Research Progress of Titanium-Based Alloys for Medical Devices
Abstract
:1. Introduction
2. Materials and Methods
2.1. Material Development
2.2. Structural Characterization Methods
2.3. Mechanical Properties
2.4. In Vivo Biocompatibility Assessment
3. Results and Discussion
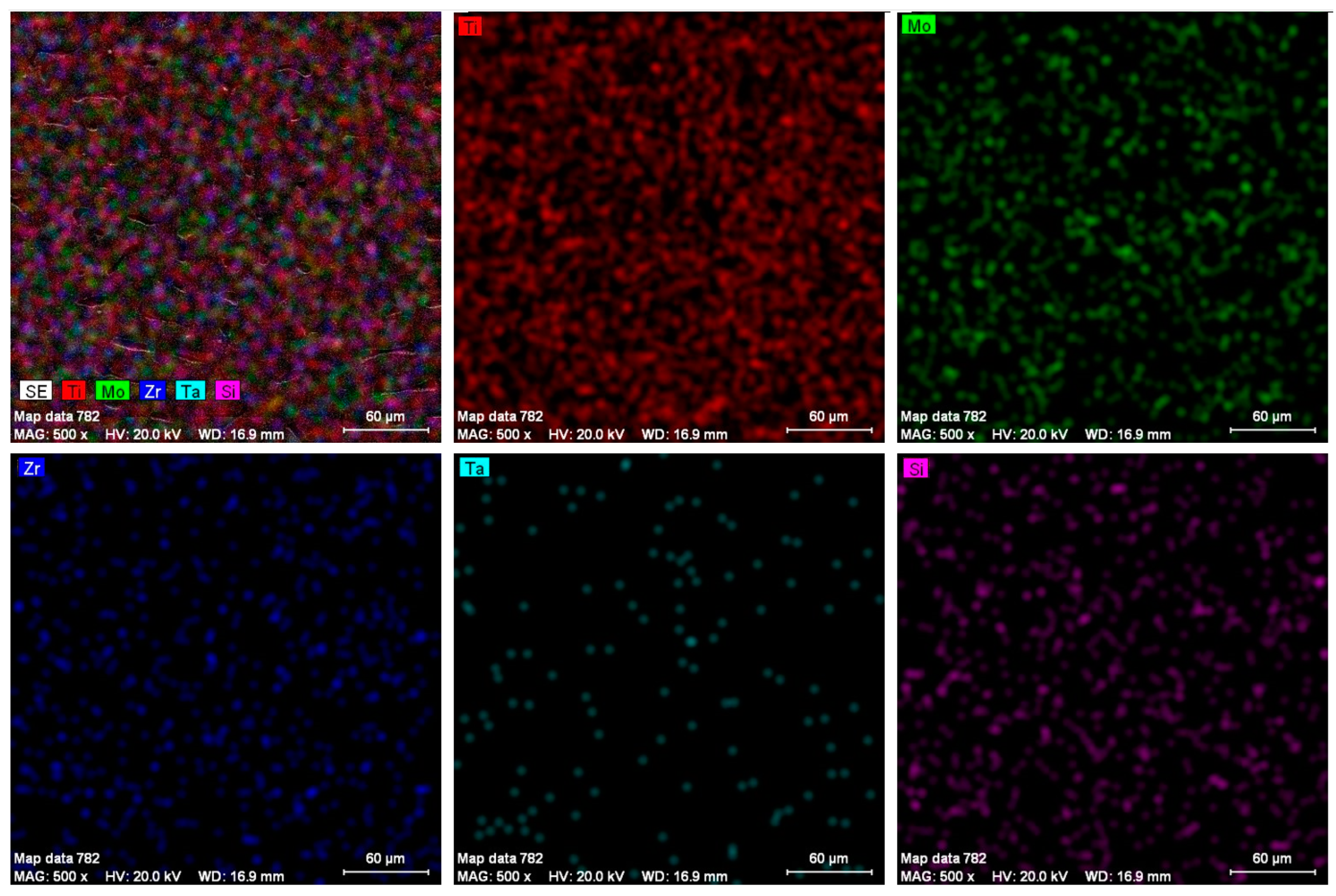
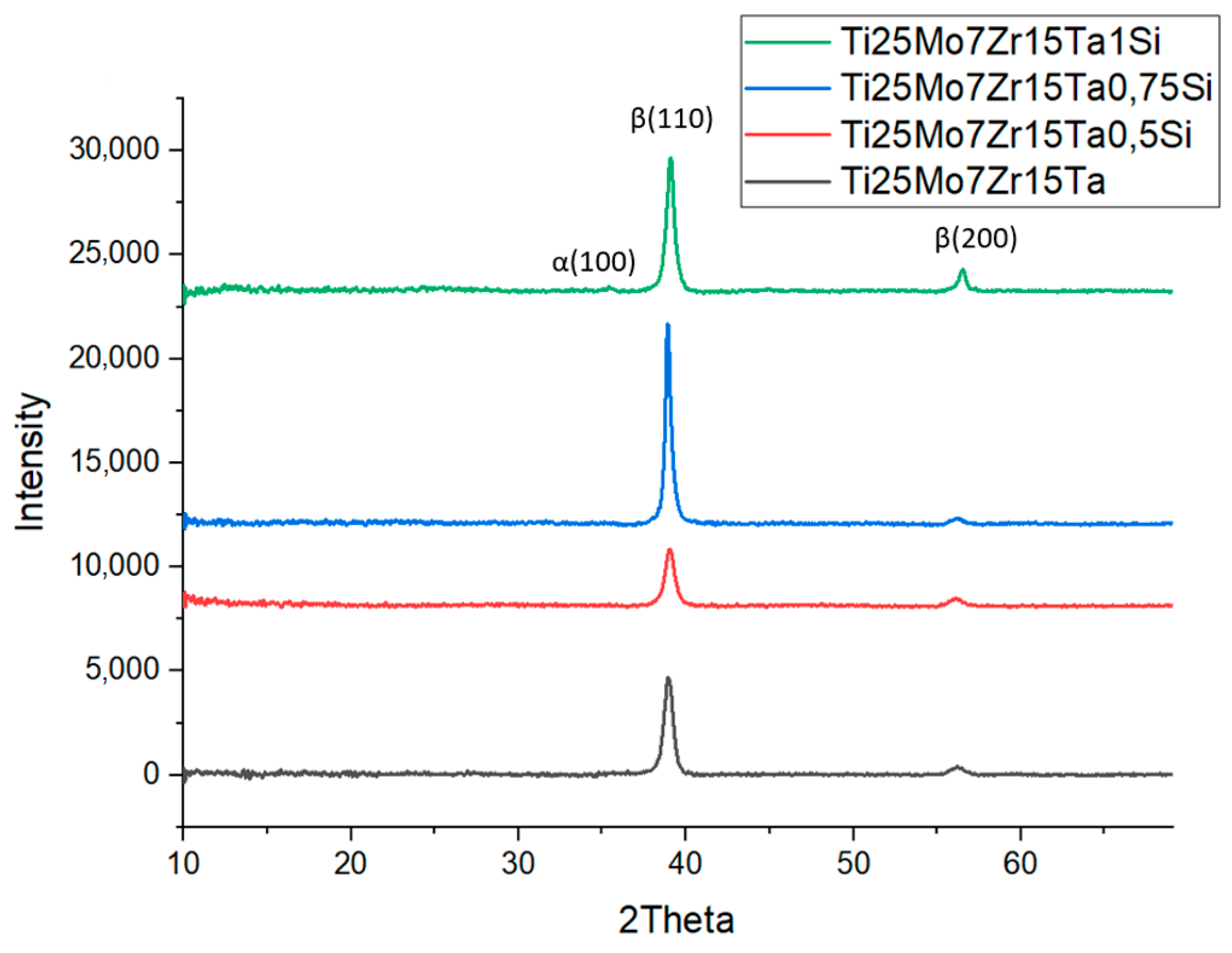
3.1. Structural Analysis
3.2. Mechanical Properties
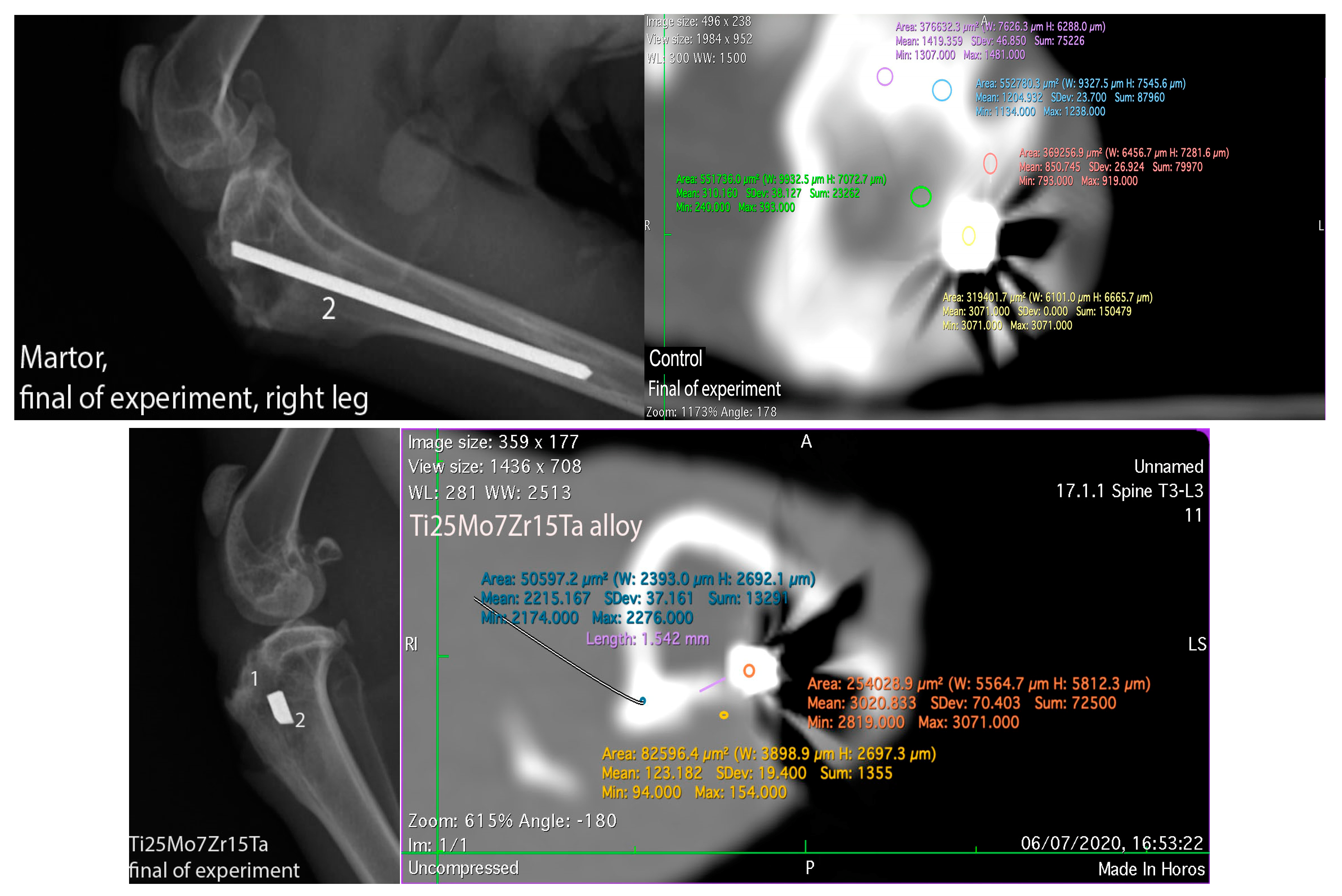
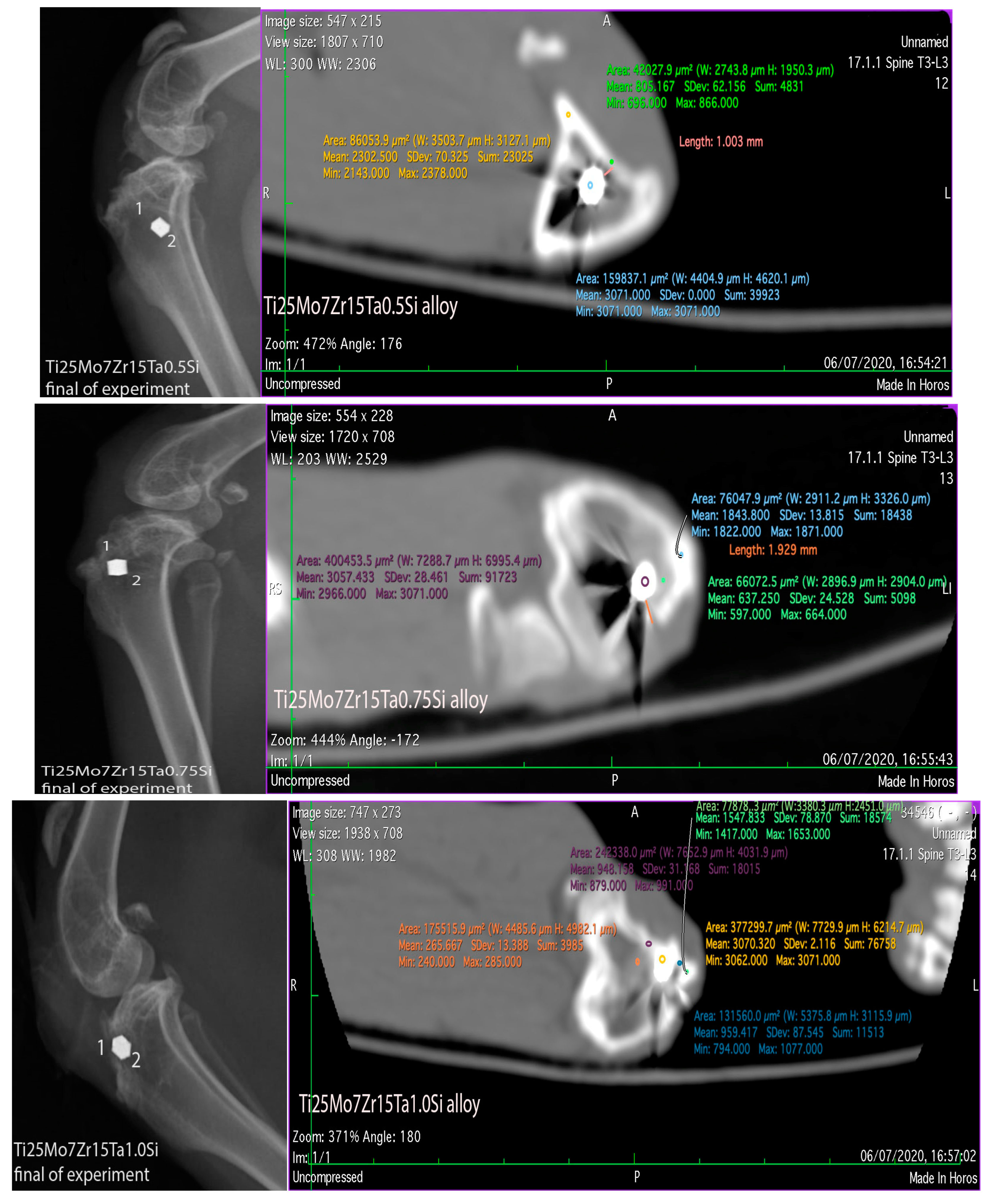
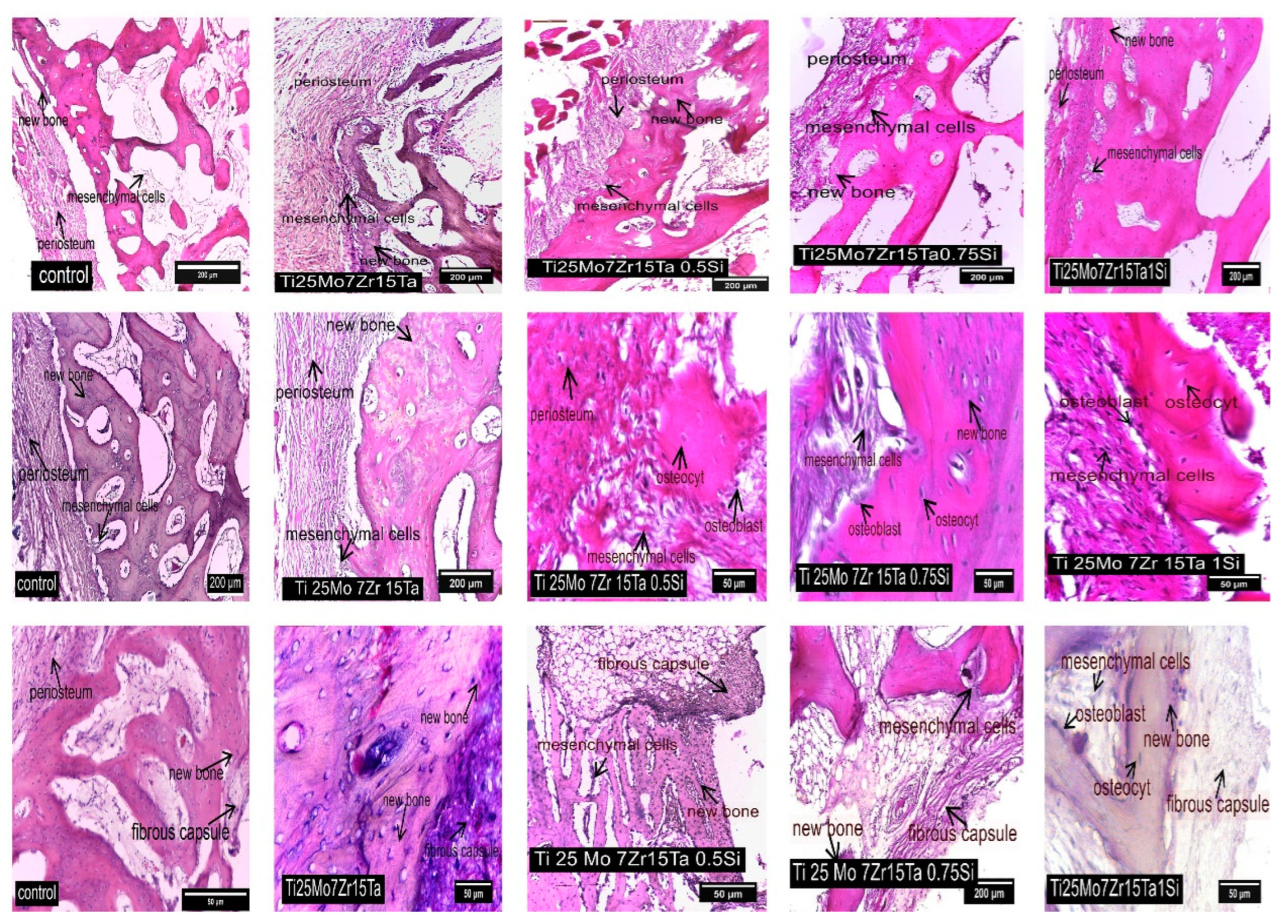
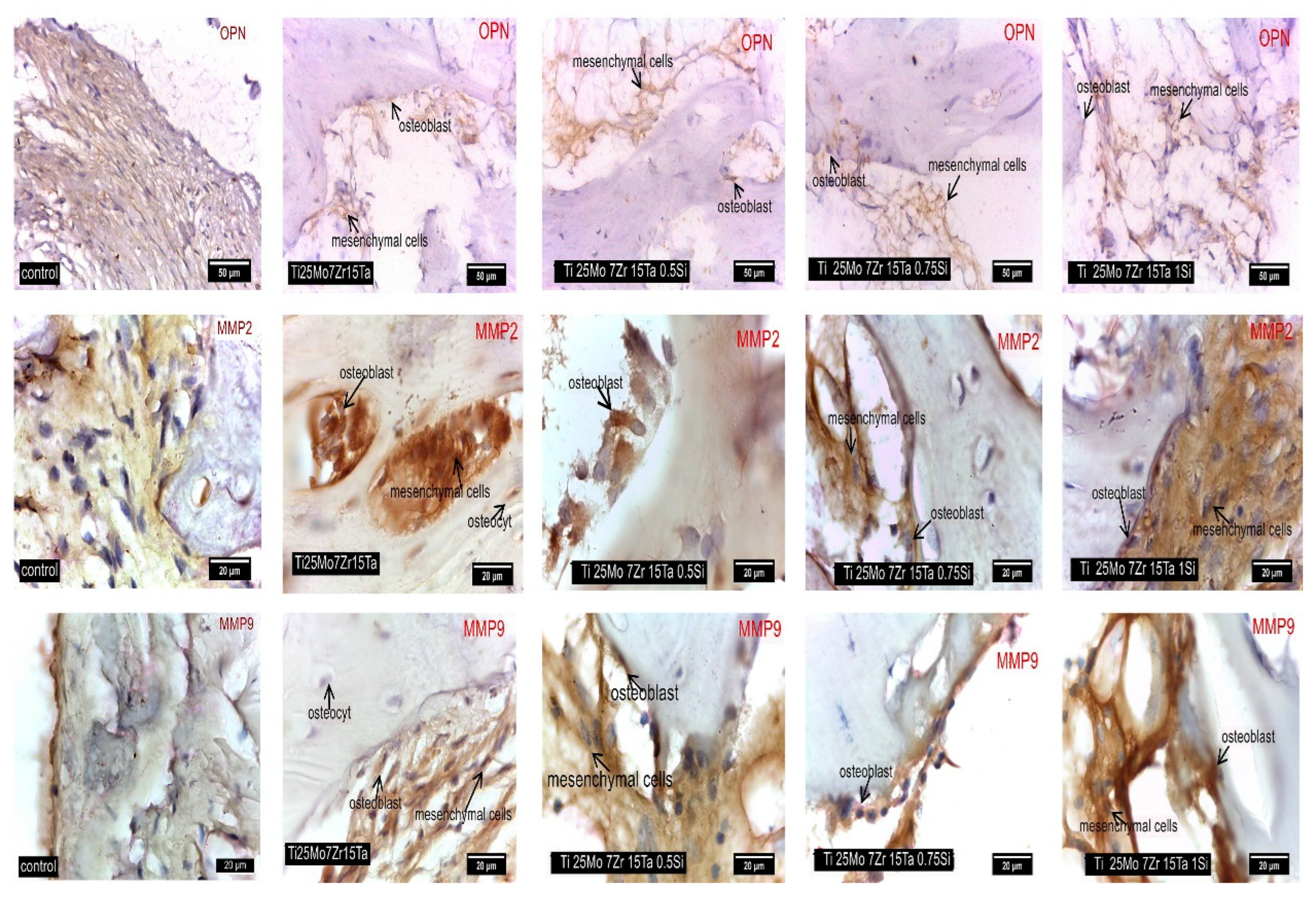
3.3. Interpretation of Results, Radiographs and Micrographs
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chen, Q.; Thouas, G.A. Metallic implant biomaterials. Mater. Sci. Eng. R Rep. 2015, 87, 1–57. [Google Scholar] [CrossRef]
- Savin, A.; Vizureanu, P.; Prevorovsky, Z.; Chlada, M.; Krofta, J.; Baltatu, M.S.; Istrate, B.; Steigmann, R. Noninvasive Evaluation of Special Alloys for Prostheses Using Complementary Methods. IOP Conf. Ser. Mater. Sci. Eng. 2018, 374, 012030. [Google Scholar] [CrossRef]
- Antoniac, I.; Miculescu, M.; Mănescu (Păltânea), V.; Stere, A.; Quan, P.H.; Păltânea, G.; Robu, A.; Earar, K. Magnesium-Based Alloys Used in Orthopedic Surgery. Materials 2022, 15, 1148. [Google Scholar] [CrossRef] [PubMed]
- Istrate, B.; Munteanu, C.; Cimpoesu, R.; Cimpoesu, N.; Popescu, O.D.; Vlad, M.D. Microstructural, Electrochemical and In Vitro Analysis of Mg-0.5Ca-xGd Biodegradable Alloys. Appl. Sci. 2021, 11, 981. [Google Scholar] [CrossRef]
- Geetha, M.; Singh, A.K.; Asokamani, R.; Gogia, A.K. Ti based biomaterials, the ultimate choice for orthopaedic implants—A review. Prog. Mater. Sci. 2009, 54, 397–425. [Google Scholar] [CrossRef]
- Sittig, C.; Textor, M.; Spencer, N.D.; Wieland, M.; Vallotton, P.H. Surface characterization of implant materials c.p. Ti, Ti-6Al-7Nb and Ti-6Al-4V with different pretreatments. J. Mater. Sci. Mater. Med. 1999, 10, 35–46. [Google Scholar] [CrossRef]
- Fellah, M.; Labaïz, M.; Assala, O.; Dekhil, L.; Taleb, A.; Rezag, H.; Iost, A. Tribological behavior of Ti-6Al-4V and Ti-6Al-7Nb Alloys for Total Hip Prosthesis. Adv. Tribol. 2014, 2014, 451387. [Google Scholar] [CrossRef]
- Khan, M.A.; Williams, R.L.; Williams, D.F. The corrosion behaviour of Ti-6Al-4V, Ti-6Al-7Nb and Ti-13Nb-13Zr in protein solutions. Biomaterials 1999, 20, 631–637. [Google Scholar] [CrossRef]
- Manivasagam, G.; Dhinasekaran, D.; Rajamanickam, A. Biomedical implants: Corrosion and its prevention—A review. Recent Pat. Corros. Sci. 2010, 2, 40–54. [Google Scholar] [CrossRef]
- Xue, P.; Li, Y.; Li, K.; Zhang, D.; Zhou, C. Superelasticity, corrosion resistance and biocompatibility of the Ti-19Zr-10Nb-1Fe alloy. Mater. Sci. Eng. C Mater. Biol. Appl. 2015, 50, 179–186. [Google Scholar] [CrossRef]
- Cordeiro, J.M.; Barão, V.A.R. Is there scientific evidence favoring the substitution of commercially pure titanium with titanium alloys for the manufacture of dental implants? Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 71, 1201–1215. [Google Scholar] [CrossRef]
- Ou, P.; Liu, J.; Hao, C.; He, R.; Chang, L.; Ruan, J. Cytocompatibility, stability and osteogenic activity of powder metallurgy Ta-xZr alloys as dental implant materials. J. Biomater. Appl. 2020, 35, 790–798. [Google Scholar] [CrossRef] [PubMed]
- Bobyn, J.D.; Stackpool, G.J.; Hacking, S.A.; Tanzer, M.; Krygier, J.J. Characteristics of bone ingrowth and interface mechanics of a new porous tantalum biomaterial. J. Bone Jt. Surg. Br. Vol. 1999, 81, 907–914. [Google Scholar] [CrossRef]
- do Prado, R.F.; Esteves, G.C.; Santos, E.L.D.S.; Bueno, D.A.G.; Cairo, C.A.A.; Vasconcellos, L.G.O.D.; Sagnori, R.S.; Tessarin, F.B.P.; Oliveira, F.E.; Oliveira, L.; et al. In vitro and in vivo biological performance of porous Ti alloys prepared by powder metallurgy. PLoS ONE 2018, 13, e0196169. [Google Scholar] [CrossRef]
- Liu, X.; Song, X.; Zhang, P.; Zhu, Z.; Xu, X. Effects of nano tantalum implants on inducing osteoblast proliferation and differentiation. Exp. Ther. Med. 2016, 12, 3541–3544. [Google Scholar] [CrossRef]
- Baltatu, M.S.; Spataru, M.C.; Verestiuc, L.; Balan, V.; Solcan, C.; Sandu, A.V.; Geanta, V.; Voiculescu, I.; Vizureanu, P. Design, Synthesis, and Preliminary Evaluation for Ti-Mo-Zr-Ta-Si Alloys for Potential Implant Applications. Materials 2021, 14, 6806. [Google Scholar] [CrossRef] [PubMed]
- Eisenbarth, E.; Velten, D.; Müller, M.; Thull, R.; Breme, J. Biocompatibility of beta-stabilizing elements of titanium alloys. Biomaterials 2004, 25, 5705–5713. [Google Scholar] [CrossRef] [PubMed]
- Hoemann, C.D.; El-Gabalawy, H.; McKee, M.D. In vitro osteogenesis assays: Influence of the primary cell source on alkaline phosphatase activity and mineralization. Pathol. Biol. 2009, 57, 318–323. [Google Scholar] [CrossRef]
- Guo, Y.; Chen, D.; Cheng, M.; Lu, W.; Wang, L.; Zhang, X. The bone tissue compatibility of a new Ti35Nb2Ta3Zr alloy with a low Young’s modulus. Int. J. Mol. Med. 2013, 31, 689–697. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, H.; Li, Q.; Ye, L.; Gan, H.; Liu, Y.; Wang, H.; Wang, Z. Biocompatibility and osteogenic properties of porous tantalum. Exp. Ther. Med. 2015, 9, 780–786. [Google Scholar] [CrossRef]
- Al Deeb, M.; Aldosari, A.A.; Anil, S. Osseointegration of Tantalum Trabecular Metal in Titanium Dental Implants: Histological and Micro-CT Study. J. Funct. Biomater. 2023, 14, 355. [Google Scholar] [CrossRef]
- Jugdaohsingh, R.; Watson, A.I.E.; Pedro, L.D.; Powel, J.J. The decrease in silicon concentration of the connective tissues with age in rats is a marker of connective tissue turnover. Bone 2015, 75, 40–48. [Google Scholar] [CrossRef]
- Spataru, M.-C.; Cojocaru, F.D.; Sandu, A.V.; Solcan, C.; Duceac, I.A.; Baltatu, M.S.; Voiculescu, I.; Geanta, V.; Vizureanu, P. Assessment of the Effects of Si Addition to a New TiMoZrTa System. Materials 2021, 14, 7610. [Google Scholar] [CrossRef]
- Qi, M.; Ma, Y.; Yang, J.; Jia, Y.; Weng, H.; Huang, S.; Zhang, R.; Qiu, J.; Lei, J.; Yang, R. Microtexture evolution effected by Mo content in α + β titanium alloys. Mater. Charact. 2022, 188, 111884. [Google Scholar] [CrossRef]
- Bagmutov, V.P.; Vodopyanov, V.I.; Zakharov, I.N.; Ivannikov, A.Y.; Bogdanov, A.I.; Romanenko, M.D.; Barinov, V.V. Features of Changes in the Surface Structure and Phase Composition of the of alpha plus beta Titanium Alloy after Electromechanical and Thermal Treatment. Metals 2022, 12, 1535. [Google Scholar] [CrossRef]
- Ho, W.F.; Ju, C.P.; Lin, J.H.C. Structure and properties of cast binary Ti-Mo alloys. Biomaterials 1999, 20, 2115–2122. [Google Scholar] [CrossRef]
- Verma, R.P. Titanium based biomaterial for bone implants: A mini review. Mater. Today Proc. 2020, 26, 3148–3151. [Google Scholar] [CrossRef]
- Popa, M.V.; Vasilescu, E.; Drob, P.; Vasilescu, C.; Drob, S.I.; Mareci, D.; Mirza Rosca, J.C. Corrosion Resistance Improvement of Titanium Base Alloys. Quim. Nova 2010, 33, 1892–1896. [Google Scholar] [CrossRef]
- Quinn, J.; McFadden, R.; Chan, C.W.; Carson, L. Titanium for Orthopedic Applications: An Overview of Surface Modification to Improve Biocompatibility and Prevent Bacterial Biofilm Formation. iScience 2020, 23, 101745. [Google Scholar] [CrossRef]
- Moshokoa, N.; Raganya, L.; Obadele, B.A.; Machaka, R.; Makhatha, M.E. Microstructural and mechanical properties of Ti-Mo alloys designed by the cluster plus glue atom model for biomedical application. Int. J. Adv. Manuf. Technol. 2020, 111, 1237–1246. [Google Scholar] [CrossRef]
- Niinomi, M. Mechanical properties of biomedical titanium alloys. Mater. Sci. Eng. A 1998, 243, 231–236. [Google Scholar] [CrossRef]
- Baltatu, M.S.; Sandu, A.V.; Nabialek, M.; Vizureanu, P.; Ciobanu, G. Biomimetic Deposition of Hydroxyapatite Layer on Titanium Alloys. Micromachines 2021, 12, 1447. [Google Scholar] [CrossRef]
- Baltatu, M.S.; Vizureanu, P.; Goanţă, V.; Tugui, C.A.; Voiculescu, I. Mechanical tests for Ti-based alloys as new medical materials. In Proceedings of the IOP Conference Series: Materials Science and Engineering, Iasi, Romania, 16–17 May 2019; p. 012029. [Google Scholar]
- Baltatu, M.S.; Chiriac-Moruzzi, C.; Vizureanu, P.; Tóth, L.; Novák, J. Effect of Heat Treatment on Some Titanium Alloys Used as Biomaterials. Appl. Sci. 2022, 12, 11241. [Google Scholar] [CrossRef]
- Ashtiani, R.E.; Alam, M.; Tavakolizadeh, S.; Abbasi, K. The Role of Biomaterials and Biocompatible Materials in ImplantSupported Dental Prosthesis. Evid.-Based Complement. Altern. Med. 2021, 2021, 3349433. [Google Scholar]
- Zhou, Y.-L.; Luo, D.-M. Microstructures and mechanical properties of Ti–Mo alloys cold-rolled and heat treated. Mater. Charact. 2011, 62, 931–937. [Google Scholar] [CrossRef]
- Zhao, E.; Sun, S.; Zhang, Y. Recent advances in silicon containing high temperature titanium alloys. J. Mater. Res. Technol. 2021, 14, 3029–3042. [Google Scholar] [CrossRef]
- Istrate, B.; Munteanu, C.; Antoniac, I.-V.; Lupescu, Ș.-C. Current Research Studies of Mg–Ca–Zn Biodegradable Alloys Used as Orthopedic Implants—Review. Crystals 2022, 12, 1468. [Google Scholar] [CrossRef]
- Bǎltatu, I.; Vizureanu, P.; Ciolacu, F.; Achiţei, D.C.; Bǎltatu, M.S.; Vlad, D. In vitro study for new Ti-Mo-Zr-Ta alloys for medical use. IOP Conf. Ser. Mater. Sci. Eng. 2019, 572, 012030. [Google Scholar] [CrossRef]
- Baltatu, M.S.; Vizureanu, P.; Balan, T.; Lohan, N.M.; Tugui, C.A. Preliminary tests for Ti-Mo-Zr-Ta alloys as potential biomaterials. IOP Conf. Ser. Mater. Sci. Eng. 2018, 374, 012023. [Google Scholar] [CrossRef]
- Melgaard, D.K.; Williamson, R.L.; Beaman, J.J. Controlling remelting processes for superalloys and aerospace Ti alloys. JOM 1998, 50, 13–17. [Google Scholar] [CrossRef]
- Information, on. Available online: https://lp.filab.fr/iso-10993-18-en (accessed on 15 September 2023).
- Directive 2010/63/EU of the European Parliament and of the Council of 22 September 2010 on the protection of animals used for scientific purposes. Off. J. Eur. Union 2010, 276, 33–79.
- Law No 43/2014 on the Protection of Animals Used for Scientific Purposes Supplemented by Law 199/2018 and the Law 149/2019. Available online: https://aldf.org/wp-content/uploads/2020/02/2019-Animal-Protection-US-State-Laws-Rankings-Report.pdf (accessed on 15 September 2023).
- Weng, W.; Biesiekierski, A.; Li, Y.; Wen, C. Effects of selected metallic and interstitial elements on the microstructure and mechanical properties of beta titanium alloys for orthopedic applications. Materialia 2019, 6, 100323. [Google Scholar] [CrossRef]
- Saha, S.; Roy, S. Metallic Dental Implants Wear Mechanisms, Materials, and Manufacturing Processes: A Literature Review. Materials 2023, 16, 161. [Google Scholar] [CrossRef]
- Razavykia, A.; Brusa, E.; Delprete, C.; Yavari, R. An overview of additive manufacturing technologies—A review to technical synthesis in numerical study of selective laser melting. Materials 2020, 13, 3895. [Google Scholar] [CrossRef]
- Zhong, C.; Liu, J.; Zhao, T.; Schopphoven, T.; Fu, J.; Gasser, A.; Schleifenbaum, J.H. Laser Metal Deposition of Ti6Al4V—A Brief Review. Appl. Sci. 2020, 10, 764. [Google Scholar] [CrossRef]
- Arisan, V.; Karabuda, Z.C.; Avsever, H.; Özdemir, T. Conventional multi-slice computed tomography (CT) and conebeam CT (CBCT) for computer-assisted implant placement. Part I: Relationship of radiographic gray density and implant stability. Clin. Implant. Dent. Relat. Res. 2013, 15, 893–906. [Google Scholar] [CrossRef]
- Schepull, T.; Aspenberg, P. Healing of human Achilles tendon ruptures: Radiodensity reflects mechanical properties. Knee Surgery Sports Traumatol. Arthrosc. 2013, 23, 884–889. [Google Scholar] [CrossRef]
- Lev, M.H.; Gonzalez, R.G. CT Angiography and CT Perfusion Imaging. In Brain Mapping: The Methods, 2nd ed.; Academic Press: San Diego, CA, USA, 2002. [Google Scholar]
- Bruder, S.P.; Kraus, K.H.; Goldberg, V.M.; Kadiyala, S. The effect of implants loaded with autologous mesenchymal stem cells on the healing of canine segmental bone defects. J. Bone. Jt. Surg. Am. 1998, 80, 985–996. [Google Scholar] [CrossRef]
- Quinn, R.K.; Armstrong, N.R. Electrochemical and surface analytical characterization of titanium and titanium hydride thin-film electrode oxidation. J. Electrochem. Soc. 1978, 125, 1790–1796. [Google Scholar] [CrossRef]
- Sidambe, A.T. Biocompatibility of Advanced Manufactured Titanium Implants—A Review. Materials 2014, 7, 8168–8188. [Google Scholar] [CrossRef]
- Spataru, M.C.; Butnaru, M.; Sandu, A.V.; Vulpe, V.; Vlad, M.D.; Baltatu, M.S.; Geanta, V.; Voiculescu, I.; Vizureanu, P.; Solcan, C. In-depth assessment of new Ti-based biocompatible materials. Mater. Chem. Phys. 2020, 258, 123959. [Google Scholar] [CrossRef]
- Verestiuc, L.; Spataru, M.-C.; Baltatu, M.S.; Butnaru, M.; Solcan, C.; Sandu, A.V.; Voiculescu, I.; Geanta, V.; Vizureanu, P. New Ti–Mo–Si materials for bone prosthesis applications. J. Mech. Behav. Biomed. Mater. 2020, 113, 104198. [Google Scholar] [CrossRef]
- Knight, M.N.; Hankenson, K.D.; Lin, T.; Kohno, Y.; Huang, J.-F.; Romero-Lopez, M.; Maruyama, M.; Ueno, M.; Pajarinen, J.; Nathan, K.; et al. Mesenchymal Stem Cells in Bone Regeneration. Adv. Wound Care 2013, 2, 306–316. [Google Scholar] [CrossRef]
- Oryan, A.; Kamali, A.; Moshiri, A.; Eslaminejad, M.B. Role of Mesenchymal Stem Cells in Bone Regenerative Medicine: What Is the Evidence? Cells Tissues Organs 2017, 204, 59–83. [Google Scholar] [CrossRef]
- Sodek, J.; Chen, J.; Nagata, T.; Kasugai, S.; Todescan, R., Jr.; Li, I.W.; Kim, R.H. Regulation of osteopontin expression in osteoblasts. Ann. N. Y. Acad. Sci. 1995, 760, 223–241. [Google Scholar] [CrossRef] [PubMed]
- Giachelli, C.M.; Schwartz, S.M.; Liaw, L. Molecular and cellular biology of osteopontin: Potential role in cardiovascular disease. Trends Cardiovasc. Med. 1995, 5, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Denhardt, D.T.; Noda, M. Osteopontin expression and function: Role in bone remodeling. J. Cell. Biochem. Suppl. 1998, 72, 92–102. [Google Scholar] [CrossRef]
- Schack, L.; Stapulionis, R.; Christensen, B.; Kofod-Olsen, E.; Sørensen, U.B.S.; Vorup-Jensen, T.; Sørensen, E.S.; Hollsberg, P. Osteopontin Enhances Phagocytosis through a Novel Osteopontin Receptor, the αXβ2 Integrin. J. Immunol. 2009, 182, 6943–6950. [Google Scholar] [CrossRef]
- Araújo, M.G.; Liljenberg, B.; Lindhe, J. Dynamics of Bio-Oss® Collagen incorporation in fresh extraction wounds: An experimental study in the dog. Clin. Oral Implant. Res. 2009, 21, 55–64. [Google Scholar] [CrossRef]
- Rullo, O.J.; Woo, J.M.; Parsa, M.F.; Hoftman, A.D.; Maranian, P.; Elashoff, D.A.; Niewold, T.B.; Grossman, J.M.; Hahn, B.H.; McMahon, M.; et al. Plasma levels of osteopontin identify patients at risk for organ damage in systemic lupus erythematosus. Thromb. Haemost. 2013, 15, R18. [Google Scholar] [CrossRef]
- Castellano, G.; Malaponte, G.; Mazzarino, M.C.; Figini, M.; Marchese, F.; Gangemi, P.; Travali, S.; Stivala, F.; Canevari, S.; Libra, M. Activation of the Osteopontin/Matrix Metalloproteinase-9 Pathway Correlates with Prostate Cancer Progression. Clin. Cancer Res. 2008, 14, 7470–7480. [Google Scholar] [CrossRef] [PubMed]
- Fiorellini, J.; Glindmann, S.; Salcedo, J.; Weber, H.-P.; Park, C.-J.; Sarmiento, H. The Effect of Osteopontin and an Osteopontin-Derived Synthetic Peptide Coating on Osseointegration of Implants in a Canine Model. Int. J. Periodontics Restor. Dent. 2016, 36, e88–e94. [Google Scholar] [CrossRef] [PubMed]
- Lieu, S.; Hansen, E.; Dedini, R.; Behonick, D.; Werb, Z.; Miclau, T.; Marcucio, R.; Colnot, C. Impaired remodeling phase of fracture repair in the absence of matrix metalloproteinase-2. Dis. Model. Mech. 2011, 4, 203–211. [Google Scholar] [CrossRef] [PubMed]
- Pivodova, V.; Frankova, J.; Ulrichova, J. Osteoblast and gingival fibroblast markers in dental implant studies. Biomed. Pap. Med. Fac. Palacky Univ. Olomouc 2011, 155, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Colnot, C.; Thompson, Z.; Miclau, T.; Werb, Z.; Helms, J.A. Altered fracture repair in the absence of MMP9. Development 2003, 130, 4123–4133. [Google Scholar] [CrossRef]
- Antoniac, I.; Adam, R.; Biță, A.; Miculescu, M.; Trante, O.; Petrescu, I.M.; Pogărășteanu, M. Comparative Assessment of In Vitro and In Vivo Biodegradation of Mg-1Ca Magnesium Alloys for Orthopedic Applications. Materials 2020, 14, 84. [Google Scholar] [CrossRef] [PubMed]


| Sample | Ti25Mo7Zr15Ta | Ti25Mo7Zr15Ta0.5Si | Ti25Mo7Zr15Ta0.75Si | Ti25Mo7Zr15Ta1Si | |
|---|---|---|---|---|---|
| Average chemical composition | Ti (wt.%) | 53.40 ± 0.60 | 52.60 ± 0.8 | 52.49 ± 0.70 | 54.00 ± 0.60 |
| Mo (wt.%) | 24.90 ± 0.30 | 24.70 ± 0.20 | 24.80 ± 0.50 | 23.60 ± 0.40 | |
| Zr (wt.%) | 6.90 ± 0.10 | 7.10 ± 0.20 | 6.90 ± 0.10 | 6.80 ± 0.30 | |
| Ta (wt.%) | 14.80 ± 0.40 | 15.20 ± 0.30 | 15.10 ± 0.20 | 14.70 ± 0.50 | |
| Si (wt.%) | - | 0.40 ± 0.01 | 0.71 ± 0.05 | 0.90 ± 0.09 | |
| Crystal System | a (Å) | b (Å) | c (Å) | Alpha (°) | Beta (°) | Gamma (°) | Calculated Density (g/cm3) | Volume of the Cell (106 pm3) | Z | RIR |
|---|---|---|---|---|---|---|---|---|---|---|
| Cubic | 3.2830 | 3.2830 | 3.2830 | 90.00 | 90.00 | 90.00 | 4.49 | 35.38 | 2.00 | 9.61 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baltatu, M.S.; Vizureanu, P.; Sandu, A.V.; Solcan, C.; Hritcu, L.D.; Spataru, M.C. Research Progress of Titanium-Based Alloys for Medical Devices. Biomedicines 2023, 11, 2997. https://doi.org/10.3390/biomedicines11112997
Baltatu MS, Vizureanu P, Sandu AV, Solcan C, Hritcu LD, Spataru MC. Research Progress of Titanium-Based Alloys for Medical Devices. Biomedicines. 2023; 11(11):2997. https://doi.org/10.3390/biomedicines11112997
Chicago/Turabian StyleBaltatu, Madalina Simona, Petrica Vizureanu, Andrei Victor Sandu, Carmen Solcan, Luminița Diana Hritcu, and Mihaela Claudia Spataru. 2023. "Research Progress of Titanium-Based Alloys for Medical Devices" Biomedicines 11, no. 11: 2997. https://doi.org/10.3390/biomedicines11112997









