Circulating Total Extracellular Vesicles Cargo Are Associated with Age-Related Oxidative Stress and Susceptibility to Cardiovascular Diseases: Exploratory Results from Microarray Data
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Circulating Extracellular Vesicle and Particle Isolation and Characterization
2.3. microRNA Analysis
2.4. Western Blot for Pro- and Antioxidant Machinery
2.5. Determination of Pro- and Antioxidant Enzyme Activities
2.6. Statistical Analysis
3. Results
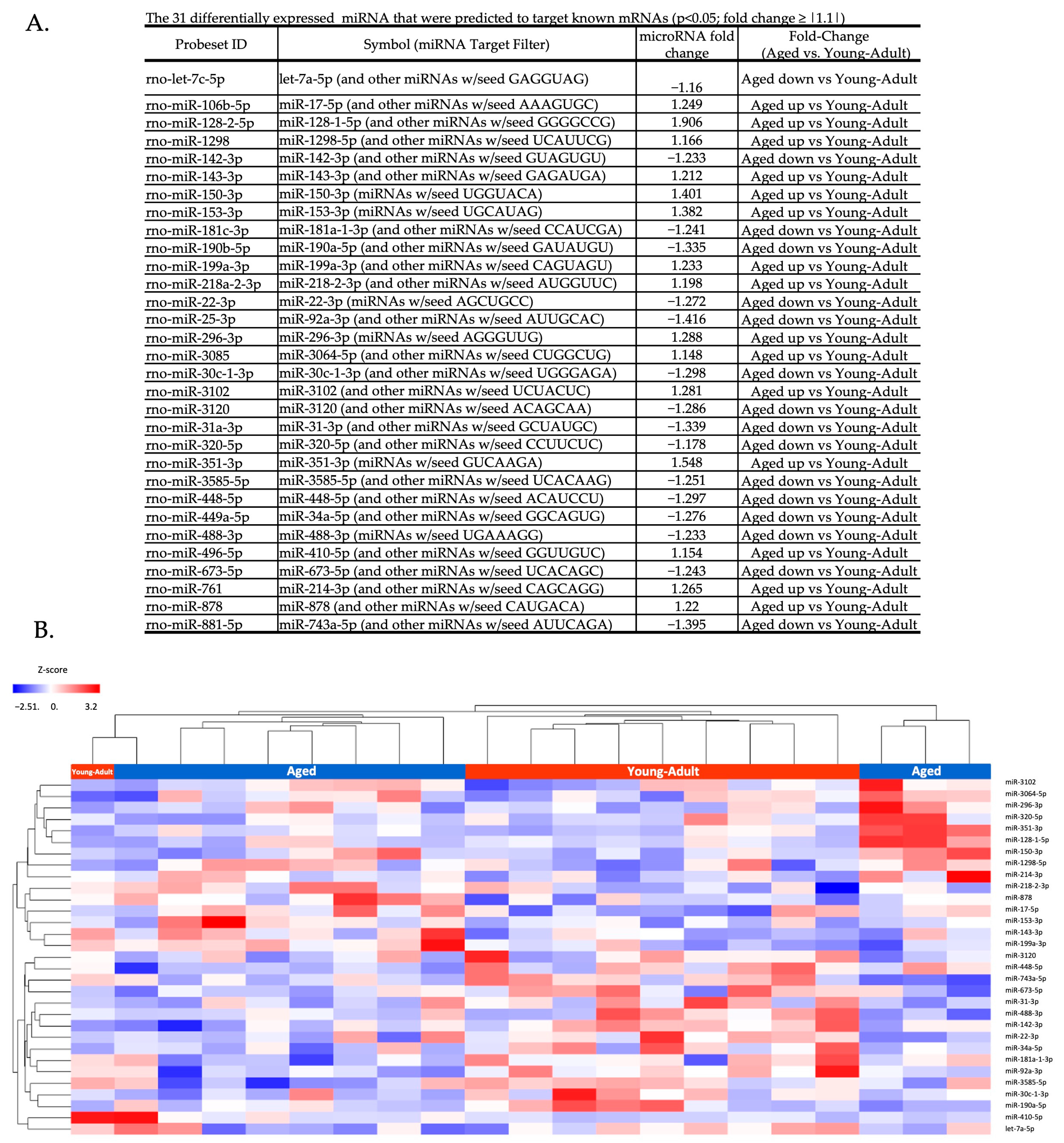
3.1. Circulating Total EV microRNA Expression Is Altered by Aging
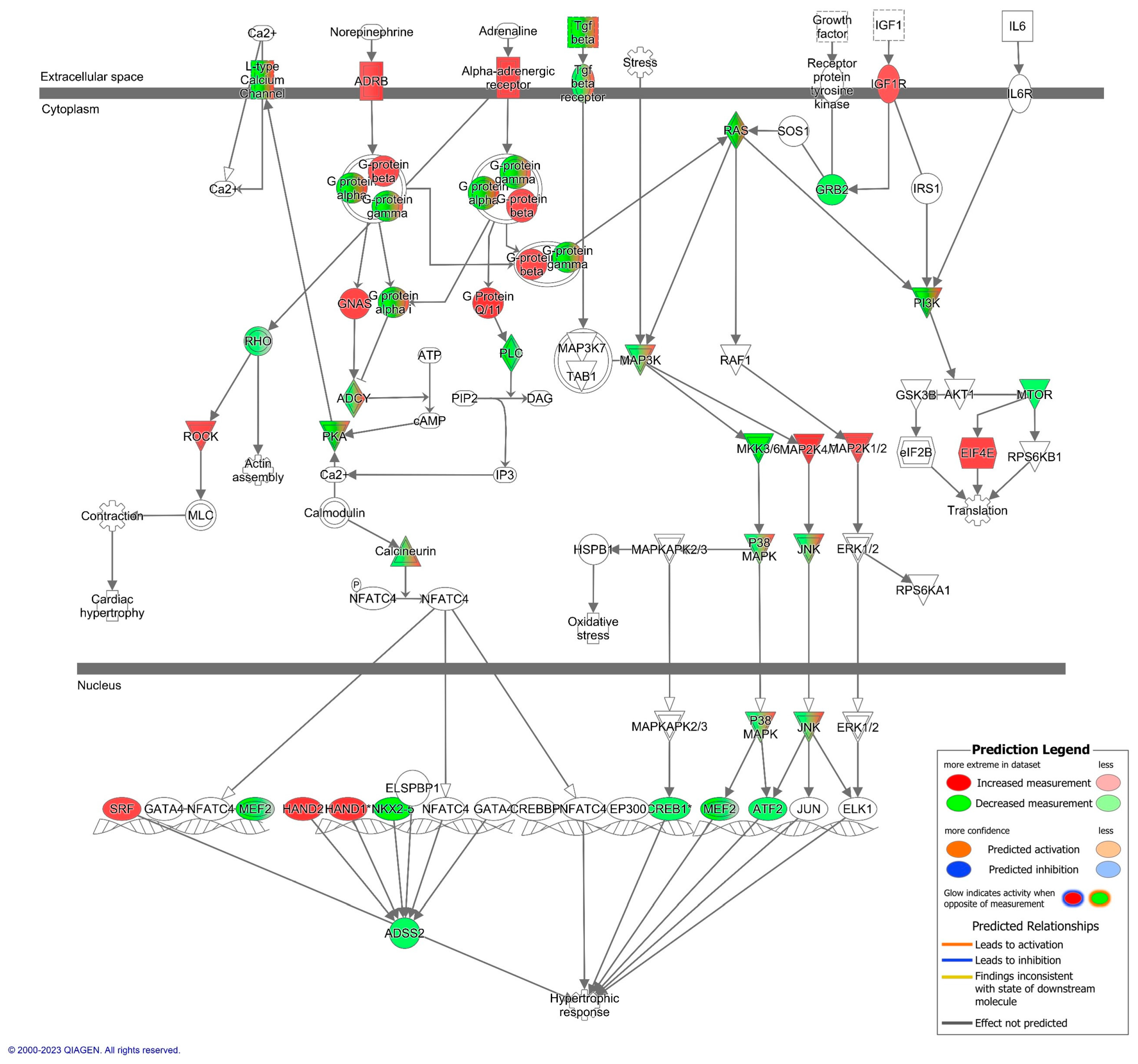
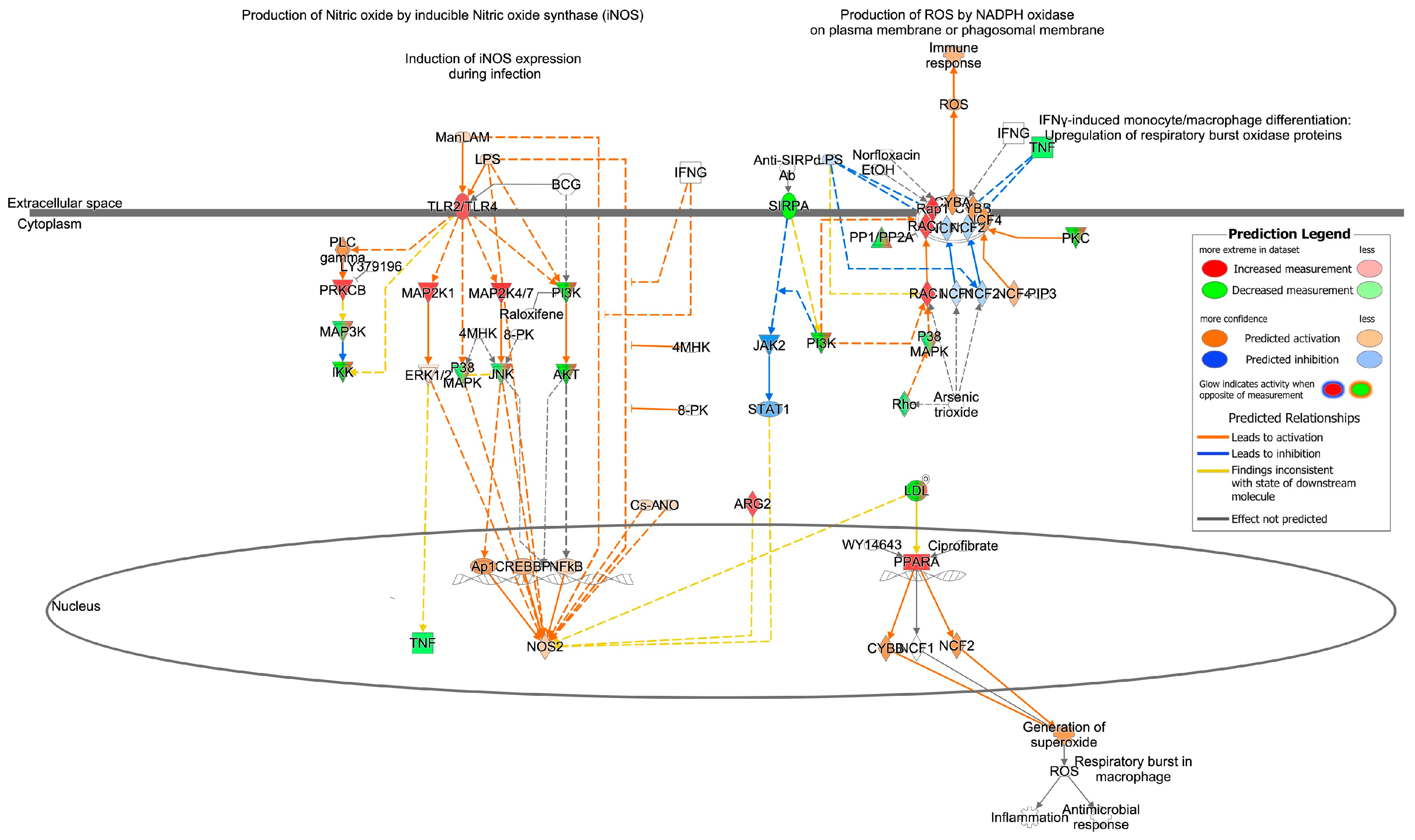
3.2. Cardiovascular Signaling and Redox Pathways Are Predicted to Be Impacted by Circulating Total EV microRNA from Aged Animals
3.3. Redox Machinery in EVPs
3.4. Characterization of Isolated EVPs
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Niccoli, T.; Partridge, L. Ageing as a Risk Factor for Disease. Curr. Biol. 2012, 22, R741–R752. [Google Scholar] [CrossRef] [PubMed]
- Bertoldi, K.; Cechinel, L.R.; Schallenberger, B.; Corssac, G.B.; Davies, S.; Guerreiro, I.C.K.; Belló-Klein, A.; Araujo, A.S.R.; Siqueira, I.R. Circulating Extracellular Vesicles in the Aging Process: Impact of Aerobic Exercise. Mol. Cell. Biochem. 2018, 440, 115–125. [Google Scholar] [CrossRef]
- Gomes de Andrade, G.; Reck Cechinel, L.; Bertoldi, K.; Galvão, F.; Valdeci Worm, P.; Rodrigues Siqueira, I. The Aging Process Alters IL-1β and CD63 Levels Differently in Extracellular Vesicles Obtained from the Plasma and Cerebrospinal Fluid. Neuroimmunomodulation 2018, 25, 18–22. [Google Scholar] [CrossRef]
- Harman, D. Free Radical Theory of Aging. Mutat. Res. 1992, 275, 257–266. [Google Scholar] [CrossRef]
- Davies, S.M.; Poljak, A.; Duncan, M.W.; Smythe, G.A.; Murphy, M.P. Measurements of Protein Carbonyls, Ortho- and Meta-Tyrosine and Oxidative Phosphorylation Complex Activity in Mitochondria from Young and Old Rats. Free Radic. Biol. Med. 2001, 31, 181–190. [Google Scholar] [CrossRef] [PubMed]
- Doğru-Abbasoğlu, S.; Tamer-Toptani, S.; Uğurnal, B.; Koçak-Toker, N.; Aykaç-Toker, G.; Uysal, M. Lipid Peroxidation and Antioxidant Enzymes in Livers and Brains of Aged Rats. Mech. Ageing Dev. 1997, 98, 177–180. [Google Scholar] [CrossRef] [PubMed]
- Evans, P.H. Free Radicals in Brain Metabolism and Pathology. Br. Med. Bull. 1993, 49, 577–587. [Google Scholar] [CrossRef]
- Siqueira, I.R.; Fochesatto, C.; de Andrade, A.; Santos, M.; Hagen, M.; Bello-Klein, A.; Netto, C.A. Total Antioxidant Capacity Is Impaired in Different Structures from Aged Rat Brain. Int. J. Dev. Neurosci. 2005, 23, 663–671. [Google Scholar] [CrossRef]
- Friedman, R.C.; Farh, K.K.-H.; Burge, C.B.; Bartel, D.P. Most Mammalian MRNAs Are Conserved Targets of MicroRNAs. Genome Res. 2009, 19, 92–105. [Google Scholar] [CrossRef]
- Borras, C.; Mas-Bargues, C.; Sanz-Ros, J.; Román-Domínguez, A.; Gimeno-Mallench, L.; Inglés, M.; Gambini, J.; Viña, J. Extracellular Vesicles and Redox Modulation in Aging. Free Radic. Biol. Med. 2020, 149, 44–50. [Google Scholar] [CrossRef]
- Janiszewski, M.; Do Carmo, A.O.; Pedro, M.A.; Silva, E.; Knobel, E.; Laurindo, F.R.M. Platelet-Derived Exosomes of Septic Individuals Possess Proapoptotic NAD(P)H Oxidase Activity: A Novel Vascular Redox Pathway. Crit. Care Med. 2004, 32, 818–825. [Google Scholar] [CrossRef]
- Brodsky, S.V.; Zhang, F.; Nasjletti, A.; Goligorsky, M.S. Endothelium-Derived Microparticles Impair Endothelial Function in Vitro. Am. J. Physiol. Heart Circ. Physiol. 2004, 286, H1910–H1915. [Google Scholar] [CrossRef] [PubMed]
- Mostefai, H.A.; Agouni, A.; Carusio, N.; Mastronardi, M.L.; Heymes, C.; Henrion, D.; Andriantsitohaina, R.; Martinez, M.C. Phosphatidylinositol 3-Kinase and Xanthine Oxidase Regulate Nitric Oxide and Reactive Oxygen Species Productions by Apoptotic Lymphocyte Microparticles in Endothelial Cells. J. Immunol. 2008, 180, 5028–5035. [Google Scholar] [CrossRef]
- Nicholls, S.J.; Hazen, S.L. Myeloperoxidase and Cardiovascular Disease. Arterioscler. Thromb. Vasc. Biol. 2005, 25, 1102–1111. [Google Scholar] [CrossRef] [PubMed]
- Brandes, R.P.; Weissmann, N.; Schröder, K. NADPH Oxidases in Cardiovascular Disease. Free Radic. Biol. Med. 2010, 49, 687–706. [Google Scholar] [CrossRef]
- Lee, B.-R.; Kim, J.-H.; Choi, E.-S.; Cho, J.-H.; Kim, E. Effect of Young Exosomes Injected in Aged Mice. Int. J. Nanomed. 2018, 13, 5335–5345. [Google Scholar] [CrossRef]
- Kura, B.; Szeiffova Bacova, B.; Kalocayova, B.; Sykora, M.; Slezak, J. Oxidative Stress-Responsive MicroRNAs in Heart Injury. Int. J. Mol. Sci. 2020, 21, 358. [Google Scholar] [CrossRef]
- Engedal, N.; Žerovnik, E.; Rudov, A.; Galli, F.; Olivieri, F.; Procopio, A.D.; Rippo, M.R.; Monsurrò, V.; Betti, M.; Albertini, M.C. From Oxidative Stress Damage to Pathways, Networks, and Autophagy via MicroRNAs. Oxid. Med. Cell. Longev. 2018, 2018, 4968321. [Google Scholar] [CrossRef]
- Gelibter, S.; Marostica, G.; Mandelli, A.; Siciliani, S.; Podini, P.; Finardi, A.; Furlan, R. The Impact of Storage on Extracellular Vesicles: A Systematic Study. J. Extracell. Vesicles 2022, 11, e12162. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Klein, D.; Kern, R.M.; Sokol, R.Z. A Method for Quantification and Correction of Proteins after Transfer to Immobilization Membranes. Biochem. Mol. Biol. Int. 1995, 36, 59–66. [Google Scholar]
- Romero-Calvo, I.; Ocón, B.; Martínez-Moya, P.; Suárez, M.D.; Zarzuelo, A.; Martínez-Augustin, O.; de Medina, F.S. Reversible Ponceau Staining as a Loading Control Alternative to Actin in Western Blots. Anal. Biochem. 2010, 401, 318–320. [Google Scholar] [CrossRef] [PubMed]
- Corssac, G.B.; Bonetto, J.P.; Campos-Carraro, C.; Cechinel, L.R.; Zimmer, A.; Parmeggiani, B.; Grings, M.; Carregal, V.M.; Massensini, A.R.; Siqueira, I.; et al. Pulmonary Arterial Hypertension Induces the Release of Circulating Extracellular Vesicles with Oxidative Content and Alters Redox and Mitochondrial Homeostasis in the Brains of Rats. Hypertens. Res. 2021, 44, 918–931. [Google Scholar] [CrossRef]
- Osteikoetxea, X.; Sódar, B.; Németh, A.; Szabó-Taylor, K.; Pálóczi, K.; Vukman, K.V.; Tamási, V.; Balogh, A.; Kittel, Á.; Pállinger, É.; et al. Differential Detergent Sensitivity of Extracellular Vesicle Subpopulations. Org. Biomol. Chem. 2015, 13, 9775–9782. [Google Scholar] [CrossRef] [PubMed]
- Prajda, N.; Weber, G. Malignant Transformation-Linked Imbalance: Decreased Xanthine Oxidase Activity in Hepatomas. FEBS Lett. 1975, 59, 245–249. [Google Scholar] [CrossRef]
- Aebi, H. Catalase in Vitro. Methods Enzymol. 1984, 105, 121–126. [Google Scholar] [PubMed]
- Krämer, A.; Green, J.; Pollard, J., Jr.; Tugendreich, S. Causal Analysis Approaches in Ingenuity Pathway Analysis. Bioinformatics 2014, 30, 523–530. [Google Scholar] [CrossRef] [PubMed]
- Siqueira, I.R.; de Souza Rodrigues, A.; Flores, M.S.; Vieira Cunha, E.L.; Goldberg, M.; Harmon, B.; Batabyal, R.; Freishtat, R.J.; Cechinel, L.R. Circulating Extracellular Vesicles and Particles Derived From Adipocytes: The Potential Role in Spreading MicroRNAs Associated With Cellular Senescence. Front. Aging 2022, 3, 867100. [Google Scholar] [CrossRef]
- Lv, L.; Li, C.; Zhang, X.; Ding, N.; Cao, T.; Jia, X.; Wang, J.; Pan, L.; Jia, H.; Li, Z.; et al. RNA Profiling Analysis of the Serum Exosomes Derived from Patients with Active and Latent Mycobacterium Tuberculosis Infection. Front. Microbiol. 2017, 8, 1051. [Google Scholar] [CrossRef] [PubMed]
- Bernstein, D.; Fajardo, G.; Zhao, M. The role OF β-adrenergic receptors in heart failure: Differential regulation of cardiotoxicity and cardioprotection. Prog. Pediatr. Cardiol. 2011, 31, 35–38. [Google Scholar] [CrossRef]
- Hordijk, P.L. Regulation of NADPH Oxidases. Circ. Res. 2006, 98, 453–462. [Google Scholar] [CrossRef]
- Lee, T.H.; D’Asti, E.; Magnus, N.; Al-Nedawi, K.; Meehan, B.; Rak, J. Microvesicles as Mediators of Intercellular Communication in Cancer--the Emerging Science of Cellular “Debris”. Semin. Immunopathol. 2011, 33, 455–467. [Google Scholar] [CrossRef] [PubMed]
- Fröhlich, D.; Kuo, W.P.; Frühbeis, C.; Sun, J.-J.; Zehendner, C.M.; Luhmann, H.J.; Pinto, S.; Toedling, J.; Trotter, J.; Krämer-Albers, E.-M. Multifaceted Effects of Oligodendroglial Exosomes on Neurons: Impact on Neuronal Firing Rate, Signal Transduction and Gene Regulation. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2014, 369, 20130510. [Google Scholar] [CrossRef] [PubMed]
- Paciaroni, E.; Fraticelli, A. Left Ventricular Hypertrophy. Prevalence in Older Patients and Management. Drugs Aging 1995, 6, 301–311. [Google Scholar] [CrossRef]
- Chiao, Y.A.; Rabinovitch, P.S. The Aging Heart. Cold Spring Harb. Perspect. Med. 2015, 5, a025148. [Google Scholar] [CrossRef]
- Leibowitz, D. Left Ventricular Hypertrophy and Chronic Renal Insufficiency in the Elderly. Cardiorenal Med. 2014, 4, 168–175. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.-M.; Liu, F.-Z.; Zhu, J.-N.; Fu, Y.-H.; Lin, Q.-X.; Deng, C.-Y.; Hu, Z.-Q.; Yang, H.; Zheng, X.-L.; Cheng, J.-D.; et al. Myocyte-Specific Enhancer Factor 2C: A Novel Target Gene of MiR-214-3p in Suppressing Angiotensin II-Induced Cardiomyocyte Hypertrophy. Sci. Rep. 2016, 6, 36146. [Google Scholar] [CrossRef]
- Rane, S.; He, M.; Sayed, D.; Yan, L.; Vatner, D.; Abdellatif, M. An Antagonism between the AKT and Beta-Adrenergic Signaling Pathways Mediated through Their Reciprocal Effects on MiR-199a-5p. Cell. Signal. 2010, 22, 1054–1062. [Google Scholar] [CrossRef]
- Yang, Y.; Ago, T.; Zhai, P.; Abdellatif, M.; Sadoshima, J. Thioredoxin 1 Negatively Regulates Angiotensin II-Induced Cardiac Hypertrophy through Upregulation of MiR-98/Let-7. Circ. Res. 2011, 108, 305–313. [Google Scholar] [CrossRef]
- Sun, D.; Li, C.; Liu, J.; Wang, Z.; Liu, Y.; Luo, C.; Chen, Y.; Wen, S. Expression Profile of MicroRNAs in Hypertrophic Cardiomyopathy and Effects of MicroRNA-20 in Inducing Cardiomyocyte Hypertrophy Through Regulating Gene MFN2. DNA Cell Biol. 2019, 38, 796–807. [Google Scholar] [CrossRef] [PubMed]
- Xue, J.; Zhou, D.; Poulsen, O.; Hartley, I.; Imamura, T.; Xie, E.X.; Haddad, G.G. Exploring MiRNA-MRNA Regulatory Network in Cardiac Pathology in Na+/H+ Exchanger Isoform 1 Transgenic Mice. Physiol. Genom. 2018, 50, 846–861. [Google Scholar] [CrossRef]
- de Lucia, C.; Eguchi, A.; Koch, W.J. New Insights in Cardiac β-Adrenergic Signaling During Heart Failure and Aging. Front. Pharmacol. 2018, 9, 904. [Google Scholar] [CrossRef] [PubMed]
- Enns, L.C.; Morton, J.F.; Treuting, P.R.; Emond, M.J.; Wolf, N.S.; Dai, D.-F.; McKnight, G.S.; Rabinovitch, P.S.; Ladiges, W.C. Disruption of Protein Kinase A in Mice Enhances Healthy Aging. PLoS ONE 2009, 4, e5963. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhu, H.; Shen, E.; Wan, L.; Arnold, J.M.O.; Peng, T. Deficiency of Rac1 Blocks NADPH Oxidase Activation, Inhibits Endoplasmic Reticulum Stress, and Reduces Myocardial Remodeling in a Mouse Model of Type 1 Diabetes. Diabetes 2010, 59, 2033–2042. [Google Scholar] [CrossRef]
- Wang, Y.; Ouyang, M.; Wang, Q.; Jian, Z. MicroRNA-142-3p Inhibits Hypoxia/Reoxygenation-induced Apoptosis and Fibrosis of Cardiomyocytes by Targeting High Mobility Group Box 1. Int. J. Mol. Med. 2016, 38, 1377–1386. [Google Scholar] [CrossRef]
- Huang, H.; Pandya, C.; Liu, C.; Al-Obaidi, N.F.; Wang, M.; Zheng, L.; Keating, S.T.; Aono, M.; Love, J.D.; Evans, B.; et al. Panoramic View of a Superfamily of Phosphatases through Substrate Profiling. Proc. Natl. Acad. Sci. USA 2015, 112, E1974–E1983. [Google Scholar] [CrossRef]
- Miyano, K.; Sumimoto, H. Assessment of the Role for Rho Family GTPases in NADPH Oxidase Activation. Methods Mol. Biol. 2012, 827, 195–212. [Google Scholar] [PubMed]
- Schupp, M.; Kintscher, U.; Fielitz, J.; Thomas, J.; Pregla, R.; Hetzer, R.; Unger, T.; Regitz-Zagrosek, V. Cardiac PPARalpha Expression in Patients with Dilated Cardiomyopathy. Eur. J. Heart Fail. 2006, 8, 290–294. [Google Scholar] [CrossRef]
- Teissier, E.; Nohara, A.; Chinetti, G.; Paumelle, R.; Cariou, B.; Fruchart, J.-C.; Brandes, R.P.; Shah, A.; Staels, B. Peroxisome Proliferator-Activated Receptor Alpha Induces NADPH Oxidase Activity in Macrophages, Leading to the Generation of LDL with PPAR-Alpha Activation Properties. Circ. Res. 2004, 95, 1174–1182. [Google Scholar] [CrossRef]
- Hervera, A.; De Virgiliis, F.; Palmisano, I.; Zhou, L.; Tantardini, E.; Kong, G.; Hutson, T.; Danzi, M.C.; Perry, R.B.-T.; Santos, C.X.C.; et al. Reactive Oxygen Species Regulate Axonal Regeneration through the Release of Exosomal NADPH Oxidase 2 Complexes into Injured Axons. Nat. Cell Biol. 2018, 20, 307–319. [Google Scholar] [CrossRef]
- Pitanga, T.N.; de Aragão França, L.; Rocha, V.C.J.; Meirelles, T.; Borges, V.M.; Gonçalves, M.S.; Pontes-de-Carvalho, L.C.; Noronha-Dutra, A.A.; dos-Santos, W.L.C. Neutrophil-Derived Microparticles Induce Myeloperoxidase-Mediated Damage of Vascular Endothelial Cells. BMC Cell Biol. 2014, 15, 21. [Google Scholar] [CrossRef]
- Aranda, R.; Doménech, E.; Rus, A.D.; Real, J.T.; Sastre, J.; Viña, J.; Pallardó, F.V. Age-Related Increase in Xanthine Oxidase Activity in Human Plasma and Rat Tissues. Free Radic. Res. 2007, 41, 1195–1200. [Google Scholar] [CrossRef] [PubMed]
- Terman, A.; Brunk, U.T. Aging as a Catabolic Malfunction. Int. J. Biochem. Cell Biol. 2004, 36, 2365–2375. [Google Scholar] [CrossRef] [PubMed]
- Johnstone, R.M.; Mathew, A.; Mason, A.B.; Teng, K. Exosome Formation during Maturation of Mammalian and Avian Reticulocytes: Evidence That Exosome Release Is a Major Route for Externalization of Obsolete Membrane Proteins. J. Cell. Physiol. 1991, 147, 27–36. [Google Scholar] [CrossRef] [PubMed]
- de Godoy, M.A.; Saraiva, L.M.; de Carvalho, L.R.P.; Vasconcelos-Dos-Santos, A.; Beiral, H.J.V.; Ramos, A.B.; de Paula Silva, L.R.; Leal, R.B.; Monteiro, V.H.S.; Braga, C.V.; et al. Mesenchymal Stem Cells and Cell-Derived Extracellular Vesicles Protect Hippocampal Neurons from Oxidative Stress and Synapse Damage Induced by Amyloid-β Oligomers. J. Biol. Chem. 2018, 293, 1957–1975. [Google Scholar] [CrossRef]
- Haney, M.J.; Klyachko, N.L.; Zhao, Y.; Gupta, R.; Plotnikova, E.G.; He, Z.; Patel, T.; Piroyan, A.; Sokolsky, M.; Kabanov, A.V.; et al. Exosomes as Drug Delivery Vehicles for Parkinson’s Disease Therapy. J. Control. Release 2015, 207, 18–30. [Google Scholar] [CrossRef]
- Loffredo, F.S.; Steinhauser, M.L.; Jay, S.M.; Gannon, J.; Pancoast, J.R.; Yalamanchi, P.; Sinha, M.; Dall’Osso, C.; Khong, D.; Shadrach, J.L.; et al. Growth Differentiation Factor 11 Is a Circulating Factor That Reverses Age-Related Cardiac Hypertrophy. Cell 2013, 153, 828–839. [Google Scholar] [CrossRef]
- Yoshida, M.; Satoh, A.; Lin, J.B.; Mills, K.F.; Sasaki, Y.; Rensing, N.; Wong, M.; Apte, R.S.; Imai, S.-I. Extracellular Vesicle-Contained ENAMPT Delays Aging and Extends Lifespan in Mice. Cell Metab. 2019, 30, 329–342.e5. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cechinel, L.R.; Batabyal, R.A.; Blume Corssac, G.; Goldberg, M.; Harmon, B.; Vallejos, V.M.R.; Bruch, G.E.; Massensini, A.R.; Belló-Klein, A.; Araujo, A.S.d.R.; et al. Circulating Total Extracellular Vesicles Cargo Are Associated with Age-Related Oxidative Stress and Susceptibility to Cardiovascular Diseases: Exploratory Results from Microarray Data. Biomedicines 2023, 11, 2920. https://doi.org/10.3390/biomedicines11112920
Cechinel LR, Batabyal RA, Blume Corssac G, Goldberg M, Harmon B, Vallejos VMR, Bruch GE, Massensini AR, Belló-Klein A, Araujo ASdR, et al. Circulating Total Extracellular Vesicles Cargo Are Associated with Age-Related Oxidative Stress and Susceptibility to Cardiovascular Diseases: Exploratory Results from Microarray Data. Biomedicines. 2023; 11(11):2920. https://doi.org/10.3390/biomedicines11112920
Chicago/Turabian StyleCechinel, Laura Reck, Rachael Ann Batabyal, Giana Blume Corssac, Madeleine Goldberg, Brennan Harmon, Virgínia Mendes Russo Vallejos, Gisele E. Bruch, André Ricardo Massensini, Adriane Belló-Klein, Alex Sander da Rosa Araujo, and et al. 2023. "Circulating Total Extracellular Vesicles Cargo Are Associated with Age-Related Oxidative Stress and Susceptibility to Cardiovascular Diseases: Exploratory Results from Microarray Data" Biomedicines 11, no. 11: 2920. https://doi.org/10.3390/biomedicines11112920





