Endostatin in 3D Fibrin Hydrogel Scaffolds Promotes Chondrogenic Differentiation in Swine Neonatal Meniscal Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Sample Collection
2.3. Cell Culture
2.4. Immunofluorescence: Basal Expression of Endogenous Markers
2.5. Scaffold Preparation of Fibrin Glue Hydrogel
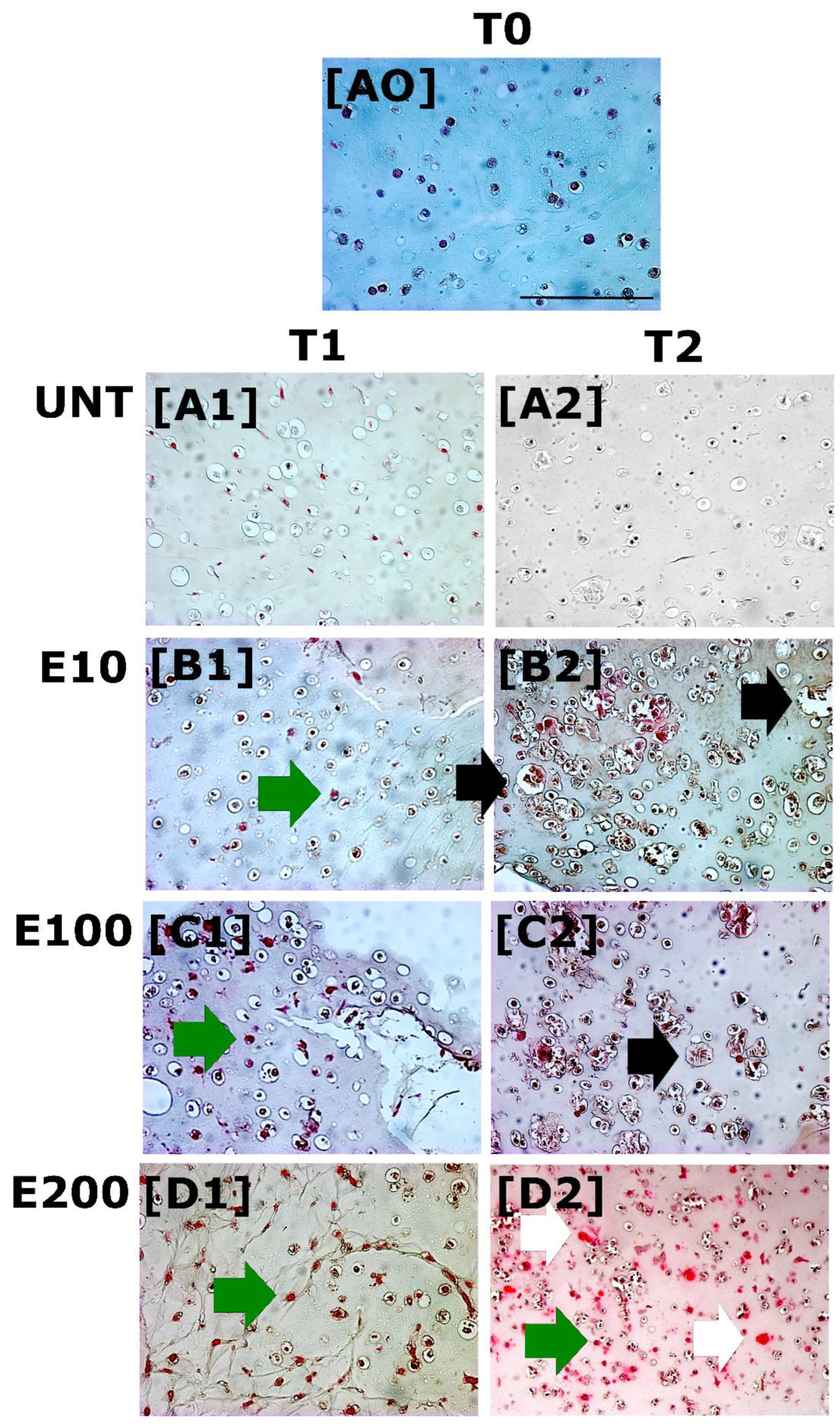
2.6. Morphological Analyses: Safranin-O Staining
2.7. Biochemical Analyses
2.8. Molecular Analyses
2.9. Statistical Analyses
3. Results
3.1. Immunofluorescence: Basal Expression of Endogenous Markers
3.2. Scaffolds Analyses
3.2.1. Safranin-O Staining: GAGs Deposition
3.2.2. Biochemical Analysis: DNA and GAGs Content
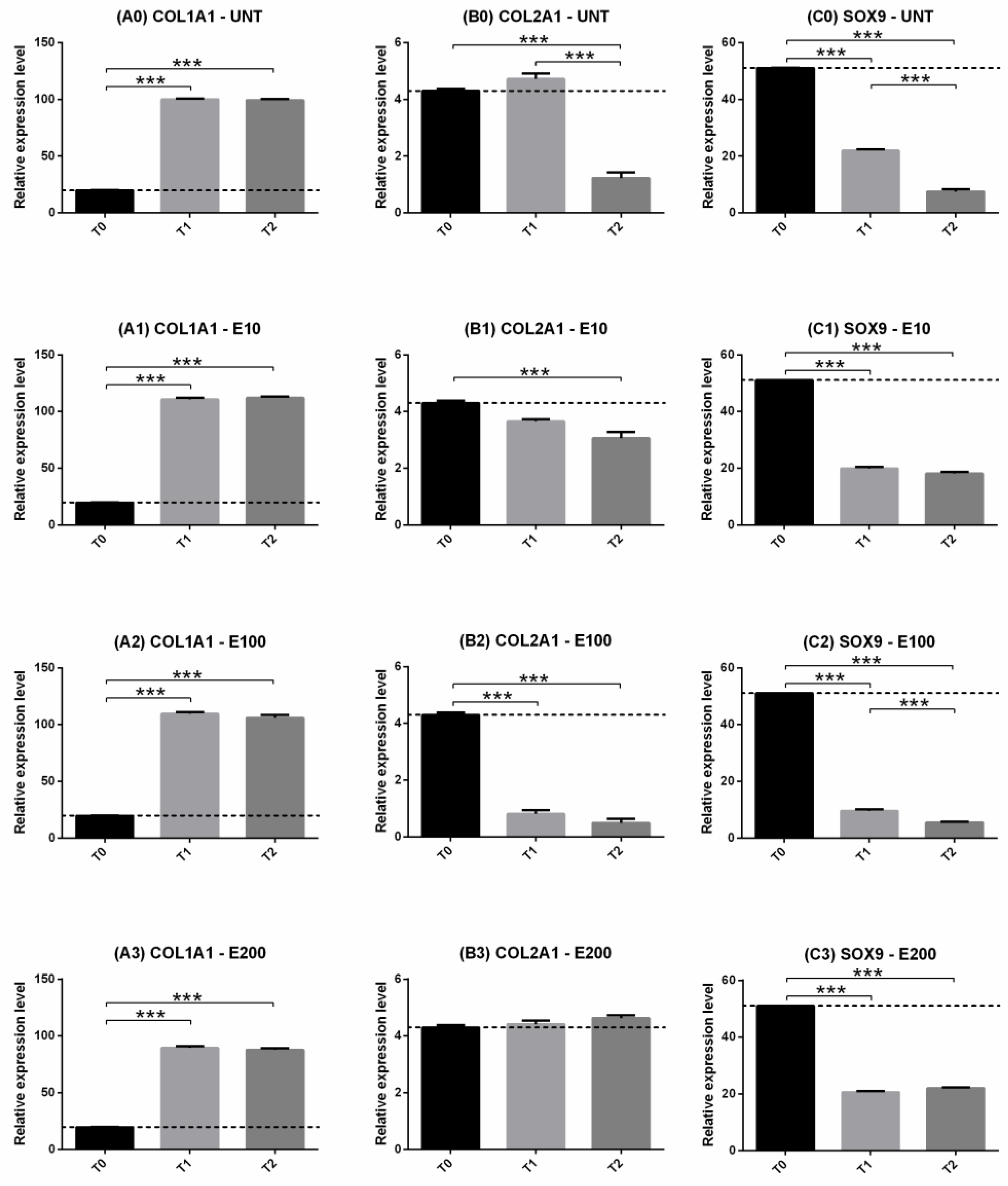
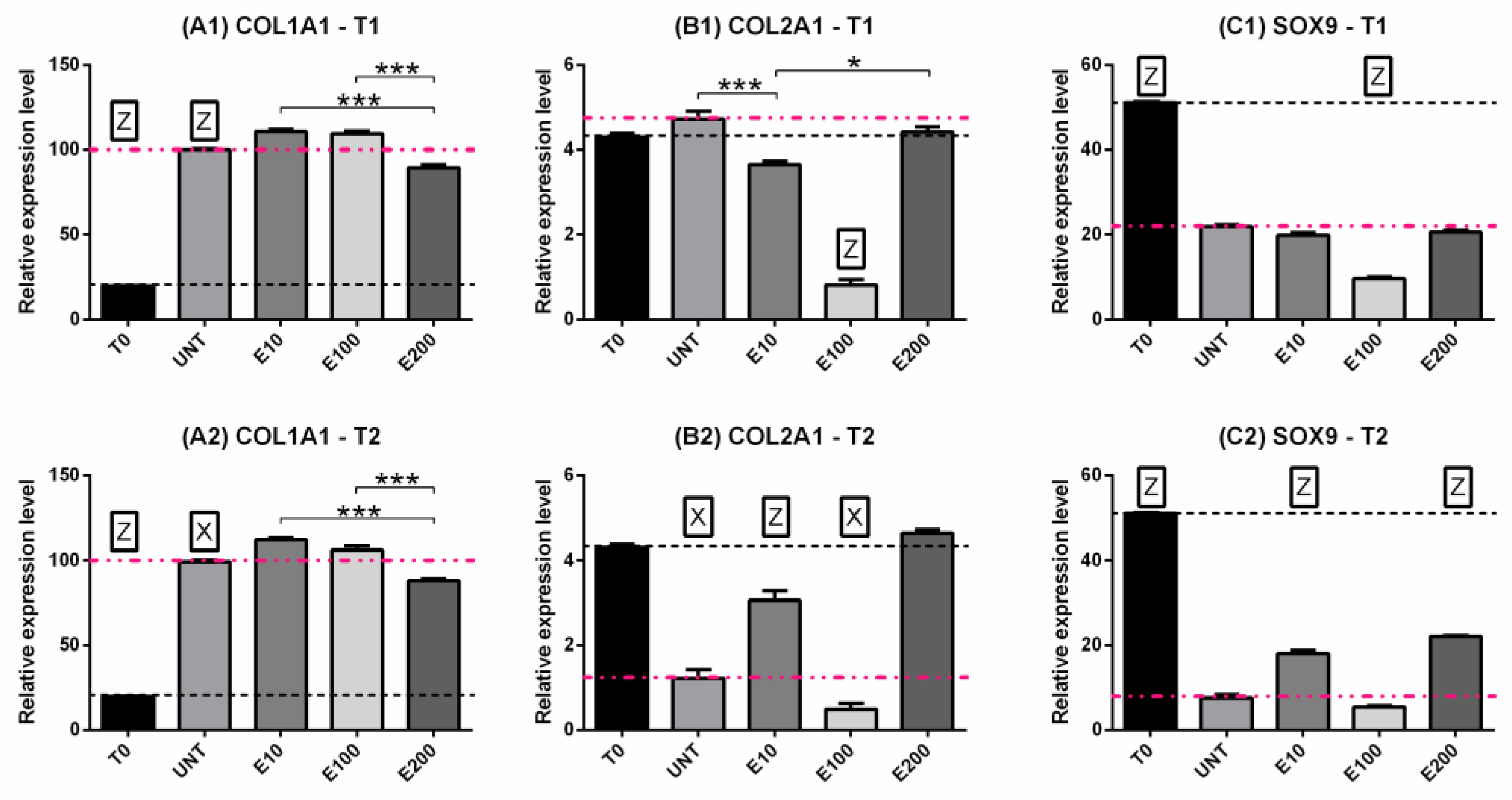
3.2.3. Gene Expression: COL1, COL2, SOX9
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Di Giancamillo, A.; Deponti, D.; Modina, S.; Tessaro, I.; Domeneghini, C.; Peretti, G.M. Age-Related Modulation of Angiogenesis-Regulating Factors In The Swine Meniscus. J. Cell. Mol. Med. 2017, 21, 3066–3075. [Google Scholar] [CrossRef] [PubMed]
- Petersen, W.; Pufe, T.; Stärke, C.; Fuchs, T.; Kopf, S.; Raschke, M.; Becker, R.; Tillmann, B. Locally Applied Angiogenic Factors—A New Therapeutic Tool for Meniscal Repair. Ann. Anat. Anat. Anzeiger. 2005, 187, 509–519. [Google Scholar] [CrossRef] [PubMed]
- Meller, R.; Schiborra, F.; Brandes, G.; Knobloch, K.; Tschernig, T.; Hankemeier, S.; Haasper, C.; Schmiedl, A.; Jagodzinski, M.; Krettek, C.; et al. Postnatal Maturation of Tendon, Cruciate Ligament, Meniscus and Articular Cartilage: A Histological Study In Sheep. Ann. Anat. Anat. Anzeiger. 2009, 191, 575–585. [Google Scholar] [CrossRef] [PubMed]
- Esparza, R.; Gortazar, A.R.; Forriol, F. Cell Study of The Three Areas of The Meniscus: Effect of Growth Factors in an Experimental Model in Sheep. J. Orthop. Res. 2012, 30, 1647–1651. [Google Scholar] [CrossRef] [PubMed]
- Kawanishi, Y.; Nakasa, T.; Shoji, T.; Hamanishi, M.; Shimizu, R.; Kamei, N.; Usman, M.A.; Ochi, M. Intra-Articular Injection of Synthetic Microrna-210 Accelerates Avascular Meniscal Healing in Rat Medial Meniscal Injured Model. Arthritis Res. Ther. 2014, 16, 488. [Google Scholar] [CrossRef]
- Fox, A.J.S.; Bedi, A.; Rodeo, S.A. The Basic Science of Human Knee Menisci: Structure, Composition, And Function. Sports Health 2012, 4, 340–351. [Google Scholar] [CrossRef] [PubMed]
- Di Giancamillo, A.; Deponti, D.; Addis, A.; Domeneghini, C.; Peretti, G.M. Meniscus Maturation in The Swine Model: Changes Occurring Along with Anterior to Posterior and Medial to Lateral Aspect During Growth. J. Cell. Mol. Med. 2014, 18, 1964–1974. [Google Scholar] [CrossRef]
- Kiani, C.; Chen, L.; Wu, Y.J.; Yee, A.J.; Yang, B.B. Structure and Function of Aggrecan. Cell Res. 2002, 12, 19–32. [Google Scholar] [CrossRef]
- Kambic, H.E.; Mcdevitt, C.A. Spatial Organization of Types I And Ii Collagen in The Canine Meniscus. J. Orthop. Res. 2005, 23, 142–149. [Google Scholar] [CrossRef]
- Aidos, L.; Modina, S.C.; Millar, V.R.H.; Peretti, G.M.; Mangiavini, L.; Ferroni, M.; Boschetti, F.; Di Giancamillo, A. Meniscus Matrix Structural and Biomechanical Evaluation: Age-Dependent Properties in A Swine Model. Bioengineering 2022, 9, 117. [Google Scholar] [CrossRef]
- Arnoczky, S.P.; Warren, R.F. Microvasculature of The Human Meniscus. Am. J. Sports Med. 1982, 10, 90–95. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Bosque, J.; Meehan, J.P.; Jamali, A.; Marder, R. Increase in Outpatient Knee Arthroscopy in The United States: A Comparison of National Surveys of Ambulatory Surgery, 1996 And 2006. JBJS 2011, 93, 994–1000. [Google Scholar] [CrossRef] [PubMed]
- Jiang, D.; Luo, X.; Ao, Y.; Gong, X.; Wang, Y.J.; Wang, H.J.; Miao, Y.; Li, N.; Zhang, J.Y.; Yu, J.K. Risk of Total/Subtotal Meniscectomy for Respective Medial and Lateral Meniscus Injury: Correlation with Tear Type, Duration Of Complaint, Age, Gender And Acl Rupture In 6034 Asian Patients. BMC Surg. 2017, 17, 127. [Google Scholar] [CrossRef]
- Englund, M.; Roos, E.M.; Roos, H.P.; Lohmander, L.S. Patient-Relevant Outcomes Fourteen Years After Meniscectomy: Influence of Type of Meniscal Tear and Size of Resection. Rheumatology 2001, 40, 631–639. [Google Scholar] [CrossRef]
- Pyne, S.W. Current Progress in Meniscal Repair and Postoperative Rehabilitation. Curr. Sports Med. Rep. 2002, 1, 265–271. [Google Scholar] [CrossRef]
- Shelbourne, K.D.; Carr, D.R. Meniscal Repair Compared with Meniscectomy for Bucket-Handle Medial Meniscal Tears In Anterior Cruciate Ligament-Reconstructed Knees. Am. J. Sports Med. 2003, 31, 718–723. [Google Scholar] [CrossRef]
- Tudor, F.; Mcdermott, I.D.; Myers, P. Meniscal Repair: A Review of Current Practice. Orthop. Trauma 2014, 28, 88–96. [Google Scholar] [CrossRef]
- Bhan, K. Meniscal Tears: Current Understanding, Diagnosis, and Management. Cureus 2020, 12, e8590. [Google Scholar] [CrossRef]
- Kopf, S.; Beaufils, P.; Hirschmann, M.T.; Rotigliano, N.; Ollivier, M.; Pereira, H.; Verdonk, R.; Darabos, N.; Ntagiopoulos, P.; Dejour, D.; et al. Management of Traumatic Meniscus Tears: The 2019 Esska Meniscus Consensus. Knee Surg. Sport Traumatol. Arthrosc. 2020, 28, 1177–1194. [Google Scholar] [CrossRef]
- Dehaven, K.E. Meniscus Repair. Am. J. Sports Med. 1999, 27, 242–250. [Google Scholar] [CrossRef]
- Turman, K.; Diduch, D. Meniscal Repair—Indications and Techniques. J. Knee Surg. 2010, 21, 154–162. [Google Scholar] [CrossRef]
- Noyes, F.R.; Barber-Westin, S.D.; Chen, R.C. Repair of Complex and Avascular Meniscal Tears and Meniscal Transplantation. Instr. Course Lect. 2011, 60, 415–437. Available online: http://www.ncbi.nlm.nih.gov/Pubmed/21553789 (accessed on 26 July 2022).
- Yan, W.; Dai, W.; Cheng, J.; Fan, Y.; Wu, T.; Zhao, F.; Zhang, J.; Hu, X.; Ao, Y. Advances in The Mechanisms Affecting Meniscal Avascular Zone Repair and Therapies. Front. Cell Dev. Biol. 2021, 9, 758217. [Google Scholar] [CrossRef]
- Lund-Olesen, K. Oxygen Tension in Synovial Fluids. Arthritis Rheum. 1970, 13, 769–776. [Google Scholar] [CrossRef]
- D’ippolito, G.; Diabira, S.; Howard, G.A.; Roos, B.A.; Schiller, P.C. Low Oxygen Tension Inhibits Osteogenic Differentiation And Enhances Stemness Of Human Miami Cells. Bone 2006, 39, 513–522. [Google Scholar] [CrossRef]
- Khan, W.S.; Adesida, A.B.; Hardingham, T.E. Hypoxic Conditions Increase Hypoxia-Inducible Transcription Factor 2α And Enhance Chondrogenesis In Stem Cells from The Infrapatellar Fat Pad of Osteoarthritis Patients. Arthritis Res. Ther. 2007, 9, R55. [Google Scholar] [CrossRef]
- Canciani, B.; Herrera Millar, V.R.; Pallaoro, M.; Aidos, L.; Cirillo, F.; Anastasia, L.; Peretti, G.M.; Modina, S.C.; Mangiavini, L.; Di Giancamillo, A. Testing Hypoxia in Pig Meniscal Culture: Biological Role of The Vascular-Related Factors in the Differentiation and Viability of Neonatal Meniscus. Int. J. Mol. Sci. 2021, 22, 12465. [Google Scholar] [CrossRef]
- Herrera Millar, V.R.; Mangiavini, L.; Polito, U.; Canciani, B.; Nguyen, V.T.; Cirillo, F.; Anastasia, L.; Peretti, G.M.; Modina, S.C.; Di Giancamillo, A. Hypoxia as A Stimulus for the Maturation of Meniscal Cells: Highway to Novel Tissue Engineering Strategies? Int. J. Mol. Sci. 2021, 22, 6905. [Google Scholar] [CrossRef]
- Domm, C.; Schünke, M.; Christesen, K.; Kurz, B. Redifferentiation Of Dedifferentiated Bovine Articular Chondrocytes in Alginate Culture Under Low Oxygen Tension. Osteoarthr. Cartil. 2002, 10, 13–22. [Google Scholar] [CrossRef]
- Schnabel, M.; Marlovits, S.; Eckhoff, G.; Fichtel, I.; Gotzen, L.; Vecsei, V.; Schlegel, J. Dedifferentiation-Associated Changes in Morphology and Gene Expression in Primary Human Articular Chondrocytes in Cell Culture. Osteoarthr. Cartil. 2002, 10, 62–70. [Google Scholar] [CrossRef]
- Stokes Dg Liu, G.; Coimbra, I.B.; Piera-Velazquez, S.; Crowl, R.M.; Jiménez, S.A. Assessment of the Gene Expression Profile of Differentiated and Dedifferentiated Human Fetal Chondrocytes by Microarray Analysis. Arthritis Rheum. 2002, 46, 404–419. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Willers, C.; Xu, J.; Zheng, M.-H. The Chondrocyte: Biology and Clinical Application. Tissue Eng. 2006, 12, 1971–1984. [Google Scholar] [CrossRef] [PubMed]
- Caron, M.M.J.; Emans, P.J.; Coolsen, M.M.E.; Voss, L.; Surtel, D.A.; Cremers, A.; van Rhijn, L.W.; Welting, T.J. Redifferentiation Of Dedifferentiated Human Articular Chondrocytes: Comparison Of 2d and 3d Cultures. Osteoarthr. Cartil. 2012, 20, 1170–1178. [Google Scholar] [CrossRef] [PubMed]
- Mellor, L.F.; Baker, T.L.; Brown, R.J.; Catlin, L.W.; Oxford, J.T. Optimal 3d Culture of Primary Articular Chondrocytes for Use In The Rotating Wall Vessel Bioreactor. Aviat. Space Environ. Med. 2014, 85, 798–804. [Google Scholar] [CrossRef]
- Ghosh, S.; Scott, A.K.; Seelbinder, B.; Barthold, J.E.; Martin, B.M.S.; Kaonis, S.; Schneider, S.E.; Henderson, J.T.; Neu, C.P. Dedifferentiation Alters Chondrocyte Nuclear Mechanics During In Vitro Culture and Expansion. Biophys. J. 2022, 121, 131–141. [Google Scholar] [CrossRef]
- Sabbagh, F.; Muhamad, I.I.; Pa’e, N.; Hashim, Z. Strategies in Improving Properties of Cellulose-Based Hydrogels for Smart Applications. Cellul. Based Superabsorbent Hydrogels 2019, 887–908. [Google Scholar] [CrossRef]
- Wu, Y.-L.; Chen, X.; Wang, W.; Loh, X.J. Engineering Bioresponsive Hydrogels Toward Healthcare Applications. Macromol. Chem. Phys. 2016, 217, 175–188. [Google Scholar] [CrossRef]
- Zheng, W.J.; Gao, J.; Wei, Z.; Zhou, J.; Chen, Y.M. Facile Fabrication of Self-Healing Carboxymethyl Cellulose Hydrogels. Eur. Polym. J. 2015, 72, 514–522. [Google Scholar] [CrossRef]
- Sannino, A.; Demitri, C.; Madaghiele, M. Biodegradable Cellulose-Based Hydrogels: Design and Applications. Materials 2009, 2, 353–373. [Google Scholar] [CrossRef]
- Wang, W.; Li, B.; Yang, J.; Xin, L.; Li, Y.; Yin, H.; Qi, Y.; Jiang, Y.; Ouyang, H.; Gao, C. The Restoration of Full-Thickness Cartilage Defects with Bmscs And Tgf-Beta 1 Loaded Plga/Fibrin Gel Constructs. Biomaterials. 2010, 31, 8964–8973. [Google Scholar] [CrossRef]
- Deponti, D.; Di Giancamillo, A.; Gervaso, F.; Domenicucci, M.; Domeneghini, C.; Sannino, A.; Peretti, G.M. Collagen Scaffold for Cartilage Tissue Engineering: The Benefit of Fibrin Glue and The Proper Culture Time In An Infant Cartilage Model. Tissue Eng. Part A 2014, 20, 1113–1126. [Google Scholar] [CrossRef] [PubMed]
- Scotti, C.; Tonnarelli, B.; Papadimitropoulos, A.; Scherberich, A.; Schaeren, S.; Schauerte, A.; Lopez-Rios, J.; Zeller, R.; Barbero, A.; Martin, I. Recapitulation of Endochondral Bone Formation Using Human Adult Mesenchymal Stem Cells as A Paradigm for Developmental Engineering. Proc. Natl. Acad. Sci. USA 2010, 107, 7251–7256. [Google Scholar] [CrossRef] [PubMed]
- Deponti, D.; Di Giancamillo, A.; Mangiavini, L.; Pozzi, A.; Fraschini, G.; Sosio, C.; Domeneghini, C.; Peretti, G.M. Fibrin-Based Model For Cartilage Regeneration: Tissue Maturation from In Vitro To In Vivo. Tissue Eng. Part A 2012, 18, 1109–1122. [Google Scholar] [CrossRef]
- Wang, Z.-H.; He, X.-J.; Yang, Z.-Q.; Tu, J.-B. Cartilage Tissue Engineering with Demineralized Bone Matrix Gelatin and Fibrin Glue Hybrid Scaffold: An In Vitro Study. Artif. Organs. 2010, 34, 161–166. [Google Scholar] [CrossRef] [PubMed]
- Mazzotti, E.; Teti, G.; Falconi, M.; Chiarini, F.; Barboni, B.; Mazzotti, A.; Muttini, A. Age-Related Alterations Affecting the Chondrogenic Differentiation Of Synovial Fluid Mesenchymal Stromal Cells In An Equine Model. Cells 2019, 8, 1116. [Google Scholar] [CrossRef]
- Kanawa, M.; Igarashi, A.; Ronald, V.s.; Higashi, Y.; Kurihara, H.; Sugiyama, M.; Saskianti, T.; Pan, H.; Kato, Y. Age-Dependent Decrease in the Chondrogenic Potential Of Human Bone Marrow Mesenchymal Stromal Cells Expanded With Fibroblast Growth Factor-2. Cytotherapy 2013, 15, 1062–1072. [Google Scholar] [CrossRef]
- Barbero, A.; Grogan, S.; Schäfer, D.; Heberer, M.; Mainil-Varlet, P.; Martin, I. Age Related Changes in Human Articular Chondrocyte Yield, Proliferation and Post-Expansion Chondrogenic Capacity. Osteoarthr. Cartil. 2004, 12, 476–484. [Google Scholar] [CrossRef]
- Vanderploeg, E.J.; Wilson, C.G.; Imler, S.M.; Ling, C.H.-Y.; Levenston, M.E. Regional Variations in The Distribution and Colocalization of Extracellular Matrix Proteins in the Juvenile Bovine Meniscus. J. Anat. 2012, 221, 174–186. [Google Scholar] [CrossRef]
- Li, Y.; Meng, H.; Liu, Y.; Lee, B.P. Fibrin Gel as an Injectable Biodegradable Scaffold and Cell Carrier for Tissue Engineering. Sci. World J. 2015, 2015, 1–10. [Google Scholar] [CrossRef]
- Peretti, G.M.; Xu, J.-W.; Bonassar, L.J.; Kirchhoff, C.H.; Yaremchuk, M.J.; Randolph, M.A. Review of Injectable Cartilage Engineering Using Fibrin Gel in Mice and Swine Models. Tissue Eng. 2006, 12, 1151–1168. [Google Scholar] [CrossRef]
- Van Susante, J.l.C.; Buma, P.; Schuman, L.; Homminga, G.n.; Van Den Berg, W.B.; Veth, R.p.h. Resurfacing Potential of Heterologous Chondrocytes Suspended in Fibrin Glue in Large Full-Thickness Defects of Femoral Articular Cartilage: An Experimental Study in the Goat. Biomaterials 1999, 20, 1167–1175. [Google Scholar] [CrossRef]
- Kaplonyi, G.; Zimmerman, I.; Frenyo, A.D.; Farkas, T.; Nemes, G. The Use of Fibrin Adhesive in the Repair of Chondral and Osteochondral Injuries. Injury 1988, 19, 267–272. [Google Scholar] [CrossRef]
- Schlag, G.; Redl, H. Fibrin Sealant in Orthopedic Surgery. Clin. Orthop. Relat. Res. 1988, 227, 269–285. Available online: http://www.ncbi.nlm.nih.gov/pubmed/2448076 (accessed on 26 July 2022). [CrossRef] [PubMed]
- Scotti, C.; Mangiavini, L.; Boschetti, F.; Vitari, F.; Domeneghini, C.; Fraschini, G.; Peretti, G.M. Effect of In Vitro Culture on a Chondrocyte-Fibrin Glue Hydrogel for Cartilage Repair. Knee Surg. Sport Traumatol. Arthrosc. 2010, 18, 1400–1406. [Google Scholar] [CrossRef]
- Ahmed, T.A.E.; Giulivi, A.; Griffith, M.; Hincke, M. Fibrin Glues in Combination with Mesenchymal Stem Cells to Develop a Tissue-Engineered Cartilage Substitute. Tissue Eng. Part A 2011, 17, 323–335. [Google Scholar] [CrossRef]
- Pei, M.; He, F.; Boyce, B.M.; Kish, V.L. Repair of Full-Thickness Femoral Condyle Cartilage Defects Using Allogeneic Synovial Cell-Engineered Tissue Constructs. Osteoarthr. Cartil. 2009, 17, 714–722. [Google Scholar] [CrossRef] [PubMed]
- Walia, A.; Yang, J.F.; Huang, Y.; Rosenblatt, M.I.; Chang, J.-H.; Azar, D.T. Endostatin’s Emerging Roles in Angiogenesis, Lymphangiogenesis, Disease, and Clinical Applications. Biochim. Biophys. Acta Gen. Subj. 2015, 1850, 2422–2438. [Google Scholar] [CrossRef]
- Hoberg, M.; Schmidt, E.L.; Tuerk, M.; Stark, V.; Aicher, W.K.; Rudert, M. Induction of Endostatin Expression in Meniscal Fibrochondrocytes by Co-Culture with Endothelial Cells. Arch. Orthop. Trauma Surg. 2009, 129, 1137–1143. [Google Scholar] [CrossRef]
- Pufe, T.; Petersen, W.J.; Miosge, N.; Goldring, M.B.; Mentlein, R.; Varoga, D.J.; Tillmann, B.N. Endostatin/Collagen Xviii—An Inhibitor of Angiogenesis—Is Expressed in Cartilage and Fibrocartilage. Matrix Biol. 2004, 23, 267–276. [Google Scholar] [CrossRef]
- Fujii, M.; Furumatsu, T.; Yokoyama, Y.; Kanazawa, T.; Kajiki, Y.; Abe, N.; Ozaki, T. Chondromodulin-I Derived from The Inner Meniscus Prevents Endothelial Cell Proliferation. J. Orthop. Res. 2013, 31, 538–543. [Google Scholar] [CrossRef]
- Hiraki, Y.; Shukunami, C. Chondromodulin-I As A Novel Cartilage-Specific Growth-Modulating Factor. Pediatr. Nephrol. 2000, 14, 602–605. [Google Scholar] [CrossRef] [PubMed]
- Klinger, P.; Surmann-Schmitt, C.; Brem, M.; Swoboda, B.; Distler, J.H.; Carl, H.D.; von der Mark, K.; Hennig, F.F.; Gelse, K. Chondromodulin 1 Stabilizes the Chondrocyte Phenotype and Inhibits Endochondral Ossification of Porcine Cartilage Repair Tissue. Arthritis Rheum. 2011, 63, 2721–2731. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Ding, D.; Ren, G.; Xu, X.; Yin, X.; Hu, Y. Sustained Delivery Of Endostatin Improves The Efficacy Of Therapy In Lewis Lung Cancer Model. J. Control. Release 2009, 134, 91–97. [Google Scholar] [CrossRef] [PubMed]
- O’reilly, M.s.; Boehm, T.; Shing, Y.; Fukai, N.; Vasios, G.; Lane, W.S.; Flynn, E.; Birkhead, J.R.; Olsen, B.R.; Folkman, J. Endostatin: An Endogenous Inhibitor of Angiogenesis and Tumor Growth. Cell 1997, 88, 277–285. [Google Scholar] [CrossRef]
- Celik, I.; Sürücü, O.; Dietz, C.; Heymach, J.V.; Force, J.; Höschele, I.; Becker, C.M.; Folkman, J.; Kisker, O. Therapeutic Efficacy of Endostatin Exhibits A Biphasic Dose-Response Curve. Cancer Res. 2005, 65, 11044–11050. [Google Scholar] [CrossRef]
- Lotz, M.; Loeser, R.F. Effects of Aging on Articular Cartilage Homeostasis. Bone 2012, 51, 241–248. [Google Scholar] [CrossRef]
- Gilmore, R.S.; Palfrey, A.J. Chondrocyte Distribution in the Articular Cartilage of Human Femoral Condyles. J. Anat. 1988, 157, 23–31. [Google Scholar]
- Meachim, G.; Collins, D.h. Cell Counts of Normal and Osteo-Arthritic Articular Cartilage in Relation to the Uptake Of Sulphate (35so4) In Vitro. Ann. Rheum. Dis. 1962, 21, 45–50. [Google Scholar] [CrossRef]
- Quintero, M.; Mitrovic, D.R.; Stankovic, A.; De Sèze, S.; Miravet, L.; Ryckewaert, A. Cellular Aspects of the Aging of Articular Cartilage. I. Condylar Cartilage with a Normal Surface Sampled from Normal Knees. Rev. Rhum. Mal. Osteoartic. 1984, 51, 375–379. [Google Scholar]
- Stockwell, R.A. The Cell Density of Human Articular and Costal Cartilage. J. Anat. 1967, 101 Pt 4, 753–763. Available online: http://www.ncbi.nlm.nih.gov/Pubmed/6059823 (accessed on 26 July 2022).
- Sun, X.-D.; Jeng, L.; Bolliet, C.; Olsen, B.R.; Spector, M. Non-Viral Endostatin Plasmid Transfection of Mesenchymal Stem Cells Via Collagen Scaffolds. Biomaterials 2009, 30, 1222–1231. [Google Scholar] [CrossRef] [PubMed]
- Jeng, L.; Olsen, B.R.; Spector, M. Engineering Endostatin-Producing Cartilaginous Constructs for Cartilage Repair Using Nonviral Transfection of Chondrocyte-Seeded and Mesenchymal-Stem-Cell-Seeded Collagen Scaffolds. Tissue Eng. Part A 2010, 16, 3011–3021. [Google Scholar] [CrossRef] [PubMed]
- Adesida, A.B.; Grady, L.M.; Khan, W.S.; Hardingham, T.E. The Matrix-Forming Phenotype of Cultured Human Meniscus Cells Is Enhanced After Culture with Fibroblast Growth Factor 2 and Is Further Stimulated by Hypoxia. Arthritis Res. Ther. 2006, 8, R61. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Verbruggen, G.; Almqvist, K.F.; Elewaut, D.; Broddelez, C.; Veys, E.M. Flow Cytometric Analysis of the Human Articular Chondrocyte Phenotype In Vitro. Osteoarthr. Cartil. 2001, 9, 73–84. [Google Scholar] [CrossRef] [Green Version]
- Karjalainen, V.-P.; Kestilä, I.; Finnilä, M.A.; Folkesson, E.; Turkiewicz, A.; Önnerfjord, P.; Hughes, V.; Tjörnstrand, J.; Englund, M.; Saarakkala, S. Quantitative Three-Dimensional Collagen Orientation Analysis of Human Meniscus Posterior Horn in Health and Osteoarthritis Using Micro-Computed Tomography. Osteoarthr. Cartil. 2021, 29, 762–772. [Google Scholar] [CrossRef]
- Pauli, C.; Grogan, S.P.; Patil, S.; Otsuki, S.; Hasegawa, A.; Koziol, J.; Lotz, M.K.; D’Lima, D.D. Macroscopic and Histopathologic Analysis of Human Knee Menisci in Aging and Osteoarthritis. Osteoarthr. Cartil. 2011, 19, 1132–1141. [Google Scholar] [CrossRef]
- Battistelli, M.; Favero, M.; Burini, D.; Trisolino, G.; Dallari, D.; De Franceschi, L.; Goldring, S.R.; Goldring, M.B.; Belluzzi, E.; Filardo, G.; et al. Morphological and Ultrastructural Analysis of Normal, Injured and Osteoarthritic Human Knee Menisci. Eur. J. Histochem. 2019, 63, 2998. [Google Scholar] [CrossRef]
- Ng, L.-J.; Wheatley, S.; Muscat, G.E.; Conway-Campbell, J.; Bowles, J.; Wright, E.; Bell, D.M.; Tam, P.P.; Cheah, K.S.; Koopman, P. Sox9 Binds Dna, Activates Transcription, and Coexpresses with Type Ii Collagen During Chondrogenesis in the Mouse. Dev. Biol. 1997, 183, 108–121. [Google Scholar] [CrossRef]
- Brew, C.J.; Clegg, P.D.; Boot-Handford, R.P.; Andrew, J.G.; Hardingham, T. Gene Expression in Human Chondrocytes in Late Osteoarthritis Is Changed in Both Fibrillated and Intact Cartilage Without Evidence of Generalised Chondrocyte Hypertrophy. Ann Rheum Dis. 2010, 69, 234–240. [Google Scholar] [CrossRef]
- Akiyama, H.; Kim, J.E.; Nakashima, K.; Balmes, G.; Iwai, N.; Deng, J.M.; Zhang, Z.; Martin, J.F.; Behringer, R.R.; Nakamura, T.; et al. Osteo-Chondroprogenitor Cells Are Derived from Sox9 Expressing Precursors. Proc. Natl. Acad. Sci. USA 2005, 102, 14665–14670. [Google Scholar] [CrossRef]
- Wang, Y.; Sul, H.s. Pref-1 Regulates Mesenchymal Cell Commitment and Differentiation Through Sox9. Cell Metab. 2009, 9, 287–302. [Google Scholar] [CrossRef] [PubMed]
- Ikegami, D.; Akiyama, H.; Suzuki, A.; Nakamura, T.; Nakano, T.; Yoshikawa, H.; Tsumaki, N. Sox9 Sustains Chondrocyte Survival and Hypertrophy in Part Through Pik3ca-Akt Pathways. Development 2011, 138, 1507–1519. [Google Scholar] [CrossRef] [PubMed]
- Sekiya, I.; Katano, H.; Ozeki, N. Characteristics of Mscs in Synovial Fluid and Mode of Action of Intra-Articular Injections of Synovial Mscs in Knee Osteoarthritis. Int. J. Mol. Sci. 2021, 22, 2838. [Google Scholar] [CrossRef] [PubMed]
- Puetzer, J.L.; Bonassar, L.J. Physiologically Distributed Loading Patterns Drive The Formation Of Zonally Organized Collagen Structures in Tissue-Engineered Meniscus. Tissue Eng. Part A 2016, 22, 907–916. [Google Scholar] [CrossRef] [PubMed]




| Gene | Forward (5′-3′) | Reverse (5′-3′) | Amplicon Size (bp) |
|---|---|---|---|
| COL1A1 | CCA ACA AGG CCA AGA AGA AG | ATG GTA CCT GAG GCC GTT CT | 64 |
| COL2A1 | CAC GGA TGG TCC CAA AGG | ATA CCA GCA GCT CCC CTC T | 102 |
| SOX9 | CCG GTG CGC GTC AAC | TGC AGG TGC GGG TAC TGAT | 119 |
| ACT | CAA GGA GAA GCT CTG CTA CG | AGA GGT CCT TCC TGA TGT CC | 245 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Herrera Millar, V.R.; Canciani, B.; Mangiavini, L.; Filipe, J.F.S.; Aidos, L.; Pallaoro, M.; Peretti, G.M.; Pocar, P.; Modina, S.C.; Di Giancamillo, A. Endostatin in 3D Fibrin Hydrogel Scaffolds Promotes Chondrogenic Differentiation in Swine Neonatal Meniscal Cells. Biomedicines 2022, 10, 2415. https://doi.org/10.3390/biomedicines10102415
Herrera Millar VR, Canciani B, Mangiavini L, Filipe JFS, Aidos L, Pallaoro M, Peretti GM, Pocar P, Modina SC, Di Giancamillo A. Endostatin in 3D Fibrin Hydrogel Scaffolds Promotes Chondrogenic Differentiation in Swine Neonatal Meniscal Cells. Biomedicines. 2022; 10(10):2415. https://doi.org/10.3390/biomedicines10102415
Chicago/Turabian StyleHerrera Millar, Valentina Rafaela, Barbara Canciani, Laura Mangiavini, Joel Fernando Soares Filipe, Lucia Aidos, Margherita Pallaoro, Giuseppe Maria Peretti, Paola Pocar, Silvia Clotilde Modina, and Alessia Di Giancamillo. 2022. "Endostatin in 3D Fibrin Hydrogel Scaffolds Promotes Chondrogenic Differentiation in Swine Neonatal Meniscal Cells" Biomedicines 10, no. 10: 2415. https://doi.org/10.3390/biomedicines10102415





