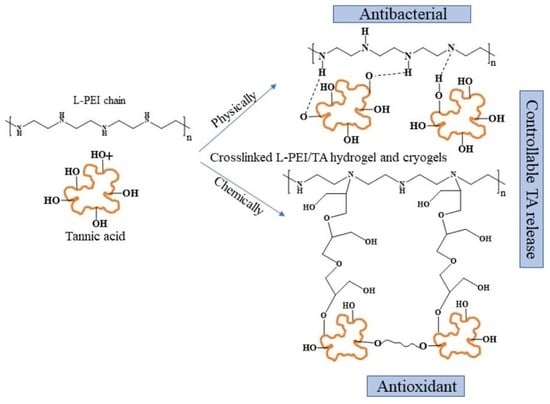
Physically and Chemically Crosslinked, Tannic Acid Embedded Linear PEI-Based Hydrogels and Cryogels with Natural Antibacterial and Antioxidant Properties
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of L-PEI
2.3. Synthesis of L-PEI-Based Hydrogels and Cryogels
2.3.1. Synthesis of Physically Crosslinked L-PEI and L-PEI/TA Hydrogels
2.3.2. Synthesis of Chemically Crosslinked L-PEI, L-PEI/TA Hydrogels, and Cryogels
2.4. Characterization
2.5. TA Release from L-PEI/TA Hydrogels and Cryogels
2.6. Antioxidant Properties of L-PEI-Based Hydrogels and Cryogels
2.6.1. Total Phenol Content Assay
2.6.2. ABTS+ Scavenging Assay
2.7. Antimicrobial Activity Tests of L-PEI-Based Hydrogels and Cryogels
2.7.1. Macro-Dilution Assay
2.7.2. Zone of Inhibition Experiments
3. Results and Discussion
3.1. Synthesis and Characterization of L-PEI-Based Hydrogels and Cryogels
3.2. Synthesis and Characterization of L-PEI/TA-Based Hydrogels and Cryogels
3.3. TA Release from L-PEI/TA-Based Hydrogels and Cryogels
3.4. Antioxidant Properties of L-PEI/TA-Based Hydrogels and Cryogels
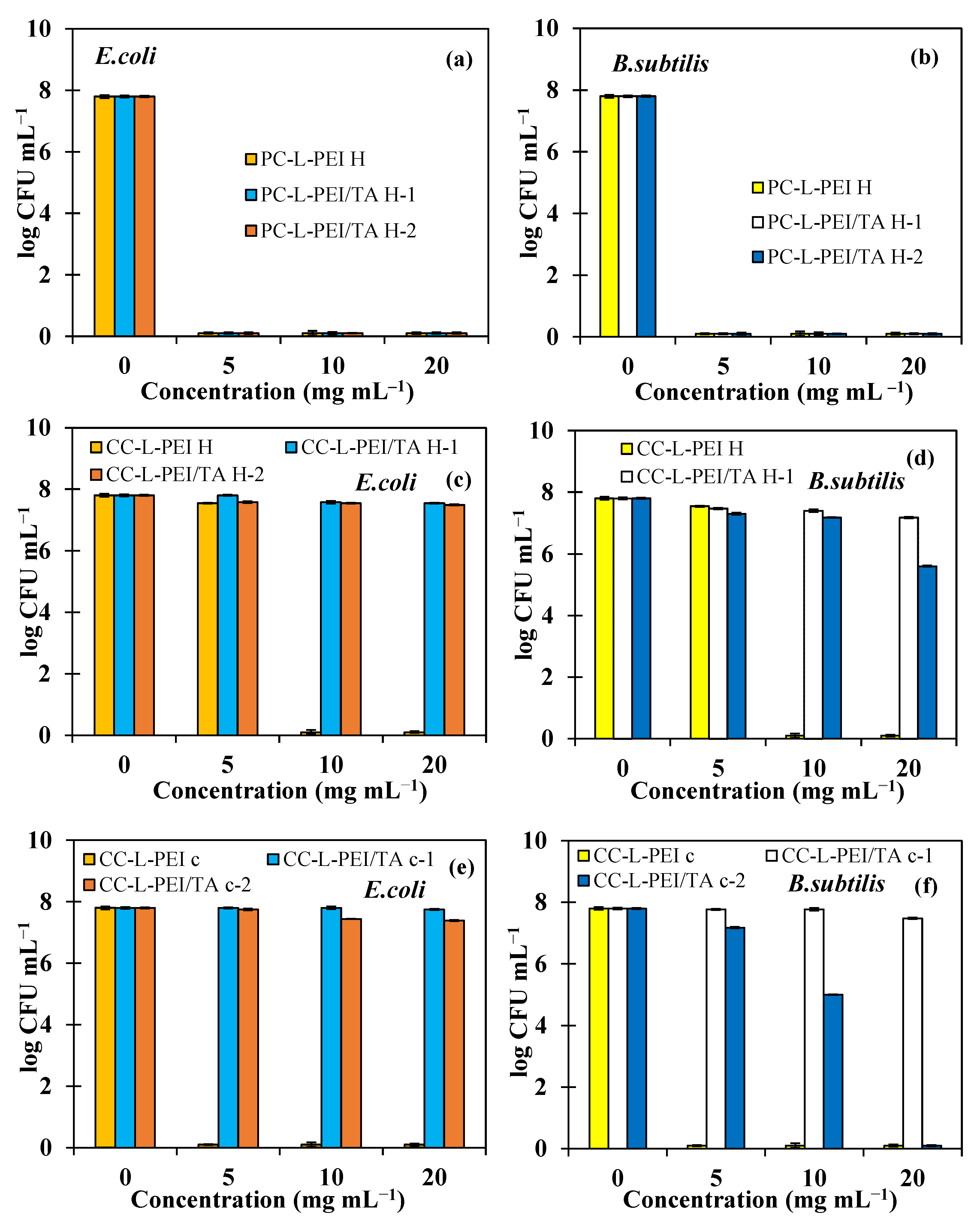
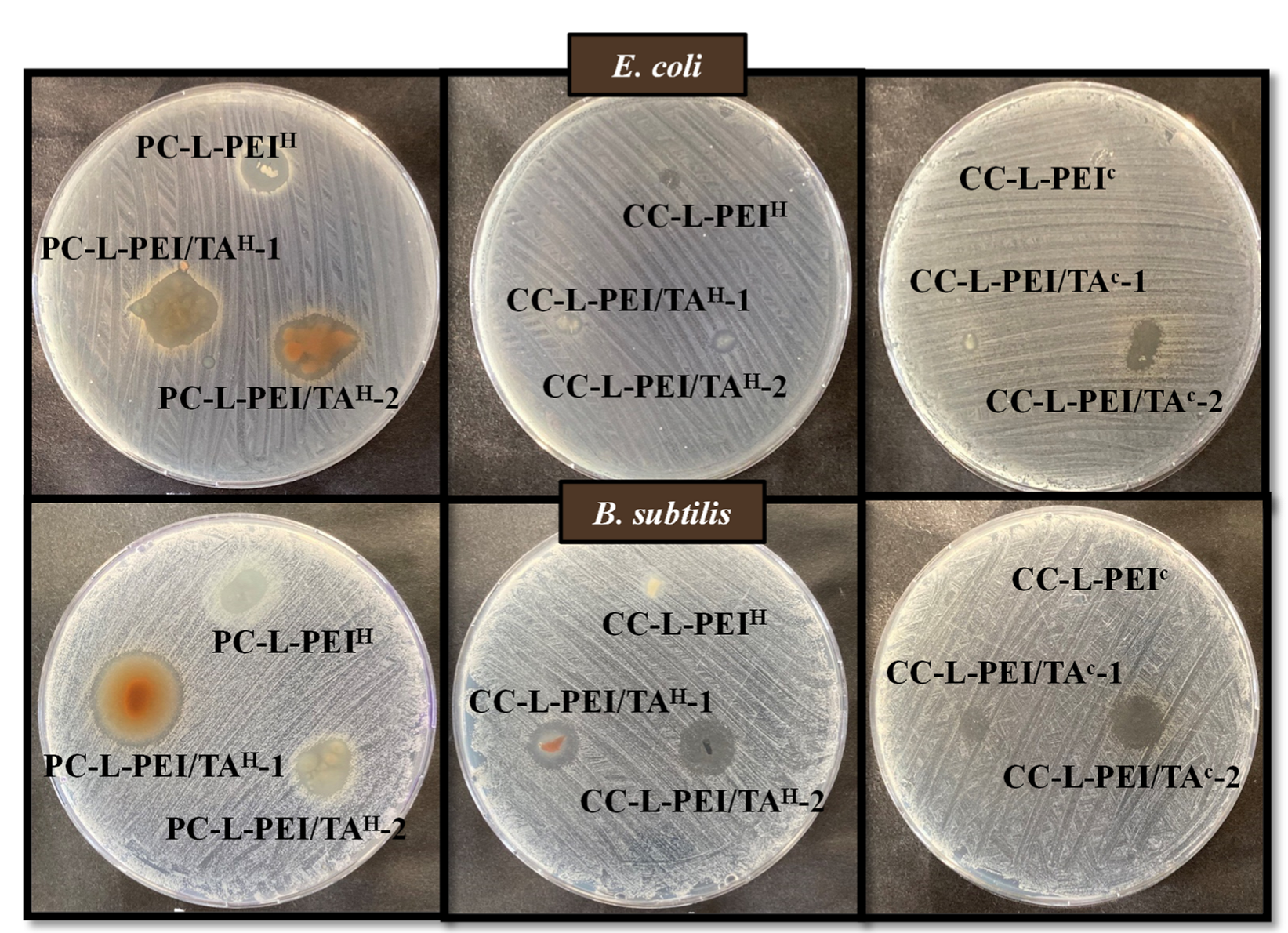
3.5. Antibacterial Properties of L-PEI and L-PEI/TA-Based Hydrogels and Cryogels
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ateia, M.; Arifuzzaman, M.; Pellizzeri, S.; Attia, M.F.; Tharayil, N.; Anker, J.N.; Karanfil, T. Cationic polymer for selective removal of GenX and short-chain PFAS from surface waters and wastewaters at ng/L levels. Water Res. 2019, 163, 114874. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Xia, K.; Liu, X.; Fang, L.; Du, H.; Zhang, X. Utilization of cationic polymer-modified fly ash for dye wastewater treatment. Clean Technol. Environ. Policy 2021, 23, 1273–1282. [Google Scholar] [CrossRef]
- Li, X.; Li, Y.; Wang, H.; Niu, Z.; He, Y.; Jin, L.; Wu, M.; Wang, H.; Chai, L.; Al-Enizi, A.M.; et al. 3D Cationic Polymeric Network Nanotrap for Efficient Collection of Perrhenate Anion from Wastewater. Small 2021, 17, 2007994. [Google Scholar] [CrossRef]
- Chen, Z.; Lv, Z.; Sun, Y.; Chi, Z.; Qing, G. Recent advancements in polyethyleneimine-based materials and their biomedical, biotechnology, and biomaterial applications. J. Mater. Chem. B 2020, 8, 2951–2973. [Google Scholar] [CrossRef] [PubMed]
- Dong, R.; Zhou, L.; Wu, J.; Tu, C.; Su, Y.; Zhu, B.; Gu, H.; Yan, D.; Zhu, X. A supramolecular approach to the preparation of charge-tunable dendritic polycations for efficient gene delivery. Chem. Commun. 2011, 47, 5473–5475. [Google Scholar] [CrossRef] [PubMed]
- Danko, M.; Kronekova, Z.; Krupa, I.; Tkac, J.; Matúš, P.; Kasak, P. Exchange Counterion in Polycationic Hydrogels: Tunability of Hydrophobicity, Water State, and Floating Capability for a Floating pH Device. Gels 2021, 7, 109. [Google Scholar] [CrossRef]
- Sahiner, N.; Demirci, S. Can PEI microgels become biocompatible upon betainization? Mater. Sci. Eng. C 2017, 77, 642–648. [Google Scholar] [CrossRef]
- Bagdat, S.; Tokay, F.; Demirci, S.; Yilmaz, S.; Sahiner, N. Removal of Cd(II), Co(II), Cr(III), Ni(II), Pb(II) and Zn(II) ions from wastewater using polyethyleneimine (PEI) cryogels. J. Environ. Manag. 2023, 329, 117002. [Google Scholar] [CrossRef]
- Lv, Y.; Yang, S.-J.; Du, Y.; Yang, H.-C.; Xu, Z.-K. Co-deposition Kinetics of Polydopamine/Polyethyleneimine Coatings: Effects of Solution Composition and Substrate Surface. Langmuir 2018, 34, 13123–13131. [Google Scholar] [CrossRef]
- Ratanajanchai, M.; Soodvilai, S.; Pimpha, N.; Sunintaboon, P. Polyethylenimine-immobilized core–shell nanoparticles: Synthesis, characterization, and biocompatibility test. Mater. Sci. Eng. C 2014, 34, 377–383. [Google Scholar] [CrossRef] [PubMed]
- Zou, Y.; Li, D.; Shen, M.; Shi, X. Polyethylenimine-Based Nanogels for Biomedical Applications. Macromol. Biosci. 2019, 19, 1900272. [Google Scholar] [CrossRef] [PubMed]
- Cai, H.; An, X.; Cui, J.; Li, J.; Wen, S.; Li, K.; Shen, M.; Zheng, L.; Zhang, G.; Shi, X. Facile Hydrothermal Synthesis and Surface Functionalization of Polyethyleneimine-Coated Iron Oxide Nanoparticles for Biomedical Applications. ACS Appl. Mater. Interfaces 2013, 5, 1722–1731. [Google Scholar] [CrossRef] [PubMed]
- Ciofani, G.; Raffa, V.; Menciassi, A.; Cuschieri, A. Cytocompatibility, interactions, and uptake of polyethyleneimine-coated boron nitride nanotubes by living cells: Confirmation of their potential for biomedical applications. Biotechnol. Bioeng. 2008, 101, 850–858. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Namgung, R.; Singha, K.; Oh, I.-K.; Kim, W.J. Graphene Oxide–Polyethylenimine Nanoconstruct as a Gene Delivery Vector and Bioimaging Tool. Bioconjug. Chem. 2011, 22, 2558–2567. [Google Scholar] [CrossRef]
- Hosu, O.; Florea, A.; Cristea, C.; Sandulescu, R. Functionalized Advanced Hybrid Materials for Biosensing Applications. In Advanced Biosensors for Health Care Applications; Elsevier: Amsterdam, The Netherlands, 2019; pp. 171–207. [Google Scholar]
- Huang, Q.; Liu, M.; Zhao, J.; Chen, J.; Zeng, G.; Huang, H.; Tian, J.; Wen, Y.; Zhang, X.; Wei, Y. Facile preparation of polyethylenimine-tannins coated SiO2 hybrid materials for Cu2+ removal. Appl. Surf. Sci. 2018, 427, 535–544. [Google Scholar] [CrossRef]
- Koopmann, A.-K.; Schuster, C.; Torres-Rodríguez, J.; Kain, S.; Pertl-Obermeyer, H.; Petutschnigg, A.; Hüsing, N. Tannin-Based Hybrid Materials and Their Applications: A Review. Molecules 2020, 25, 4910. [Google Scholar] [CrossRef]
- Guo, Z.; Xie, W.; Lu, J.; Guo, X.; Xu, J.; Xu, W.; Chi, Y.; Takuya, N.; Wu, H.; Zhao, L. Tannic acid-based metal phenolic networks for bio-applications: A review. J. Mater. Chem. B 2021, 9, 4098–4110. [Google Scholar] [CrossRef]
- Kaczmarek, B. Tannic Acid with Antiviral and Antibacterial Activity as A Promising Component of Biomaterials—A Minireview. Materials 2020, 13, 3224. [Google Scholar] [CrossRef] [PubMed]
- Nakayama, M.; Suzuki, K.; Toda, M.; Okubo, S.; Hara, Y.; Shimamura, T. Inhibition of the infectivity of influenza virus by tea polyphenols. Antivir. Res. 1993, 21, 289–299. [Google Scholar] [CrossRef]
- Youness, R.; Kamel, R.; Elkasabgy, N.; Shao, P.; Farag, M. Recent Advances in Tannic Acid (Gallotannin) Anticancer Activities and Drug Delivery Systems for Efficacy Improvement; A Comprehensive Review. Molecules 2021, 26, 1486. [Google Scholar] [CrossRef]
- Chung, K.-T.; Wong, T.Y.; Wei, C.-I.; Huang, Y.-W.; Lin, Y. Tannins and Human Health: A Review. Crit. Rev. Food Sci. Nutr. 1998, 38, 421–464. [Google Scholar] [CrossRef]
- Fraga-Corral, M.; Otero, P.; Cassani, L.; Echave, J.; Garcia-Oliveira, P.; Carpena, M.; Chamorro, F.; Lourenço-Lopes, C.; Prieto, M.A.; Simal-Gandara, J. Traditional Applications of Tannin Rich Extracts Supported by Scientific Data: Chemical Composition, Bioavailability and Bioaccessibility. Foods 2021, 10, 251. [Google Scholar] [CrossRef] [PubMed]
- Hatami, E.; Nagesh, P.; Chowdhury, P.; Chauhan, S.; Jaggi, M.; Samarasinghe, A.; Yallapu, M. Tannic Acid-Lung Fluid Assemblies Promote Interaction and Delivery of Drugs to Lung Cancer Cells. Pharmaceutics 2018, 10, 111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, Y.; Wu, D.; Bao, M.; Li, B.; Liang, H. Coordination driven self-assembly for enhancing the biological stability of nobiletin. J. Mol. Liq. 2019, 292, 111420. [Google Scholar] [CrossRef]
- Chowdhury, P.; Nagesh, P.K.B.; Hatami, E.; Wagh, S.; Dan, N.; Tripathi, M.K.; Khan, S.; Hafeez, B.B.; Meibohm, B.; Chauhan, S.C.; et al. Tannic acid-inspired paclitaxel nanoparticles for enhanced anticancer effects in breast cancer cells. J. Colloid Interface Sci. 2019, 535, 133–148. [Google Scholar] [CrossRef]
- Liang, X.; Cao, K.; Li, W.; Li, X.; McClements, D.J.; Hu, K. Tannic acid-fortified zein-pectin nanoparticles: Stability, properties, antioxidant activity, and in vitro digestion. Food Res. Int. 2021, 145, 110425. [Google Scholar] [CrossRef]
- Leite, L.S.F.; Pham, C.; Bilatto, S.; Azeredo, H.M.C.; Cranston, E.D.; Moreira, F.K.; Mattoso, L.H.C.; Bras, J. Effect of Tannic Acid and Cellulose Nanocrystals on Antioxidant and Antimicrobial Properties of Gelatin Films. ACS Sustain. Chem. Eng. 2021, 9, 8539–8549. [Google Scholar] [CrossRef]
- Soradech, S.; Williams, A.C.; Khutoryanskiy, V.V. Physically Cross-Linked Cryogels of Linear Polyethyleneimine: Influence of Cooling Temperature and Solvent Composition. Macromolecules 2022, 55, 9537–9546. [Google Scholar] [CrossRef]
- Kowalczyk, A.; Ruszkiewicz, M.; Biskup, I. Total phenolic content and antioxidant capacity of polish apple ciders. Indian J. Pharm. Sci. 2015, 77, 637. [Google Scholar] [CrossRef] [Green Version]
- Ivanauskas, R.; Bronusiene, A.; Ivanauskas, A.; Šarkinas, A.; Ancutiene, I. Antibacterial Activity of Copper Particles Embedded in Knitted Fabrics. Materials 2022, 15, 7147. [Google Scholar] [CrossRef]
- Ari, B.; Sahiner, M.; Demirci, S.; Sahiner, N. Poly(Vinyl alcohol)-tannic Acid Cryogel Matrix as Antioxidant and Antibacterial Material. Polymers 2022, 14, 70. [Google Scholar] [CrossRef]
- Reisner, B.S.; Woods, G.L. Times to Detection of Bacteria and Yeasts in BACTEC 9240 Blood Culture Bottles. J. Clin. Microbiol. 1999, 37, 2024–2026. [Google Scholar] [CrossRef] [Green Version]
- Reller, L.B. Endocarditis Caused by Bacillus subtilis. Am. J. Clin. Pathol. 1973, 60, 714–718. [Google Scholar] [CrossRef]
- Sedlacek, O.; Janouskova, O.; Verbraeken, B.; Hoogenboom, R. Straightforward Route to Superhydrophilic Poly(2-oxazoline)s via Acylation of Well-Defined Polyethylenimine. Biomacromolecules 2019, 20, 222–230. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shan, X.; Williams, A.C.; Khutoryanskiy, V.V. Polymer structure and property effects on solid dispersions with haloperidol: Poly(N-vinyl pyrrolidone) and poly(2-oxazolines) studies. Int. J. Pharm. 2020, 590, 119884. [Google Scholar] [CrossRef] [PubMed]
- Lozinsky, V.I. Polymeric cryogels as a new family of macroporous and supermacroporous materials for biotechnological purposes. Russ. Chem. Bull. 2008, 57, 1015–1032. [Google Scholar] [CrossRef]
- Lozinsky, V.I.; Galaev, I.Y.; Plieva, F.M.; Savina, I.N.; Jungvid, H.; Mattiasson, B. Polymeric cryogels as promising materials of biotechnological interest. Trends Biotechnol. 2003, 21, 445–451. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Fan, J.; Li, A.; Li, G. Biobased Tannic Acid Cross-Linked Epoxy Thermosets with Hierarchical Molecular Structure and Tunable Properties: Damping, Shape Memory, and Recyclability. ACS Sustain. Chem. Eng. 2020, 8, 874–883. [Google Scholar] [CrossRef]
- Zhou, S.; Yan, J.; Chen, J.; Yan, H.; Zhang, Y.; Huang, J.; Zhao, G.; Zhang, Q.; Liu, Y. Polydopamine/polyethyleneimine co-crosslinked graphene oxide for the enhanced tribological performance of epoxy resin coatings. J. Mater. Sci. Technol. 2023, 136, 13–20. [Google Scholar] [CrossRef]
- Nimer, N.A. Nosocomial Infection and Antibiotic-Resistant Threat in the Middle East. Infect. Drug Resist. 2022, 15, 631–639. [Google Scholar] [CrossRef] [PubMed]


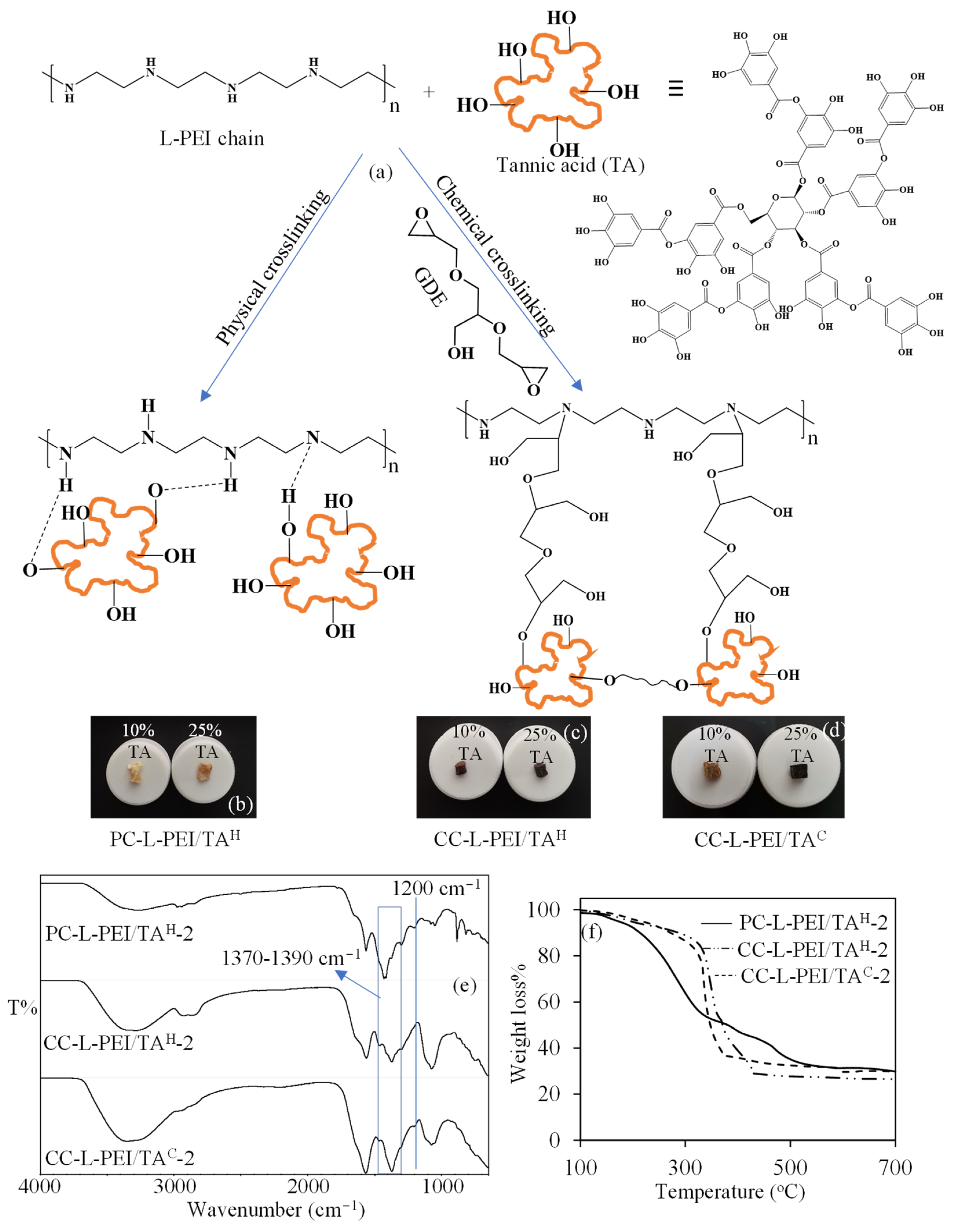

| Materials | S% | P% | PV% | Gel Yield% |
|---|---|---|---|---|
| PC-L-PEIH | 512 ± 54 | 51 ± 4 | 34 ± 3 | 95 ± 2% |
| CC-L-PEIH | 976 ± 107 | 19 ± 2 | 7 ± 1 | 96 ± 1% |
| CC-L-PEIC | 1154 ± 142 | 63 ± 5 | 57 ± 2 | 96 ± 1% |
| Samples | Concentration (mg/mL) | TPC (µg/mL) | TEAC (µmole TE/g) |
|---|---|---|---|
| PC-L-PEI/TAH-1 | 12.17 | 24.28 ± 2 | 0.29 ± 0.01 |
| PC-L-PEI/TAH-2 | 48.51 | 53.64 ± 5.3 | 0.48 ± 0.02 |
| CC-L-PEI/TAH-1 | 3.08 | 8.94 ± 1.45 | 0.05 ± 0.01 |
| CC-L-PEI/TAH-2 | 12.32 | 25.46 ± 4 | 0.26 ± 0.01 |
| CC-L-PEI/TAC-1 | 5.10 | 8.25 ± 1.9 | 0.04 ± 0.01 |
| CC-L-PEI/TAC-2 | 12.57 | 23.07 ± 2.5 | 0.24 ± 0.03 |
| Bacteria | E. coli | B. subtilis | ||
|---|---|---|---|---|
| Antimicrobial Materials | MIC (mg/mL) | MBC (mg/mL) | MIC (mg/mL) | MBC (mg/mL) |
| PC-L-PEIH | 5 | 5 | 5 | 5 |
| PC-L-PEI/TAH-1 | 5 | 5 | 5 | 5 |
| PC-L-PEI/TAH-2 | 5 | 5 | 5 | 5 |
| CC-L-PEIH | 5 | 10 | 5 | 10 |
| CC-L-PEI/TAH-1 | 20 | ND | 5 | 20 |
| CC-L-PEI/TAH-2 | 10 | ND | 5 | ND |
| CC-L-PEIC | 5 | 5 | 5 | 5 |
| CC-L-PEI/TAC-1 | ND | ND | 20 | 20 |
| CC-L-PEI/TAC-2 | 10 | ND | 5 | 20 |
| Materials | Zone Diameter (mm) | |
|---|---|---|
| E. coli | B. subtilis | |
| PC-L-PEIH | 10 ± 0.5 | 10 ± 1.5 |
| PC-L-PEI/TAH-1 | 16 ± 0.5 | 21 ± 0.5 |
| PC-L-PEI/TAH-2 | 14 ± 0.5 | 12 ± 1.2 |
| CC-L-PEIH | 3 ± 0.5 | 5 ± 0.5 |
| CC-L-PEI/TAH-1 | 5 ± 0.5 | 11 ± 1 |
| CC-L-PEI/TAH-2 | 4 ± 0.5 | 12 ± 0.5 |
| CC-L-PEIC | 3 ± 0.5 | 1 ± 0.5 |
| CC-L-PEI/TAC-1 | 2 ± 0.5 | 6 ± 1 |
| CC-L-PEI/TAC-2 | 7 ± 0.5 | 11 ± 0.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sahiner, M.; Yilmaz, A.S.; Demirci, S.; Sahiner, N. Physically and Chemically Crosslinked, Tannic Acid Embedded Linear PEI-Based Hydrogels and Cryogels with Natural Antibacterial and Antioxidant Properties. Biomedicines 2023, 11, 706. https://doi.org/10.3390/biomedicines11030706
Sahiner M, Yilmaz AS, Demirci S, Sahiner N. Physically and Chemically Crosslinked, Tannic Acid Embedded Linear PEI-Based Hydrogels and Cryogels with Natural Antibacterial and Antioxidant Properties. Biomedicines. 2023; 11(3):706. https://doi.org/10.3390/biomedicines11030706
Chicago/Turabian StyleSahiner, Mehtap, Aynur Sanem Yilmaz, Sahin Demirci, and Nurettin Sahiner. 2023. "Physically and Chemically Crosslinked, Tannic Acid Embedded Linear PEI-Based Hydrogels and Cryogels with Natural Antibacterial and Antioxidant Properties" Biomedicines 11, no. 3: 706. https://doi.org/10.3390/biomedicines11030706