Quantification of Bile Acids in Cerebrospinal Fluid: Results of an Observational Trial
Abstract
:1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shulpekova, Y.; Zharkova, M.; Tkachenko, P.; Tikhonov, I.; Stepanov, A.; Synitsyna, A.; Izotov, A.; Butkova, T.; Shulpekova, N.; Lapina, N.; et al. The Role of Bile Acids in the Human Body and in the Development of Diseases. Molecules 2022, 27, 3401. [Google Scholar] [CrossRef] [PubMed]
- Xie, A.J.; Mai, C.T.; Zhu, Y.Z.; Liu, X.C.; Xie, Y. Bile acids as regulatory molecules and potential targets in metabolic diseases. Life Sci. 2021, 287, 120152. [Google Scholar] [CrossRef] [PubMed]
- Harnisch, L.O.; Mihaylov, D.; Bein, T.; Apfelbacher, C.; Kiehntopf, M.; Bauer, M.; Moerer, O.; Quintel, M. Determination of individual bile acids in acute respiratory distress syndrome reveals a specific pattern of primary and secondary bile acids and a shift to the acidic pathway as an adaptive response to the critical condition. Clin. Chem. Lab. Med. 2022, 60, 891–900. [Google Scholar] [CrossRef]
- Fiorucci, S.; Distrutti, E. The Pharmacology of Bile Acids and Their Receptors. Handb. Exp. Pharmacol. 2019, 256, 3–18. [Google Scholar] [CrossRef]
- Deckmyn, B.; Domenger, D.; Blondel, C.; Ducastel, S.; Nicolas, E.; Dorchies, E.; Caron, E.; Charton, J.; Vallez, E.; Deprez, B.; et al. Farnesoid X Receptor Activation in Brain Alters Brown Adipose Tissue Function via the Sympathetic System. Front. Mol. Neurosci. 2021, 14, 808603. [Google Scholar] [CrossRef] [PubMed]
- St-Pierre, M.V.; Kullak-Ublick, G.A.; Hagenbuch, B.; Meier, P.J. Transport of bile acids in hepatic and non-hepatic tissues. J. Exp. Biol. 2001, 204, 1673–1686. [Google Scholar] [CrossRef]
- Higashi, T.; Watanabe, S.; Tomaru, K.; Yamazaki, W.; Yoshizawa, K.; Ogawa, S.; Nagao, H.; Minato, K.; Maekawa, M.; Mano, N. Unconjugated bile acids in rat brain: Analytical method based on LC/ESI-MS/MS with chemical derivatization and estimation of their origin by comparison to serum levels. Steroids 2017, 125, 107–113. [Google Scholar] [CrossRef]
- Parry, G.J.; Rodrigues, C.M.; Aranha, M.M.; Hilbert, S.J.; Davey, C.; Kelkar, P.; Low, W.C.; Steer, C.J. Safety, tolerability, and cerebrospinal fluid penetration of ursodeoxycholic Acid in patients with amyotrophic lateral sclerosis. Clin. Neuropharmacol. 2010, 33, 17–21. [Google Scholar] [CrossRef]
- Mano, N.; Goto, T.; Uchida, M.; Nishimura, K.; Ando, M.; Kobayashi, N.; Goto, J. Presence of protein-bound unconjugated bile acids in the cytoplasmic fraction of rat brain. J. Lipid Res. 2004, 45, 295–300. [Google Scholar] [CrossRef]
- Klaassen, C.D.; Aleksunes, L.M. Xenobiotic, bile acid, and cholesterol transporters: Function and regulation. Pharmacol. Rev. 2010, 62, 1–96. [Google Scholar] [CrossRef]
- Meaney, S.; Heverin, M.; Panzenboeck, U.; Ekstrom, L.; Axelsson, M.; Andersson, U.; Diczfalusy, U.; Pikuleva, I.; Wahren, J.; Sattler, W.; et al. Novel route for elimination of brain oxysterols across the blood-brain barrier: Conversion into 7alpha-hydroxy-3-oxo-4-cholestenoic acid. J. Lipid Res. 2007, 48, 944–951. [Google Scholar] [CrossRef] [PubMed]
- Nagata, K.; Seyama, Y.; Shimizu, T. Changes in the level of 7 alpha-hydroxy-3-oxo-4-cholestenoic acid in cerebrospinal fluid after subarachnoid hemorrhage. Neurol. Med.-Chir. 1995, 35, 294–297. [Google Scholar] [CrossRef] [PubMed]
- Bron, B.; Waldram, R.; Silk, D.B.; Williams, R. Serum, cerebrospinal fluid, and brain levels of bile acids in patients with fulminant hepatic failure. Gut 1977, 18, 692–696. [Google Scholar] [CrossRef]
- Grant, S.M.; DeMorrow, S. Bile Acid Signaling in Neurodegenerative and Neurological Disorders. Int. J. Mol. Sci. 2020, 21, 5982. [Google Scholar] [CrossRef] [PubMed]
- Kiriyama, Y.; Nochi, H. The Biosynthesis, Signaling, and Neurological Functions of Bile Acids. Biomolecules 2019, 9, 232. [Google Scholar] [CrossRef]
- McMillin, M.; DeMorrow, S. Effects of bile acids on neurological function and disease. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2016, 30, 3658–3668. [Google Scholar] [CrossRef]
- Weng, Z.B.; Chen, Y.R.; Lv, J.T.; Wang, M.X.; Chen, Z.Y.; Zhou, W.; Shen, X.C.; Zhan, L.B.; Wang, F. A Review of Bile Acid Metabolism and Signaling in Cognitive Dysfunction-Related Diseases. Oxidative Med. Cell. Longev. 2022, 2022, 4289383. [Google Scholar] [CrossRef]
- Monteiro-Cardoso, V.F.; Corliano, M.; Singaraja, R.R. Bile Acids: A Communication Channel in the Gut-Brain Axis. Neuromol. Med. 2021, 23, 99–117. [Google Scholar] [CrossRef]
- DeMorrow, S. Bile Acids in Hepatic Encephalopathy. J. Clin. Exp. Hepatol. 2019, 9, 117–124. [Google Scholar] [CrossRef]
- Varma, V.R.; Wang, Y.; An, Y.; Varma, S.; Bilgel, M.; Doshi, J.; Legido-Quigley, C.; Delgado, J.C.; Oommen, A.M.; Roberts, J.A.; et al. Bile acid synthesis, modulation, and dementia: A metabolomic, transcriptomic, and pharmacoepidemiologic study. PLoS Med. 2021, 18, e1003615. [Google Scholar] [CrossRef]
- Zangerolamo, L.; Vettorazzi, J.F.; Rosa, L.R.O.; Carneiro, E.M.; Barbosa, H.C.L. The bile acid TUDCA and neurodegenerative disorders: An overview. Life Sci. 2021, 272, 119252. [Google Scholar] [CrossRef] [PubMed]
- Ogundare, M.; Theofilopoulos, S.; Lockhart, A.; Hall, L.J.; Arenas, E.; Sjovall, J.; Brenton, A.G.; Wang, Y.; Griffiths, W.J. Cerebrospinal fluid steroidomics: Are bioactive bile acids present in brain? J. Biol. Chem. 2010, 285, 4666–4679. [Google Scholar] [CrossRef] [PubMed]
- Hemmerich, W. Statistikguru. Available online: https://statistikguru.de/rechner/winsorizing-rechner.html (accessed on 30 October 2021).
- DeMorrow, S.; Frampton, G.; Galindo, C.; Pae, H.Y.; Quinn, M. Increased serum bile acids after extrahepatic biliary obstruction causes leakiness to the blood brain barrier via the disruption of tight junctions. FASEB J. 2012, 26, 1110.7. [Google Scholar] [CrossRef]
- Quinn, M.; McMillin, M.; Galindo, C.; Frampton, G.; Pae, H.Y.; DeMorrow, S. Bile acids permeabilize the blood brain barrier after bile duct ligation in rats via Rac1-dependent mechanisms. Dig. Liver Dis. 2014, 46, 527–534. [Google Scholar] [CrossRef] [PubMed]
- Kamp, F.; Hamilton, J.A.; Kamp, F.; Westerhoff, H.V.; Hamilton, J.A. Movement of fatty acids, fatty acid analogues, and bile acids across phospholipid bilayers. Biochemistry 1993, 32, 11074–11086. [Google Scholar] [CrossRef]
- Marchi, N.; Rasmussen, P.; Kapural, M.; Fazio, V.; Kight, K.; Mayberg, M.R.; Kanner, A.; Ayumar, B.; Albensi, B.; Cavaglia, M.; et al. Peripheral markers of brain damage and blood-brain barrier dysfunction. Restor. Neurol. Neurosci. 2003, 21, 109–121. [Google Scholar]
- Reiber, H. Proteins in cerebrospinal fluid and blood: Barriers, CSF flow rate and source-related dynamics. Restor. Neurol. Neurosci. 2003, 21, 79–96. [Google Scholar]
- Sandoval, K.E.; Witt, K.A. Blood-brain barrier tight junction permeability and ischemic stroke. Neurobiol. Dis. 2008, 32, 200–219. [Google Scholar] [CrossRef]
- Alluri, H.; Wiggins-Dohlvik, K.; Davis, M.L.; Huang, J.H.; Tharakan, B. Blood-brain barrier dysfunction following traumatic brain injury. Metab. Brain Dis. 2015, 30, 1093–1104. [Google Scholar] [CrossRef]
- Barzó, P.; Marmarou, A.; Fatouros, P.; Corwin, F.; Dunbar, J. Magnetic resonance imaging—Monitored acute blood-brain barrier changes in experimental traumatic brain injury. J. Neurosurg. 1996, 85, 1113–1121. [Google Scholar] [CrossRef]
- Hay, J.R.; Johnson, V.E.; Young, A.M.; Smith, D.H.; Stewart, W. Blood-Brain Barrier Disruption Is an Early Event That May Persist for Many Years After Traumatic Brain Injury in Humans. J. Neuropathol. Exp. Neurol. 2015, 74, 1147–1157. [Google Scholar] [CrossRef] [PubMed]
- Dietschy, J.M. Central nervous system: Cholesterol turnover, brain development and neurodegeneration. Biol. Chem. 2009, 390, 287–293. [Google Scholar] [CrossRef] [PubMed]
- Gao, B.; Hagenbuch, B.; Kullak-Ublick, G.A.; Benke, D.; Aguzzi, A.; Meier, P.J. Organic anion-transporting polypeptides mediate transport of opioid peptides across blood-brain barrier. J. Pharmacol. Exp. Ther. 2000, 294, 73–79. [Google Scholar] [PubMed]
- McNeilly, A.D.; Macfarlane, D.P.; O’Flaherty, E.; Livingstone, D.E.; Mitic, T.; McConnell, K.M.; McKenzie, S.M.; Davies, E.; Reynolds, R.M.; Thiesson, H.C.; et al. Bile acids modulate glucocorticoid metabolism and the hypothalamic-pituitary-adrenal axis in obstructive jaundice. J. Hepatol. 2010, 52, 705–711. [Google Scholar] [CrossRef]
- Mertens, K.L.; Kalsbeek, A.; Soeters, M.R.; Eggink, H.M. Bile Acid Signaling Pathways from the Enterohepatic Circulation to the Central Nervous System. Front. Neurosci. 2017, 11, 617. [Google Scholar] [CrossRef]
- Copple, B.L.; Li, T. Pharmacology of bile acid receptors: Evolution of bile acids from simple detergents to complex signaling molecules. Pharmacol. Res. 2016, 104, 9–21. [Google Scholar] [CrossRef]
- Pavek, P. Pregnane X Receptor (PXR)-Mediated Gene Repression and Cross-Talk of PXR with Other Nuclear Receptors via Coactivator Interactions. Front. Pharmacol. 2016, 7, 456. [Google Scholar] [CrossRef]
- Bauer, B.; Yang, X.; Hartz, A.M.; Olson, E.R.; Zhao, R.; Kalvass, J.C.; Pollack, G.M.; Miller, D.S. In vivo activation of human pregnane X receptor tightens the blood-brain barrier to methadone through P-glycoprotein up-regulation. Mol. Pharmacol. 2006, 70, 1212–1219. [Google Scholar] [CrossRef]
- Lemmen, J.; Tozakidis, I.E.; Galla, H.J. Pregnane X receptor upregulates ABC-transporter Abcg2 and Abcb1 at the blood-brain barrier. Brain Res. 2013, 1491, 1–13. [Google Scholar] [CrossRef]
- Ebert, E.C. Hypoxic liver injury. Mayo Clin. Proc. 2006, 81, 1232–1236. [Google Scholar] [CrossRef]
- Kullak-Ublick, G.A.; Meier, P.J. Mechanisms of cholestasis. Clin. Liver Dis. 2000, 4, 357–385. [Google Scholar] [CrossRef] [PubMed]
- Beumer, C.; Wulferink, M.; Raaben, W.; Fiechter, D.; Brands, R.; Seinen, W. Calf intestinal alkaline phosphatase, a novel therapeutic drug for lipopolysaccharide (LPS)-mediated diseases, attenuates LPS toxicity in mice and piglets. J. Pharmacol. Exp. Ther. 2003, 307, 737–744. [Google Scholar] [CrossRef] [PubMed]
- Bilski, J.; Mazur-Bialy, A.; Wojcik, D.; Zahradnik-Bilska, J.; Brzozowski, B.; Magierowski, M.; Mach, T.; Magierowska, K.; Brzozowski, T. The Role of Intestinal Alkaline Phosphatase in Inflammatory Disorders of Gastrointestinal Tract. Mediat. Inflamm. 2017, 2017, 9074601. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.B.; Lin, H.C. Role of Intestinal Alkaline Phosphatase in Innate Immunity. Biomolecules 2021, 11, 1784. [Google Scholar] [CrossRef] [PubMed]
- Harnisch, L.O.; Baumann, S.; Mihaylov, D.; Kiehntopf, M.; Bauer, M.; Moerer, O.; Quintel, M. Biomarkers of Cholestasis and Liver Injury in the Early Phase of Acute Respiratory Distress Syndrome and Their Pathophysiological Value. Diagnostics 2021, 11, 2356. [Google Scholar] [CrossRef]


| Parameter | Value |
|---|---|
| Age (years) | 60 [54, 64] |
| Female:male | 23:31 |
| Delirium no. (%) | 11 (20.37) |
| Time insult–sample (h) | 19.75 [9.25, 47.0) |
| Time EVD–sample (min) | 630 [300, 810] |
| Bilirubin (mg/dL) | 0.6 [0.5, 0.8] |
| Bilirubin conjugated (mg/dL) | 0.3 [0.3, 0.5] |
| Bilirubin unconjugated (mg/dL) | 0.3 [0.3, 0.4] |
| Aspartate-Aminotransferase (U/L) | 25 [23, 31] |
| Alanine-Aminotransferase (U/L) | 17 [15, 27] |
| Gamma-Glutamyltransferase (U/L) | 30 [21, 50] |
| Alkaline phosphatase (U/L) | 68.5 [61, 80] |
| Albumin serum (mg/dL) | 2.95 [2.70, 3.20] |
| Red blood cells (RBCs) in CSF (n) | 3521 [1514, 5322] |
| Intraventricular blood volume (mL) | 313.5 [149, 2176] |
| Cerebral edema in CT no. (%) | 21 (39%) |
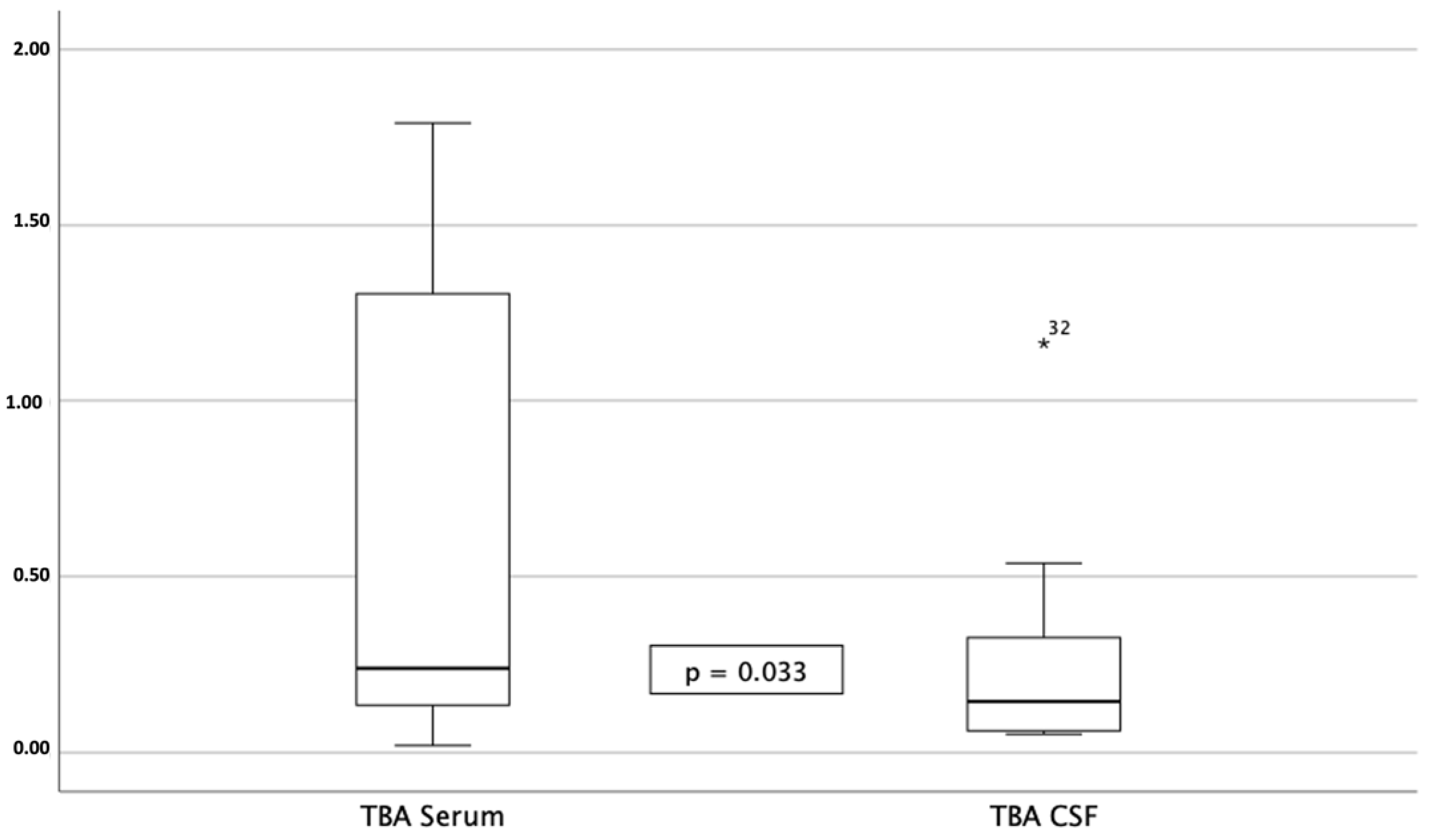
| Bile Acid Serum (µmol/L) | Median [95% CI] | Bile Acid CSF (µmol/L) | Median [95% CI] |
| TBA serum (µmol/L) | 0.37 [0.24, 0.89] | TBA CSF (µmol/L) | 0.14 [0.05, 0.43] |
| CA serum (µmol/L) | 0.09 [0.07, 1.01] | CA CSF (µmol/L) | n.d. |
| TCA serum (µmol/L) | 0.21 [0.08, 0.33] | TCA CSF (µmol/L) | n.d. |
| GCA serum (µmol/L) | 0.20 [0.15, 0.39] | GCA CSF (µmol/L) | 0.06 |
| CDCA serum (µmol/L) | 0.12 [0.08, 0.16] | CDCA CSF (µmol/L) | 0.08 [0.07, 0.08] |
| TCDCA serum (µmol/L) | 0.20 [0.13, 0.79] | TCDCA CSF (µmol/L) | 0.07 [0.05, 0.09] |
| GCDCA serum (µmol/L) | 0.21 [0.14, 0.54] | GCDCA CSF (µmol/L) | 0.36 |
| DCA serum (µmol/L) | 0.14 [0.08, 0.23] | DCA CSF (µmol/L) | 0.07 [0.06, 0.17] |
| TDCA serum (µmol/L) | 0.16 [0.14, 0.48] | TDCA CSF (µmol/L) | 0.06 [0.05, 0.09] |
| GDCA serum (µmol/L) | 0.17 [0.12, 0.31] | GDCA CSF (µmol/L) | 0.09 |
| UDCA serum (µmol/L) | 0.05 | UDCA CSF (µmol/L) | 0.08 [0.08, 0.23] |
| TUDCA serum (µmol/L) | n.d. | TUDCA CSF (µmol/L) | n.d. |
| GUDCA serum (µmol/L) | 0.16 [0.10, 0.28] | GUDCA CSF (µmol/L) | n.d. |
| LCA serum (µmol/L) | n.d. | LCA CSF (µmol/L) | n.d. |
| TLCA serum (µmol/L) | n.d. | TLCA CSF (µmol/L) | n.d. |
| GLCA serum (µmol/L) | n.d. | GLCA CSF (µmol/L) | n.d. |
| G/T-ratio TBA serum | 3.30 [1.79, 4.79] | G/T-ratio TBA CSF | n/a |
| Ratio Serum/CSF TBA | 3.10 [0.94, 14.64] | ||
| Ratio Serum/CSF CA | n/a | ||
| Ratio Serum/CSF TCA | 3.05 | ||
| Ratio Serum/CSF GCA | 14.30 [1.11, 27.13] | ||
| Ratio Serum/CSF CDCA | 0.0 | ||
| Ratio Serum/CSF TCDCA | 2.19 | ||
| Ratio Serum/CSF GCDCA | 1.91 [0.68, 8.64] | ||
| Ratio Serum/CSF DCA | 0.77 [0.0, 13.79] | ||
| Ratio Serum/CSF TDCA | n/a | ||
| Ratio Serum/CSF GDCA | n/a | ||
| Ratio Serum/CSF UDCA | n/a | ||
| Ratio Serum/CSF TUDCA | n/a | ||
| Ratio Serum/CSF GUDCA | n/a | ||
| Ratio Serum/CSF LCA | n/a | ||
| Ratio Serum/CSF TLCA | n/a | ||
| Ratio Serum/CSF GLCA | n/a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Harnisch, L.-O.; Neugebauer, S.; Mihaylov, D.; Eidizadeh, A.; Zechmeister, B.; Maier, I.; Moerer, O. Quantification of Bile Acids in Cerebrospinal Fluid: Results of an Observational Trial. Biomedicines 2023, 11, 2947. https://doi.org/10.3390/biomedicines11112947
Harnisch L-O, Neugebauer S, Mihaylov D, Eidizadeh A, Zechmeister B, Maier I, Moerer O. Quantification of Bile Acids in Cerebrospinal Fluid: Results of an Observational Trial. Biomedicines. 2023; 11(11):2947. https://doi.org/10.3390/biomedicines11112947
Chicago/Turabian StyleHarnisch, Lars-Olav, Sophie Neugebauer, Diana Mihaylov, Abass Eidizadeh, Bozena Zechmeister, Ilko Maier, and Onnen Moerer. 2023. "Quantification of Bile Acids in Cerebrospinal Fluid: Results of an Observational Trial" Biomedicines 11, no. 11: 2947. https://doi.org/10.3390/biomedicines11112947





