrossRef]
- Hoffmann, C.; Schmitt, K.; Brandenburg, A.; Hartmann, S. Rapid protein expression analysis with an interferometric biosensor for monitoring protein production. Anal. Bioanal. Chem. 2007, 387, 1921–1932. [Google Scholar] [CrossRef] [PubMed]
- Nagel, T.; Ehrentreich-Forster, E.; Singh, M.; Schmitt, K.; Brandenburg, A.; Berka, A.; Bier, F.F. Direct detection of tuberculosis infection in blood serum using three optical label-free approaches. Sens. Actuators B: Chem. 2008, 129, 934–940. [Google Scholar] [CrossRef]
- Ymeti, A.; Kanger, J.S.; Greve, J.; Lambeck, P.V.; Wijn, R.; Heideman, R.G. Realization of a multichannel integrated Young interferometer chemical sensor. Appl. Opt. 2003, 42, 5649–5660. [Google Scholar] [CrossRef] [PubMed]
- Ymeti, A.; Greve, J.; Lambeck, P.V.; Wijn, R.; Heideman, R.G.; Kanger, J.S. Drift correction in a multichannel integrated optical Young interferometer. Appl. Opt. 2005, 44, 3409–3412. [Google Scholar] [CrossRef] [PubMed]
- Ymeti, A.; Kanger, J.S.; Greve, J.; Besselink, G.A.J.; Lambeck, P.V.; Wijn, R.; Heideman, R.G. Integration of microfluidics with a four-channel integrated optical Young interferometer immunosensor. Biosens. Bioelectron. 2005, 20, 1417–1421. [Google Scholar] [CrossRef] [PubMed]
- Hiltunen, M.; Hiltunen, J.; Stenberg, P.; Aikio, S.; Kurki, L.; Vahimaa, P.; Karioja, P. Polymeric slot waveguide interferometer for sensor applications. Opt Exp. 2014, 22, 7229–7237. [Google Scholar] [CrossRef] [PubMed]
- Aikio, S.; Zeilinger, M.; Hiltunen, J.; Hakalahti, L.; Hiitola-Keinanen, J.; Hiltunen, M.; Kontturi, V.; Siitonen, S.; Puustinen, J.; Lieberzeit, P.; et al. Disposable (bio)chemical integrated optical waveguide sensors implemented on roll-to-roll produced platforms. RSC Adv. 2016, 6, 50414–50422. [Google Scholar] [CrossRef]
- Zinoviev, K.E.; Gonzalez-Guerrero, A.B.; Domınguez, C.; Lechuga, L.M. Integrated bimodal waveguide interferometric biosensor for label-free analysis. J. Lightwave Technol. 2011, 29, 1926–1930. [Google Scholar] [CrossRef]
- Duval, D.; Gonzalez-Guerrero, A.B.; Dante, S.; Osmond, J.; Monge, R.; Fernandez, L.J.; Zinoviev, K.E.; Domınguez, C.; Lechuga, L.M. Nanophotonic lab-on-a-chip platforms including novel bimodal interferometers, microfluidics and grating couplers. Lab Chip 2012, 12, 1987–1994. [Google Scholar] [CrossRef] [PubMed]
- González-Guerrero, A.B.; Maldonado, J.; Dante, S.; Grajales, D.; Lechuga, L.M. Direct and label-free detection of the human growth hormone in urine by an ultrasensitive bimodal waveguide biosensor. J. Biophotonics 2016, 10, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Maldonado, J.; González-Guerrero, A.B.; Domínguez, C.; Lechuga, L.M. Label-free bimodal waveguide immunosensor for rapid diagnosis of bacterial infections in cirrhotic patients. Biosens. Bioelectron. 2016, 85, 310–316. [Google Scholar] [CrossRef] [PubMed]
- Huertas, C.S.; Fariña, D.; Lechuga, L.M. Direct and label-free quantification of micro-RNA-181a at attomolar level in complex media using a nanophotonic biosensor. ACS Sens. 2016, 1, 748–756. [Google Scholar] [CrossRef]
- Chocarro-Ruiz, B.; Herranz, S.; Fernández Gavela, A.; Sanchís, J.; Farré, M.; Marco, M.P.; Lechuga, L.M. Interferometric nanoimmunosensor for label-free and real-time monitoring of Irgarol 1051 in seawater. Biosens. Bioelectron. 2018, 117, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Szydzik, C.; Gavela, A.F.; Herranz, S.; Roccisano, J.; Knoerzer, M.; Thurgood, P.; Khoshmanesh, K.; Mitchell, A.; Lechuga, L.M. An automated optofluidic biosensor platform combining interferometric sensors and injection moulded microfluidics. Lab Chip 2017, 17, 2793–2804. [Google Scholar] [CrossRef] [PubMed]
- Luo, D.H.; Levy, R.A.; Hor, Y.F.; Federici, J.F.; Pafchek, R.M. An integrated photonic sensor for in situ monitoring of hazardous organics. Sens Actuator B 2003, 92, 121–126. [Google Scholar] [CrossRef]
- Li, Y.; Harris, E.; Chen, L.; Bao, X. Application of spectrum differential integration method in an in-line fiber Mach-Zehnder refractive index sensor. Opt Express 2010, 18, 8135–8143. [Google Scholar] [CrossRef] [PubMed]
- Martens, D.; Ramirez-Priego, P.; Murib, M.S.; Elamin, A.A.; Gonzalez-Guerrero, A.B.; Stehr, M.; Jonas, F.; Anton, B.; Hlawatsch, N.; Soetaert, P.; et al. A low-cost integrated biosensing platform based on SiN nanophotonics for biomarker detection in urine. Anal. Method 2018, 10, 3066–3073. [Google Scholar] [CrossRef]
- Mulder, H.K.P.; Ymeti, A.; Subramaniam, V.; Kanger, J.S. Size-selective detection in integrated optical interferometric biosensors. Opt. Exp. 2012, 20, 20934. [Google Scholar] [CrossRef] [PubMed]
- Nagata, T.; Namba, T.; Kuroda, Y.; Miyake, K.; Miyamoto, T.; Yokoyama, S.; Miyazaki, S.; Koyanagi, M.; Hirose, M. Single-chip integration of light-emitting diode, waveguide and micromirrors. Jpn. J. Appl. Phys. 1995, 34, 1282–1285. [Google Scholar] [CrossRef]
- Misiakos, K.; Kakabakos, S.E.; Petrou, P.S.; Ruf, H.H. A monolithic silicon optoelectronic transducer as a real-time affinity biosensor. Anal. Chem. 2004, 76, 1366–1373. [Google Scholar] [CrossRef] [PubMed]
- Chynoweth, A.G.; McKay, K.G. Photon emission from avalanche breakdown in silicon. Phys. Rev. 1956, 102, 369–376. [Google Scholar] [CrossRef]
- Petrou, P.S.; Kakabakos, S.E.; Misiakos, K. Silicon optocouplers for biosensing. Int. J. Nanotechnol. 2009, 6, 4–17. [Google Scholar] [CrossRef]
- Misiakos, K.; Petrou, P.S.; Kakabakos, S.E.; Yannoukakos, D.; Contopanagos, H.; Knoll, T.; Velten, T.; DeFazio, M.; Schiavo, L.; Passamano, M.; et al. Fully integrated monolithic optoelectronic transducer for real-time protein and DNA detection: The NEMOSLAB approach. Biosens. Bioelectron. 2010, 26, 1528–1535. [Google Scholar] [CrossRef] [PubMed]
- Kitsara, M.; Raptis, I.; Misiakos, K.; Makarona, E. Broad-band mach-zehnder interferometry as a detection principle for label-free biochemical sensing. In Proceedings of the IEEE Sensor 2008, Lecce, Italy, 26–29 October 2008; pp. 934–937. [Google Scholar]
- Misiakos, K.; Raptis, I.; Salapatas, A.; Makarona, E.; Botsialas, A.; Hoekman, M.; Stoffer, R.; Jobst, G. Broad-band Mach-Zehnder interferometers as high performance refractive index sensors: Theory and monolithic implementation. Opt. Exp. 2014, 22, 8856–8870. [Google Scholar] [CrossRef] [PubMed]
- Misiakos, K.; Raptis, I.; Makarona, E.; Botsialas, A.; Salapatas, A.; Oikonomou, P.; Psarouli, A.; Petrou, P.S.; Kakabakos, S.E.; Tukkiniemi, K.; et al. All-silicon monolithic Mach-Zehnder interferometer as a refractive index and biochemical sensor. Opt. Exp. 2014, 22, 26803–26813. [Google Scholar] [CrossRef] [PubMed]
- Psarouli, A.; Salapatas, A.; Botsialas, A.; Petrou, P.S.; Raptis, I.; Makarona, E.; Jobst, G.; Tukkiniemi, K.; Sopanen, M.; Stoffer, R.; et al. Monolithically integrated broad-band Mach-Zehnder interferometers for highly sensitive label-free detection of biomolecules through dual polarization optics. Sci. Rep. 2015, 5, 17600. [Google Scholar] [CrossRef] [PubMed]
- Angelopoulou, Μ.; Botsialas, A.; Salapatas, A.; Petrou, P.S.; Haasnoot, W.; Makarona, E.; Jobst, G.; Goustouridis, D.; Siafaka-Kapadai, A.; Raptis, I.; et al. Assessment of goat milk adulteration with a label-free monolithically integrated optoelectronic biosensor. Anal. Bioanal. Chem. 2015, 407, 3995–4004. [Google Scholar] [CrossRef] [PubMed]
- Pagkali, V.; Petrou, P.S.; Salapatas, A.; Makarona, E.; Peters, J.; Haasnoot, W.; Jobst, G.; Economou, A.; Misiakos, K.; Raptis, I.; et al. Detection of ochratoxin A in beer samples with a label-free monolithically integrated optoelectronic biosensor. J. Hazard. Mater. 2017, 323, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Psarouli, A.; Botsialas, A.; Salapatas, A.; Stefanitsis, G.; Nikita, D.; Jobst, G.; Chaniotakis, N.; Goustouridis, D.; Makarona, E.; Petrou, P.S.; et al. Fast label-free detection of C-reactive protein using broad-band Mach Zehnder interferometers integrated on silicon chips. Talanta 2017, 165, 458–465. [Google Scholar] [CrossRef] [PubMed]
- Pagkali, V.; Petrou, P.S.; Makarona, E.; Peters, J.; Haasnoot, W.; Jobst, G.; Moser, I.; Gajos, K.; Budkowski, A.; Economou, A.; et al. Simultaneous determination of aflatoxin B1, fumonisin B1 and deoxynivalenol in beer samples with a label-free monolithically integrated optoelectronic biosensor. J. Hazard. Mater. 2018, 359, 445–453. [Google Scholar] [CrossRef] [PubMed]
- Angelopoulou, M.; Petrou, P.S.; Makarona, E.; Haasnoot, W.; Moser, I.; Jobst, G.; Goustouridis, D.; Lees, M.; Kalatzi, K.; Raptis, I.; et al. Ultrafast multiplexed-allergen detection through advanced fluidic design and monolithic interferometric silicon chips. Anal. Chem. 2018, 90, 9559–9567. [Google Scholar] [CrossRef] [PubMed]
- Makarona, E.; Salapatas, A.; Raptis, I.; Petrou, P.; Kakabakos, S.; Stavra, E.; Malainou, A.; Misiakos, K. Broadband Young interferometry for simultaneous dual polarization bioanalytics. J. Opt. Soc. Am. B: Opt. Phys. 2017, 34, 1691–1698. [Google Scholar] [CrossRef]
- Savra, E.; Malainou, A.; Salapatas, A.; Botsialas, A.; Petrou, P.; Raptis, I.; Makarona, E.; Kakabakos, S.E.; Misiakos, K. Monolithically-integrated Young interferometers for label-free and multiplexed detection of biomolecules. Proc. SPIE 2016, 9752, 97520N. [Google Scholar]












| Sensor Type | Detection Resolution (RIU) | Multiplexing Capability | Portability | Overall Cost |
|---|---|---|---|---|
| SPR | 10−5 to 10−7 | + | + | +++ |
| LSPR | 10−4 to 10−6 | +++ | ++ | ++ |
| Nanohole arrays | 10−4 to 10−6 | +++ | ++ | ++ |
| Grating couplers | 10−6 to 10−7 | ++ | ++ | ++ |
| Microring resonators | 10−6 to 10−8 | +++ | ++ | ++ |
| Photonic crystals | 10−5 to 10−7 | +++ | ++ | ++ |
| MZIs | 10−5 to 10−7 | +++ | ++ | ++ |
| YIs | 10−8 to 10−9 | +++ | ++ | ++ |
| Bimodal Interferometers | 5 × 10−7 | +++ | ++ | ++ |
| BB-MZIs (semi-integrated) | 5 × 10−6 | +++ | +++ | + |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Angelopoulou, M.; Kakabakos, S.; Petrou, P. Label-Free Biosensors Based onto Monolithically Integrated onto Silicon Optical Transducers. Chemosensors 2018, 6, 52. https://doi.org/10.3390/chemosensors6040052
Angelopoulou M, Kakabakos S, Petrou P. Label-Free Biosensors Based onto Monolithically Integrated onto Silicon Optical Transducers. Chemosensors. 2018; 6(4):52. https://doi.org/10.3390/chemosensors6040052
Chicago/Turabian StyleAngelopoulou, Michailia, Sotirios Kakabakos, and Panagiota Petrou. 2018. "Label-Free Biosensors Based onto Monolithically Integrated onto Silicon Optical Transducers" Chemosensors 6, no. 4: 52. https://doi.org/10.3390/chemosensors6040052
APA StyleAngelopoulou, M., Kakabakos, S., & Petrou, P. (2018). Label-Free Biosensors Based onto Monolithically Integrated onto Silicon Optical Transducers. Chemosensors, 6(4), 52. https://doi.org/10.3390/chemosensors6040052







