Interleukin-4 Receptor Targeting Peptide Decorated Extracellular Vesicles as a Platform for In Vivo Drug Delivery to Thyroid Cancer
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Isolation of EVs and Purification
2.3. Western Blotting
2.4. Peptides
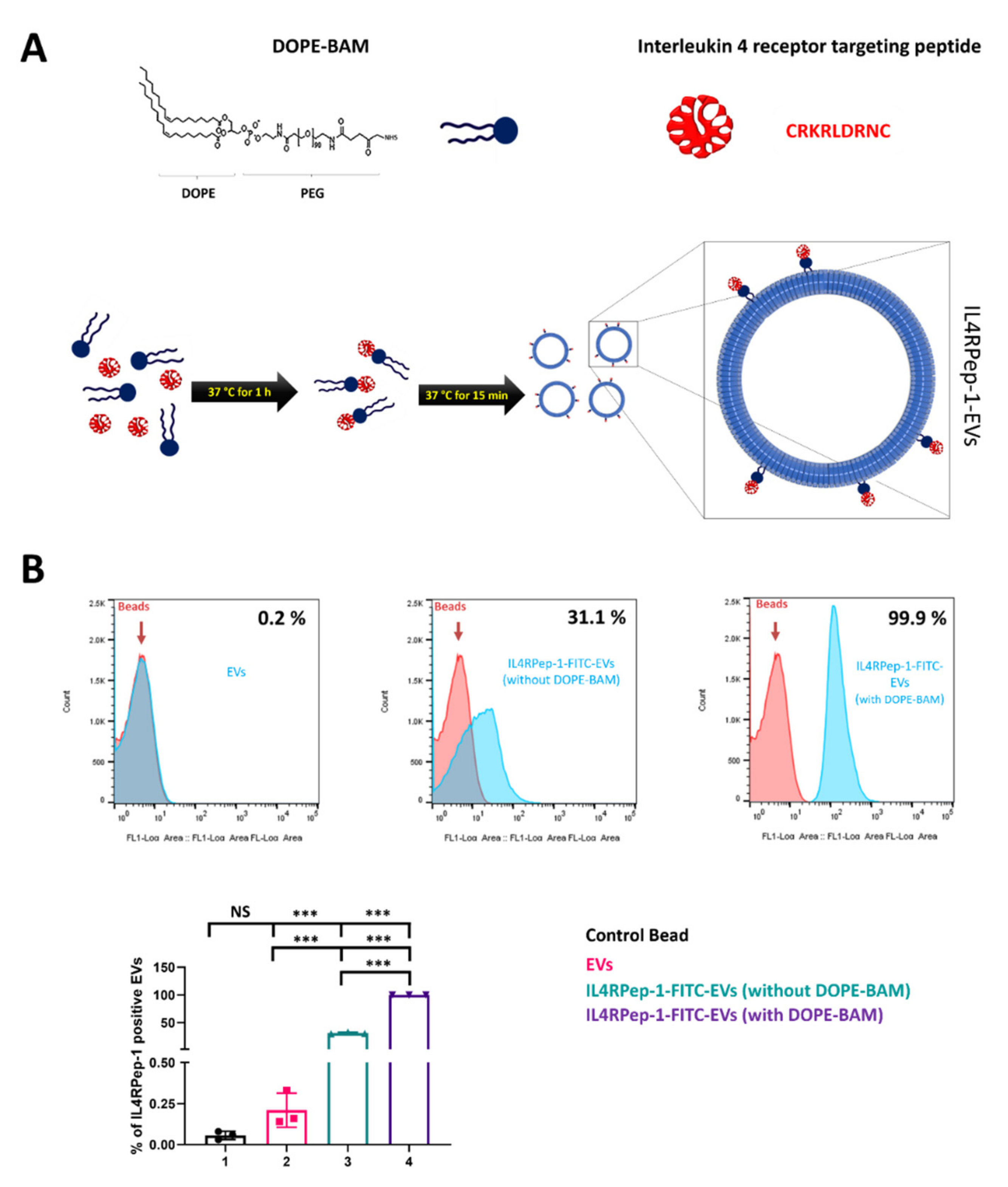
2.5. Labeling of EVs with Peptide Using DOPE-BAM
2.6. Analysis of IL4RPep-1-EVs by Flow Cytometry
2.7. Transmission Electron Microscopy (TEM)
2.8. Nanoparticle Tracking Analysis (NTA)
2.9. Immunofluorescence (IF) Assay
2.10. Cellular Binding of Peptides
2.11. Cytotoxicity Assay
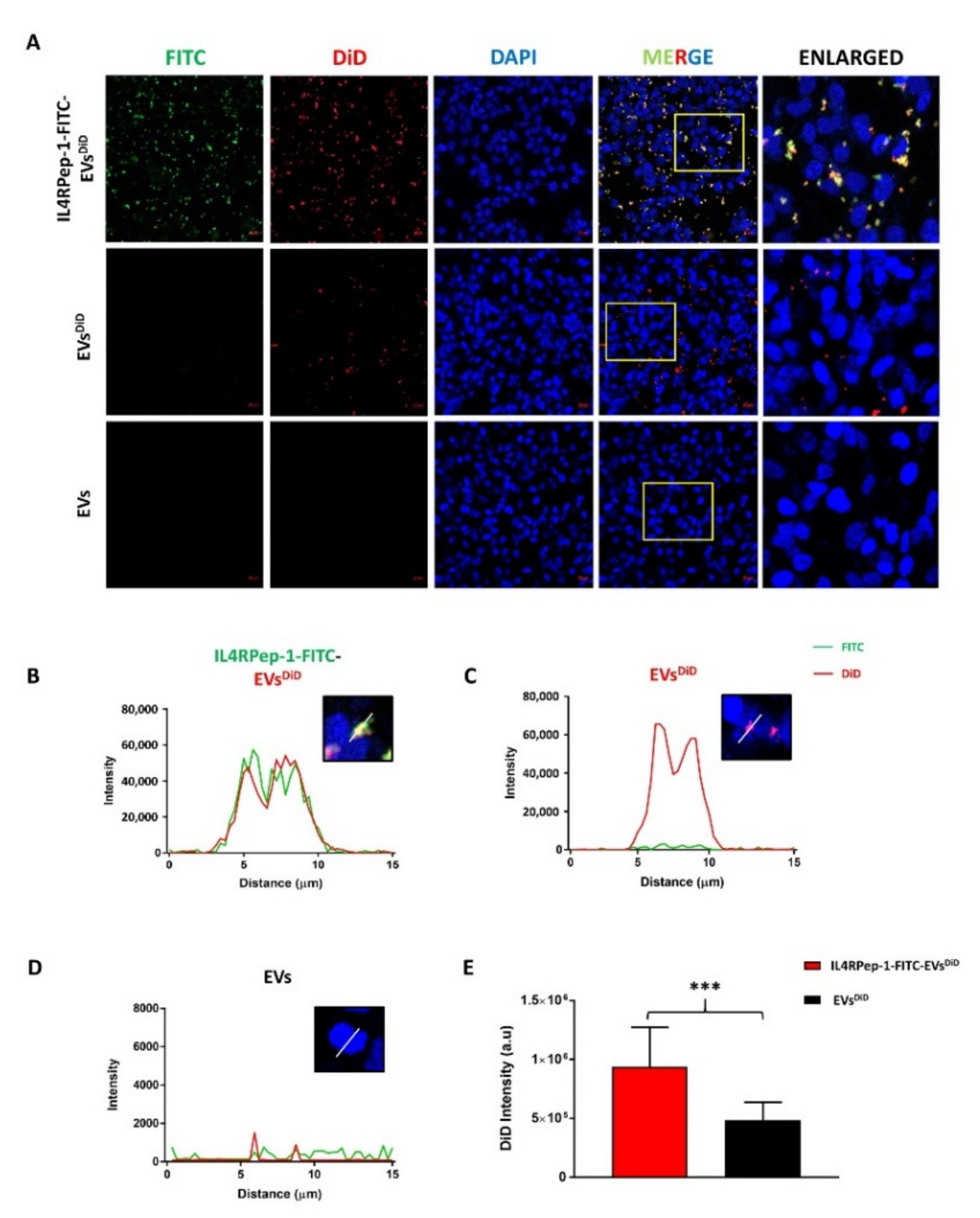
2.12. In Vitro Internalization of EVs by Cal-62
2.13. In Vivo Targeting of IL4RPep-1 to Cal-62 Xenograft Tumor in Mice
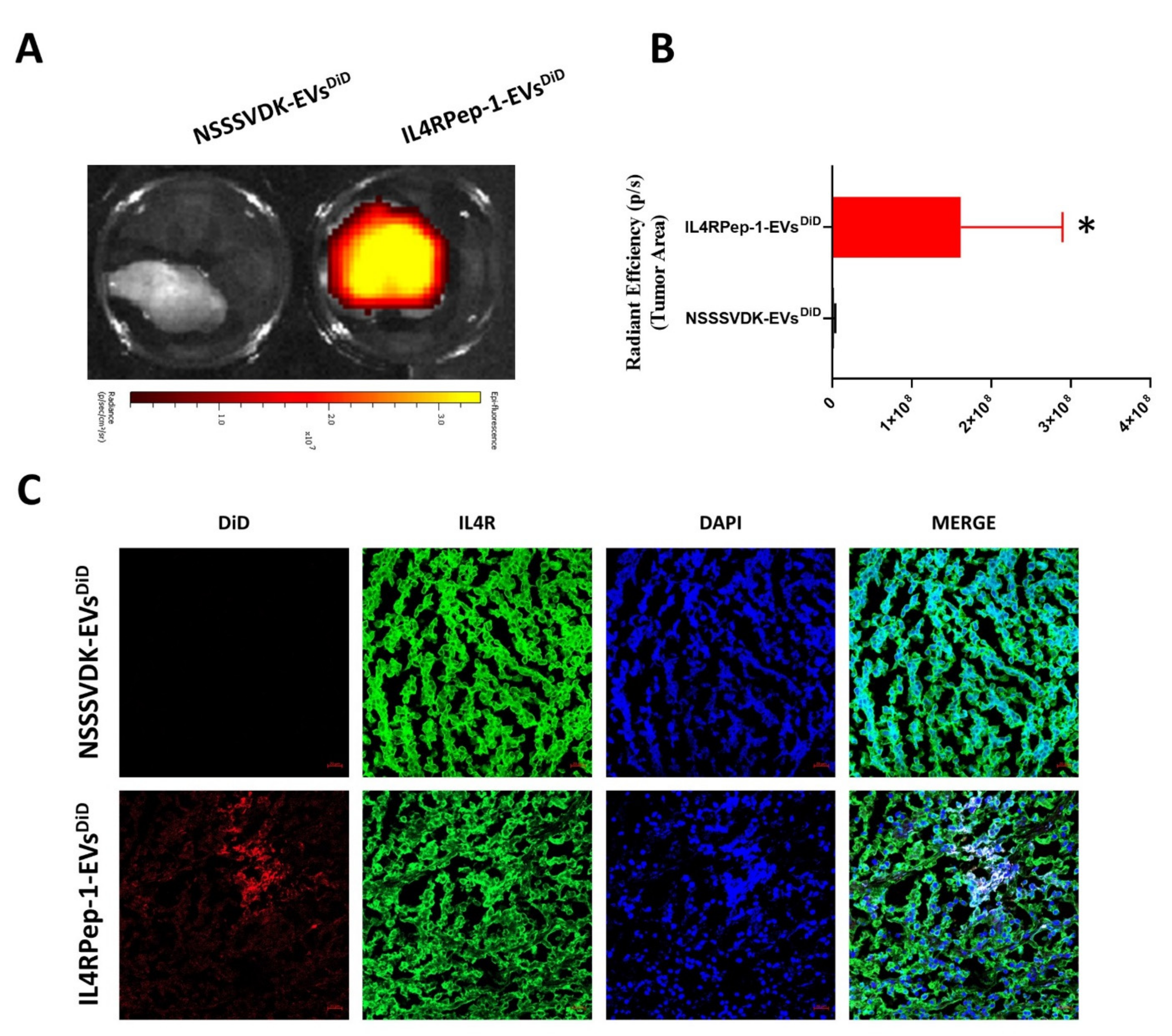
2.14. In Vivo Targeting of IL4RPep-1-EVsDiD to Cal-62 Xenograft Tumor in Mice
2.15. Immunofluorescent Analysis (IFA) of Tissues
2.16. EV-TRACK Knowledgebase
2.17. Statistical Analysis
3. Results
3.1. Characterization of EVs
3.2. Labeling of EVs with IL4RPep-1
3.3. Morphology, Size, and Distribution of EVs after IL4RPep-1 Labeling
3.4. In Vitro, Expression of IL4Rα in Thyroid Cancer Cells and Preferential Binding of IL4RPep-1 to IL4Rα-Expressing Cancer Cells
3.5. Enhanced Internalization of IL4RPep-1-EVs into Cal-62 Cells by IL4RPep-1 Labeling
3.6. In Vivo Monitoring of i.v. Injected IL4RPep-1 Targeting of Cal-62 Tumor in Mice Using Fluorescent Imaging
3.7. In Vivo Monitoring of i.v. Injected IL4RPep-1-EV Targeting of Cal-62 Tumor in Mice Using Fluorescent Imaging
3.8. Ex Vivo Fluorescent and Microscopic Imaging of IL4RPep-1-EVs Using Cal-62 Tumor
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Doyle, L.M.; Wang, M.Z. Overview of Extracellular Vesicles, Their Origin, Composition, Purpose, and Methods for Exosome Isolation and Analysis. Cells 2019, 8, 727. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liu, Y.; Liu, H.; Tang, W.H. Exosomes: Biogenesis, Biologic Function and Clinical Potential. Cell Biosci. 2019, 9, 19. [Google Scholar] [CrossRef] [PubMed]
- Cufaro, M.C.; Pieragostino, D.; Lanuti, P.; Rossi, C.; Cicalini, I.; Federici, L.; De Laurenzi, V.; Del Boccio, P. Extracellular Vesicles and Their Potential Use in Monitoring Cancer Progression and Therapy: The Contribution of Proteomics. J. Oncol. 2019, 2019, 1639854. [Google Scholar] [CrossRef] [PubMed]
- Shetgaonkar, G.G.; Marques, S.M.; DCruz, C.E.M.; Vibhavari, R.J.A.; Kumar, L.; Shirodkar, R.K. Exosomes as Cell-Derivative Carriers in the Diagnosis and Treatment of Central Nervous System Diseases. Drug Deliv. Transl. Res. 2022, 12, 1047–1079. [Google Scholar] [CrossRef]
- Paolicelli, R.C.; Bergamini, G.; Rajendran, L. Cell-to-Cell Communication by Extracellular Vesicles: Focus on Microglia. Neuroscience 2018, 405, 148–157. [Google Scholar] [CrossRef]
- Teng, F.; Fussenegger, M. Shedding Light on Extracellular Vesicle Biogenesis and Bioengineering. Adv. Sci. 2021, 8, 2003505. [Google Scholar] [CrossRef]
- Yokoi, A.; Ochiya, T. Exosomes and Extracellular Vesicles: Rethinking the Essential Values in Cancer Biology. Semin. Cancer Biol. 2021, 74, 79–91. [Google Scholar] [CrossRef]
- Veziroglu, E.M.; Mias, G.I. Characterizing Extracellular Vesicles and Their Diverse RNA Contents. Front. Genet. 2020, 11, 700. [Google Scholar] [CrossRef]
- Gurunathan, S.; Kang, M.-H.; Jeyaraj, M.; Qasim, M.; Kim, J.-H. Review of the Isolation, Characterization, Biological Function, and Multifarious Therapeutic Approaches of Exosomes. Cells 2019, 8, 307. [Google Scholar] [CrossRef]
- Skotland, T.; Hessvik, N.P.; Sandvig, K.; Llorente, A. Exosomal Lipid Composition and the Role of Ether Lipids and Phosphoinositides in Exosome Biology. J. Lipid Res. 2019, 60, 9–18. [Google Scholar] [CrossRef]
- Pegtel, D.M.; Peferoen, L.; Amor, S. Extracellular Vesicles as Modulators of Cell-to-Cell Communication in the Healthy and Diseased Brain. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2014, 369, 20130516. [Google Scholar] [CrossRef]
- Tetta, C.; Ghigo, E.; Silengo, L.; Deregibus, M.C.; Camussi, G. Extracellular Vesicles as an Emerging Mechanism of Cell-to-Cell Communication. Endocrine 2013, 44, 11–19. [Google Scholar] [CrossRef]
- Ha, D.; Yang, N.; Nadithe, V. Exosomes as Therapeutic Drug Carriers and Delivery Vehicles across Biological Membranes: Current Perspectives and Future Challenges. Acta Pharm. Sin. B 2016, 6, 287–296. [Google Scholar] [CrossRef]
- de Jong, B.; Barros, E.R.; Hoenderop, J.G.J.; Rigalli, J.P. Recent Advances in Extracellular Vesicles as Drug Delivery Systems and Their Potential in Precision Medicine. Pharmaceutics 2020, 12, 1006. [Google Scholar] [CrossRef]
- Herrmann, I.K.; Wood, M.J.A.; Fuhrmann, G. Extracellular Vesicles as a Next-Generation Drug Delivery Platform. Nat. Nanotechnol. 2021, 16, 748–759. [Google Scholar] [CrossRef]
- Kalimuthu, S.; Gangadaran, P.; Rajendran, R.L.; Zhu, L.; Oh, J.M.; Lee, H.W.; Gopal, A.; Baek, S.H.; Jeong, S.Y.; Lee, S.-W.; et al. A New Approach for Loading Anticancer Drugs into Mesenchymal Stem Cell-Derived Exosome Mimetics for Cancer Therapy. Front. Pharmacol. 2018, 9, 1116. [Google Scholar] [CrossRef]
- Johnsen, K.B.; Gudbergsson, J.M.; Skov, M.N.; Pilgaard, L.; Moos, T.; Duroux, M. A Comprehensive Overview of Exosomes as Drug Delivery Vehicles—Endogenous Nanocarriers for Targeted Cancer Therapy. Biochim. Biophys. Acta (BBA) Rev. Cancer 2014, 1846, 75–87. [Google Scholar] [CrossRef]
- Luan, X.; Sansanaphongpricha, K.; Myers, I.; Chen, H.; Yuan, H.; Sun, D. Engineering Exosomes as Refined Biological Nanoplatforms for Drug Delivery. Acta Pharmacol. Sin. 2017, 38, 754–763. [Google Scholar] [CrossRef]
- Yang, T.; Martin, P.; Fogarty, B.; Brown, A.; Schurman, K.; Phipps, R.; Yin, V.P.; Lockman, P.; Bai, S. Exosome Delivered Anticancer Drugs across the Blood-Brain Barrier for Brain Cancer Therapy in Danio Rerio. Pharm. Res. 2015, 32, 2003–2014. [Google Scholar] [CrossRef]
- Usman, W.M.; Pham, T.C.; Kwok, Y.Y.; Vu, L.T.; Ma, V.; Peng, B.; Chan, Y.S.; Wei, L.; Chin, S.M.; Azad, A.; et al. Efficient RNA Drug Delivery Using Red Blood Cell Extracellular Vesicles. Nat. Commun. 2018, 9, 2359. [Google Scholar] [CrossRef]
- Ohno, S.; Takanashi, M.; Sudo, K.; Ueda, S.; Ishikawa, A.; Matsuyama, N.; Fujita, K.; Mizutani, T.; Ohgi, T.; Ochiya, T.; et al. Systemically Injected Exosomes Targeted to EGFR Deliver Antitumor MicroRNA to Breast Cancer Cells. Mol. Ther. 2013, 21, 185–191. [Google Scholar] [CrossRef]
- Gangadaran, P.; Li, X.J.; Lee, H.W.; Oh, J.M.; Kalimuthu, S.; Rajendran, R.L.; Son, S.H.; Baek, S.H.; Singh, T.D.; Zhu, L.; et al. A New Bioluminescent Reporter System to Study the Biodistribution of Systematically Injected Tumor-Derived Bioluminescent Extracellular Vesicles in Mice. Oncotarget 2017, 8, 109894–109914. [Google Scholar] [CrossRef]
- Manca, S.; Giraud, D.; Zempleni, J. Bioavailability and Biodistribution of Fluorophore-Labeled Exosomes from Cow’s Milk after Intravenous and Oral Administration in C57Bl/6J Mice. FASEB J. 2016, 30, 690.8. [Google Scholar] [CrossRef]
- Kang, M.; Jordan, V.; Blenkiron, C.; Chamley, L.W. Biodistribution of Extracellular Vesicles Following Administration into Animals: A Systematic Review. J. Extracell. Vesicles 2021, 10, e12085. [Google Scholar] [CrossRef]
- Németh, K.; Varga, Z.; Lenzinger, D.; Visnovitz, T.; Koncz, A.; Hegedűs, N.; Kittel, Á.; Máthé, D.; Szigeti, K.; Lörincz, P.; et al. Extracellular Vesicle Release and Uptake by the Liver under Normo- and Hyperlipidemia. Cell. Mol. Life Sci. 2021, 78, 7589–7604. [Google Scholar] [CrossRef]
- Wang, Q.; Zhuang, X.; Sriwastva, M.K.; Mu, J.; Teng, Y.; Deng, Z.; Zhang, L.; Sundaram, K.; Kumar, A.; Miller, D.; et al. Blood Exosomes Regulate the Tissue Distribution of Grapefruit-Derived Nanovector via CD36 and IGFR1 Pathways. Theranostics 2018, 8, 4912–4924. [Google Scholar] [CrossRef]
- Yang, C.-Y.; Liu, H.-W.; Tsai, Y.-C.; Tseng, J.-Y.; Liang, S.-C.; Chen, C.-Y.; Lian, W.-N.; Wei, M.-C.; Lu, M.; Lu, R.-H.; et al. Interleukin-4 Receptor-Targeted Liposomal Doxorubicin as a Model for Enhancing Cellular Uptake and Antitumor Efficacy in Murine Colorectal Cancer. Cancer Biol. Ther. 2015, 16, 1641–1650. [Google Scholar] [CrossRef]
- Conticello, C.; Pedini, F.; Zeuner, A.; Patti, M.; Zerilli, M.; Stassi, G.; Messina, A.; Peschle, C.; De Maria, R. IL-4 Protects Tumor Cells from Anti-CD95 and Chemotherapeutic Agents via up-Regulation of Antiapoptotic Proteins. J. Immunol. 2004, 172, 5467–5477. [Google Scholar] [CrossRef]
- Guruprasath, P.; Kim, J.; Gunassekaran, G.R.; Chi, L.; Kim, S.; Park, R.-W.; Kim, S.-H.; Baek, M.-C.; Bae, S.M.; Kim, S.-Y.; et al. Interleukin-4 Receptor-Targeted Delivery of Bcl-XL SiRNA Sensitizes Tumors to Chemotherapy and Inhibits Tumor Growth. Biomaterials 2017, 142, 101–111. [Google Scholar] [CrossRef]
- Kawakami, M.; Kawakami, K.; Stepensky, V.A.; Maki, R.A.; Robin, H.; Muller, W.; Husain, S.R.; Puri, R.K. Interleukin 4 Receptor on Human Lung Cancer: A Molecular Target for Cytotoxin Therapy. Clin. Cancer Res. 2002, 8, 3503–3511. [Google Scholar]
- Tian, Y.; Li, S.; Song, J.; Ji, T.; Zhu, M.; Anderson, G.J.; Wei, J.; Nie, G. A Doxorubicin Delivery Platform Using Engineered Natural Membrane Vesicle Exosomes for Targeted Tumor Therapy. Biomaterials 2014, 35, 2383–2390. [Google Scholar] [CrossRef] [PubMed]
- Obiri, N.I.; Siegel, J.P.; Varricchio, F.; Puri, R.K. Expression of High-Affinity IL-4 Receptors on Human Melanoma, Ovarian and Breast Carcinoma Cells. Clin. Exp. Immunol. 1994, 95, 148–155. [Google Scholar] [CrossRef] [PubMed]
- Husain, S.R.; Gill, P.; Kreitman, R.J.; Pastan, I.; Puri, R.K. Interleukin-4 Receptor Expression on AIDS-Associated Kaposi’s Sarcoma Cells and Their Targeting by a Chimeric Protein Comprised of Circularly Permuted Interleukin-4 and Pseudomonas Exotoxin. Mol. Med. 1997, 3, 327–338. [Google Scholar] [CrossRef] [PubMed]
- Obiri, N.I.; Hillman, G.G.; Haas, G.P.; Sud, S.; Puri, R.K. Expression of High Affinity Interleukin-4 Receptors on Human Renal Cell Carcinoma Cells and Inhibition of Tumor Cell Growth In Vitro by Interleukin-4. J. Clin. Investig. 1993, 91, 88–93. [Google Scholar] [CrossRef]
- Seto, K.; Shoda, J.; Horibe, T.; Warabi, E.; Ishige, K.; Yamagata, K.; Kohno, M.; Yanagawa, T.; Bukawa, H.; Kawakami, K. Interleukin-4 Receptor α-Based Hybrid Peptide Effectively Induces Antitumor Activity in Head and Neck Squamous Cell Carcinoma. Oncol. Rep. 2013, 29, 2147–2153. [Google Scholar] [CrossRef]
- Puri, R.K.; Leland, P.; Kreitman, R.J.; Pastan, I. Human Neurological Cancer Cells Express Interleukin-4 (IL-4) Receptors Which Are Targets for the Toxic Effects of IL4-Pseudomonas Exotoxin Chimeric Protein. Int. J. Cancer 1994, 58, 574–581. [Google Scholar] [CrossRef]
- Rand, R.W.; Kreitman, R.J.; Patronas, N.; Varricchio, F.; Pastan, I.; Puri, R.K. Intratumoral Administration of Recombinant Circularly Permuted Interleukin-4-Pseudomonas Exotoxin in Patients with High-Grade Glioma. Clin. Cancer Res. 2000, 6, 2157–2165. [Google Scholar]
- Yang, L.; Horibe, T.; Kohno, M.; Haramoto, M.; Ohara, K.; Puri, R.K.; Kawakami, K. Targeting Interleukin-4 Receptor α with Hybrid Peptide for Effective Cancer Therapy. Mol. Cancer Ther. 2012, 11, 235–243. [Google Scholar] [CrossRef]
- Kawakami, K.; Kawakami, M.; Husain, S.R.; Puri, R.K. Targeting Interleukin-4 Receptors for Effective Pancreatic Cancer Therapy. Cancer Res. 2002, 62, 3575–3580. [Google Scholar]
- Weber, F.; Asher, A.; Bucholz, R.; Berger, M.; Prados, M.; Chang, S.; Bruce, J.; Hall, W.; Rainov, N.G.; Westphal, M.; et al. Safety, Tolerability, and Tumor Response of IL4-Pseudomonas Exotoxin (NBI-3001) in Patients with Recurrent Malignant Glioma. J. Neurooncol. 2003, 64, 125–137. [Google Scholar] [CrossRef]
- Garland, L.; Gitlitz, B.; Ebbinghaus, S.; Pan, H.; de Haan, H.; Puri, R.K.; Von Hoff, D.; Figlin, R. Phase I Trial of Intravenous IL-4 Pseudomonas Exotoxin Protein (NBI-3001) in Patients with Advanced Solid Tumors That Express the IL-4 Receptor. J. Immunother. 2005, 28, 376–381. [Google Scholar] [CrossRef]
- Vadevoo, S.M.P.; Gurung, S.; Khan, F.; Haque, M.E.; Gunassekaran, G.R.; Chi, L.; Permpoon, U.; Lee, B. Peptide-Based Targeted Therapeutics and Apoptosis Imaging Probes for Cancer Therapy. Arch. Pharm. Res. 2019, 42, 150–158. [Google Scholar] [CrossRef]
- Vella, V.; Mineo, R.; Frasca, F.; Mazzon, E.; Pandini, G.; Vigneri, R.; Belfiore, A. Interleukin-4 Stimulates Papillary Thyroid Cancer Cell Survival: Implications in Patients with Thyroid Cancer and Concomitant Graves’ Disease. J. Clin. Endocrinol. Metab. 2004, 89, 2880–2889. [Google Scholar] [CrossRef]
- Hong, H.; Lee, H.Y.; Kwak, W.; Yoo, J.; Na, M.-H.; So, I.S.; Kwon, T.-H.; Park, H.-S.; Huh, S.; Oh, G.T.; et al. Phage Display Selection of Peptides That Home to Atherosclerotic Plaques: IL-4 Receptor as a Candidate Target in Atherosclerosis. J. Cell. Mol. Med. 2008, 12, 2003–2014. [Google Scholar] [CrossRef]
- Kato, K.; Itoh, C.; Yasukouchi, T.; Nagamune, T. Rapid Protein Anchoring into the Membranes of Mammalian Cells Using Oleyl Chain and Poly(Ethylene Glycol) Derivatives. Biotechnol. Prog. 2004, 20, 897–904. [Google Scholar] [CrossRef]
- He, X.; Bonaparte, N.; Kim, S.; Acharya, B.; Lee, J.-Y.; Chi, L.; Lee, H.-J.; Paik, Y.-K.; Moon, P.A.; Baek, M.-C.; et al. Enhanced Delivery of T Cells to Tumor after Chemotherapy Using Membrane-Anchored, Apoptosis-Targeted Peptide. J. Control. Release 2012, 162, 521–528. [Google Scholar] [CrossRef]
- Gangadaran, P.; Rajendran, R.L.; Lee, H.W.; Kalimuthu, S.; Hong, C.M.; Jeong, S.Y.; Lee, S.-W.; Lee, J.; Ahn, B.-C. Extracellular Vesicles from Mesenchymal Stem Cells Activates VEGF Receptors and Accelerates Recovery of Hindlimb Ischemia. J. Control. Release 2017, 264, 112–126. [Google Scholar] [CrossRef]
- Osteikoetxea, X.; Sódar, B.; Németh, A.; Szabó-Taylor, K.; Pálóczi, K.; Vukman, K.V.; Tamási, V.; Balogh, A.; Kittel, Á.; Pállinger, É.; et al. Differential Detergent Sensitivity of Extracellular Vesicle Subpopulations. Org. Biomol. Chem. 2015, 13, 9775–9782. [Google Scholar] [CrossRef]
- Jogalekar, M.P.; Serrano, E.E. Morphometric Analysis of a Triple Negative Breast Cancer Cell Line in Hydrogel and Monolayer Culture Environments. PeerJ 2018, 6, e4340. [Google Scholar] [CrossRef]
- Alam, H.; Bhate, A.V.; Gangadaran, P.; Sawant, S.S.; Salot, S.; Sehgal, L.; Dange, P.P.; Chaukar, D.A.; D’cruz, A.K.; Kannanl, S.; et al. Fascin Overexpression Promotes Neoplastic Progression in Oral Squamous Cell Carcinoma. BMC Cancer 2012, 12, 32. [Google Scholar] [CrossRef]
- EV-TRACK Consortium; Van Deun, J.; Mestdagh, P.; Agostinis, P.; Akay, Ö.; Anand, S.; Anckaert, J.; Martinez, Z.A.; Baetens, T.; Beghein, E.; et al. EV-TRACK: Transparent Reporting and Centralizing Knowledge in Extracellular Vesicle Research. Nat. Methods 2017, 14, 228–232. [Google Scholar] [CrossRef]
- Vadevoo, S.M.P.; Kim, J.-E.; Gunassekaran, G.R.; Jung, H.-K.; Chi, L.; Kim, D.E.; Lee, S.-H.; Im, S.-H.; Lee, B. IL4 Receptor-Targeted Proapoptotic Peptide Blocks Tumor Growth and Metastasis by Enhancing Antitumor Immunity. Mol. Cancer Ther. 2017, 16, 2803–2816. [Google Scholar] [CrossRef]
- Remesh, A. Toxicities of Anticancer Drugs and Its Management. Int. J. Basic Clin. Pharmacol. 2017, 1, 2–12. [Google Scholar] [CrossRef]
- Damjanov, N. Anticancer Drug Toxicity: Prevention, Management, and Clinical Pharmacokinetics. Mod. Pathol. 2000, 13, 953. [Google Scholar] [CrossRef]
- Yi, Y.W.; Lee, J.H.; Kim, S.-Y.; Pack, C.-G.; Ha, D.H.; Park, S.R.; Youn, J.; Cho, B.S. Advances in Analysis of Biodistribution of Exosomes by Molecular Imaging. Int. J. Mol. Sci. 2020, 21, 665. [Google Scholar] [CrossRef]
- Ortega, A.; Martinez-Arroyo, O.; Forner, M.J.; Cortes, R. Exosomes as Drug Delivery Systems: Endogenous Nanovehicles for Treatment of Systemic Lupus Erythematosus. Pharmaceutics 2020, 13, 3. [Google Scholar] [CrossRef]
- Fu, S.; Wang, Y.; Xia, X.; Zheng, J.C. Exosome Engineering: Current Progress in Cargo Loading and Targeted Delivery. NanoImpact 2020, 20, 100261. [Google Scholar] [CrossRef]
- Walker, S.; Busatto, S.; Pham, A.; Tian, M.; Suh, A.; Carson, K.; Quintero, A.; Lafrence, M.; Malik, H.; Santana, M.X.; et al. Extracellular Vesicle-Based Drug Delivery Systems for Cancer Treatment. Theranostics 2019, 9, 8001–8017. [Google Scholar] [CrossRef]
- Gangadaran, P.; Hong, C.M.; Ahn, B.-C. An Update on In Vivo Imaging of Extracellular Vesicles as Drug Delivery Vehicles. Front. Pharmacol. 2018, 9, 169. [Google Scholar] [CrossRef]
- Gangadaran, P.; Hong, C.M.; Oh, J.M.; Rajendran, R.L.; Kalimuthu, S.; Son, S.H.; Gopal, A.; Zhu, L.; Baek, S.H.; Jeong, S.Y.; et al. In Vivo Non-Invasive Imaging of Radio-Labeled Exosome-Mimetics Derived from Red Blood Cells in Mice. Front. Pharmacol. 2018, 9, 817. [Google Scholar] [CrossRef]
- Vader, P.; Mol, E.A.; Pasterkamp, G.; Schiffelers, R.M. Extracellular Vesicles for Drug Delivery. Adv. Drug Deliv. Rev. 2016, 106, 148–156. [Google Scholar] [CrossRef] [PubMed]
- Rajendran, R.L.; Gangadaran, P.; Bak, S.S.; Oh, J.M.; Kalimuthu, S.; Lee, H.W.; Baek, S.H.; Zhu, L.; Sung, Y.K.; Jeong, S.Y.; et al. Extracellular Vesicles Derived from MSCs Activates Dermal Papilla Cell In Vitro and Promotes Hair Follicle Conversion from Telogen to Anagen in Mice. Sci. Rep. 2017, 7, 15560. [Google Scholar] [CrossRef] [PubMed]
- Vandergriff, A.; Huang, K.; Shen, D.; Hu, S.; Hensley, M.T.; Caranasos, T.G.; Qian, L.; Cheng, K. Targeting Regenerative Exosomes to Myocardial Infarction Using Cardiac Homing Peptide. Theranostics 2018, 8, 1869–1878. [Google Scholar] [CrossRef] [PubMed]
- Ayo, A.; Laakkonen, P. Peptide-Based Strategies for Targeted Tumor Treatment and Imaging. Pharmaceutics 2021, 13, 481. [Google Scholar] [CrossRef]
- Gunassekaran, G.R.; Poongkavithai Vadevoo, S.M.; Baek, M.-C.; Lee, B. M1 Macrophage Exosomes Engineered to Foster M1 Polarization and Target the IL-4 Receptor Inhibit Tumor Growth by Reprogramming Tumor-Associated Macrophages into M1-like Macrophages. Biomaterials 2021, 278, 121137. [Google Scholar] [CrossRef]
- Zhu, L.; Kalimuthu, S.; Gangadaran, P.; Oh, J.M.; Lee, H.W.; Baek, S.H.; Jeong, S.Y.; Lee, S.-W.; Lee, J.; Ahn, B.-C. Exosomes Derived From Natural Killer Cells Exert Therapeutic Effect in Melanoma. Theranostics 2017, 7, 2732–2745. [Google Scholar] [CrossRef]
- Kalimuthu, S.; Gangadaran, P.; Li, X.J.; Oh, J.M.; Lee, H.W.; Jeong, S.Y.; Lee, S.-W.; Lee, J.; Ahn, B.-C. In Vivo Therapeutic Potential of Mesenchymal Stem Cell-Derived Extracellular Vesicles with Optical Imaging Reporter in Tumor Mice Model. Sci. Rep. 2016, 6, 30418. [Google Scholar] [CrossRef]
- Joshi, B.H.; Suzuki, A.; Fujisawa, T.; Leland, P.; Varrichio, F.; Lababidi, S.; Lloyd, R.; Kasperbauer, J.; Puri, R.K. Identification, Characterization, and Targeting of IL-4 Receptor by IL-4-Pseudomonas Exotoxin in Mouse Models of Anaplastic Thyroid Cancer. Discov. Med. 2015, 20, 273–284. [Google Scholar]
- Oh, J.M.; Kalimuthu, S.; Gangadaran, P.; Baek, S.H.; Zhu, L.; Lee, H.W.; Rajendran, R.L.; Hong, C.M.; Jeong, S.Y.; Lee, S.-W.; et al. Reverting Iodine Avidity of Radioactive-Iodine Refractory Thyroid Cancer with a New Tyrosine Kinase Inhibitor (K905-0266) Excavated by High-Throughput NIS (Sodium Iodide Symporter) Enhancer Screening Platform Using Dual Reporter Gene System. Oncotarget 2018, 9, 7075–7087. [Google Scholar] [CrossRef]
- Alvarez-Erviti, L.; Seow, Y.; Yin, H.; Betts, C.; Lakhal, S.; Wood, M.J.A. Delivery of SiRNA to the Mouse Brain by Systemic Injection of Targeted Exosomes. Nat. Biotechnol. 2011, 29, 341–345. [Google Scholar] [CrossRef]
- Sarangthem, V.; Kim, Y.; Singh, T.D.; Seo, B.-Y.; Cheon, S.-H.; Lee, Y.-J.; Lee, B.-H.; Park, R.-W. Multivalent Targeting Based Delivery of Therapeutic Peptide Using AP1-ELP Carrier for Effective Cancer Therapy. Theranostics 2016, 6, 2235–2249. [Google Scholar] [CrossRef]
- de Araujo Farias, V.; O’Valle, F.; Serrano-Saenz, S.; Anderson, P.; Andrés, E.; López-Peñalver, J.; Tovar, I.; Nieto, A.; Santos, A.; Martín, F.; et al. Exosomes Derived from Mesenchymal Stem Cells Enhance Radiotherapy-Induced Cell Death in Tumor and Metastatic Tumor Foci. Mol. Cancer 2018, 17, 122. [Google Scholar] [CrossRef]
- Munich, S.; Sobo-Vujanovic, A.; Buchser, W.J.; Beer-Stolz, D.; Vujanovic, N.L. Dendritic Cell Exosomes Directly Kill Tumor Cells and Activate Natural Killer Cells via TNF Superfamily Ligands. Oncoimmunology 2012, 1, 1074–1083. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gangadaran, P.; Gunassekaran, G.R.; Rajendran, R.L.; Oh, J.M.; Vadevoo, S.M.P.; Lee, H.W.; Hong, C.M.; Lee, B.; Lee, J.; Ahn, B.-C. Interleukin-4 Receptor Targeting Peptide Decorated Extracellular Vesicles as a Platform for In Vivo Drug Delivery to Thyroid Cancer. Biomedicines 2022, 10, 1978. https://doi.org/10.3390/biomedicines10081978
Gangadaran P, Gunassekaran GR, Rajendran RL, Oh JM, Vadevoo SMP, Lee HW, Hong CM, Lee B, Lee J, Ahn B-C. Interleukin-4 Receptor Targeting Peptide Decorated Extracellular Vesicles as a Platform for In Vivo Drug Delivery to Thyroid Cancer. Biomedicines. 2022; 10(8):1978. https://doi.org/10.3390/biomedicines10081978
Chicago/Turabian StyleGangadaran, Prakash, Gowri Rangaswamy Gunassekaran, Ramya Lakshmi Rajendran, Ji Min Oh, Sri Murugan Poongkavithai Vadevoo, Ho Won Lee, Chae Moon Hong, Byungheon Lee, Jaetae Lee, and Byeong-Cheol Ahn. 2022. "Interleukin-4 Receptor Targeting Peptide Decorated Extracellular Vesicles as a Platform for In Vivo Drug Delivery to Thyroid Cancer" Biomedicines 10, no. 8: 1978. https://doi.org/10.3390/biomedicines10081978







