Epoxy- versus Glutaraldehyde-Treated Bovine Jugular Vein Conduit for Pulmonary Valve Replacement: A Comparison of Morphological Changes in a Pig Model
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Preparation of the Conduit
- (1)
- For 14 days at room temperature in 5% buffered (0.05 M phosphate buffer, pH 7.4) DE solution, changed once, on day 2;
- (2)
- For 14 days at room temperature in 0.625% buffered (0.05 M phosphate buffer, pH 7.4) GA solution, with conservative replacement on days 2, 4, and 8.
2.3. Experimental Conduit Implantation
2.3.1. Anesthesia and Mechanical Ventilation
2.3.2. Cardiopulmonary Bypass
2.3.3. Operative Techniques
2.3.4. Postoperative Management
2.4. Macroscopic and Microscopic Study of the Conduits
2.5. Scanning Electronic Microscopy (SEM)
2.6. Statistical Analyses
3. Results
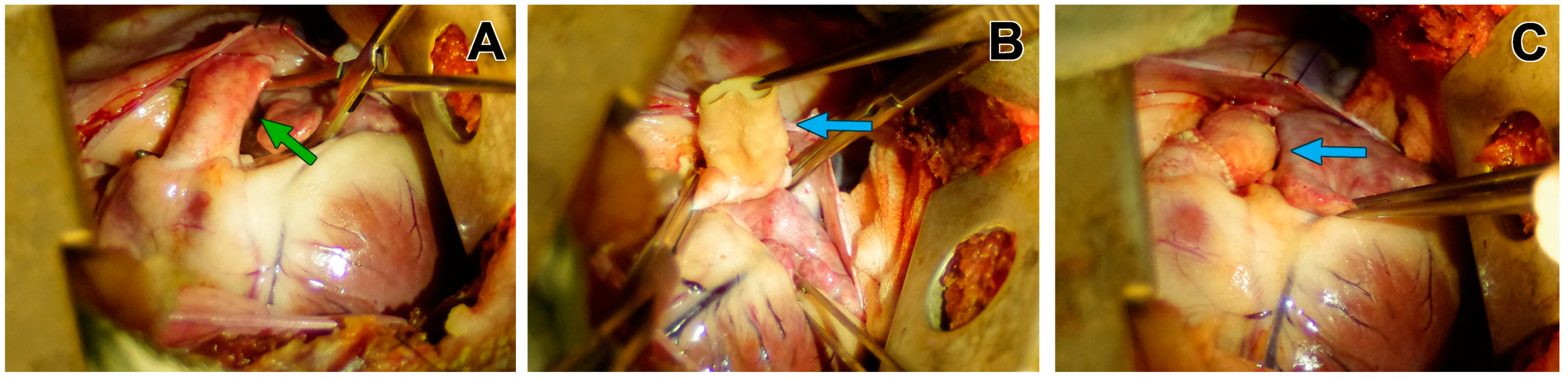
3.1. Macroscopic Findings
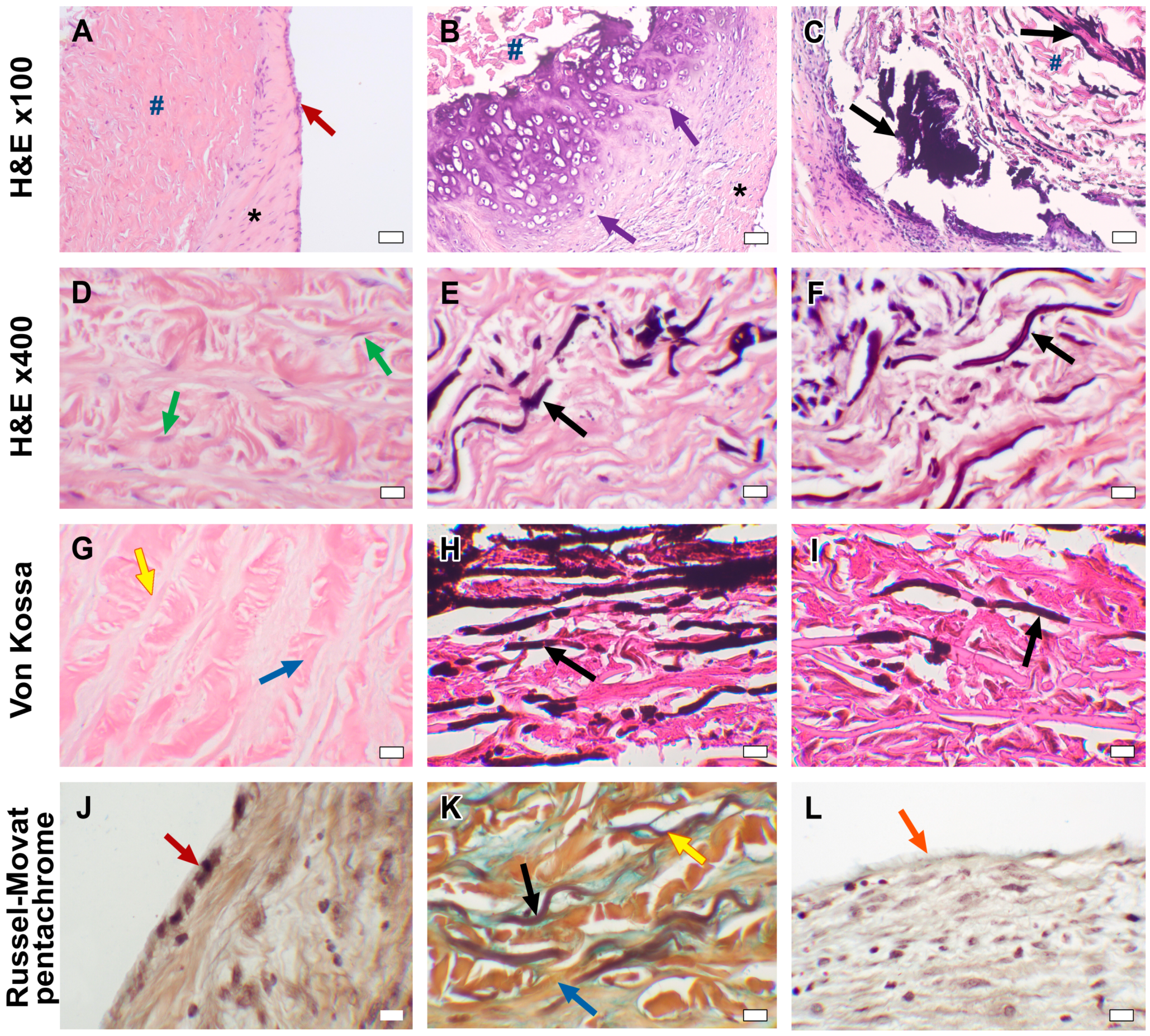
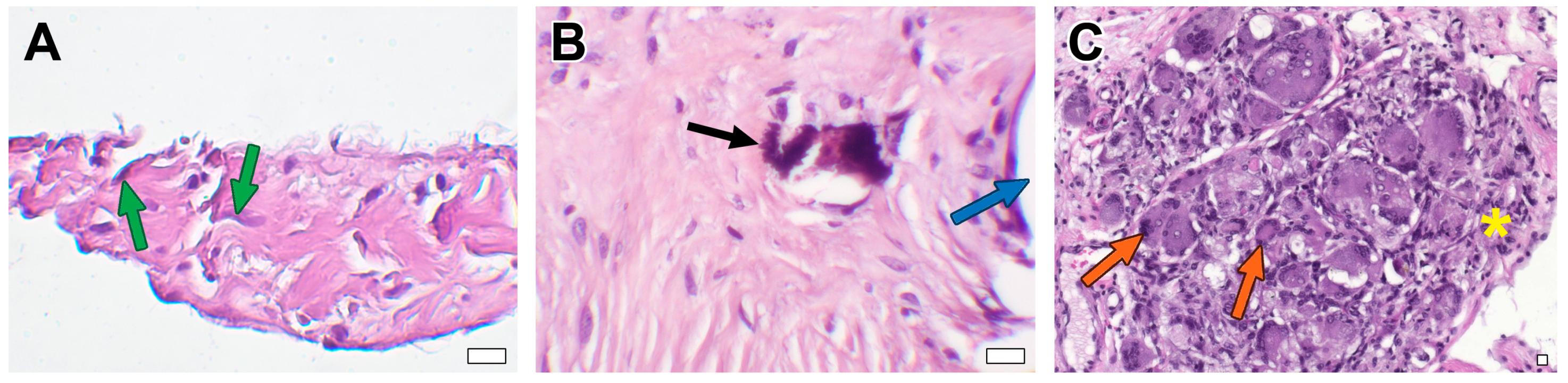
3.2. Microscopic Examination
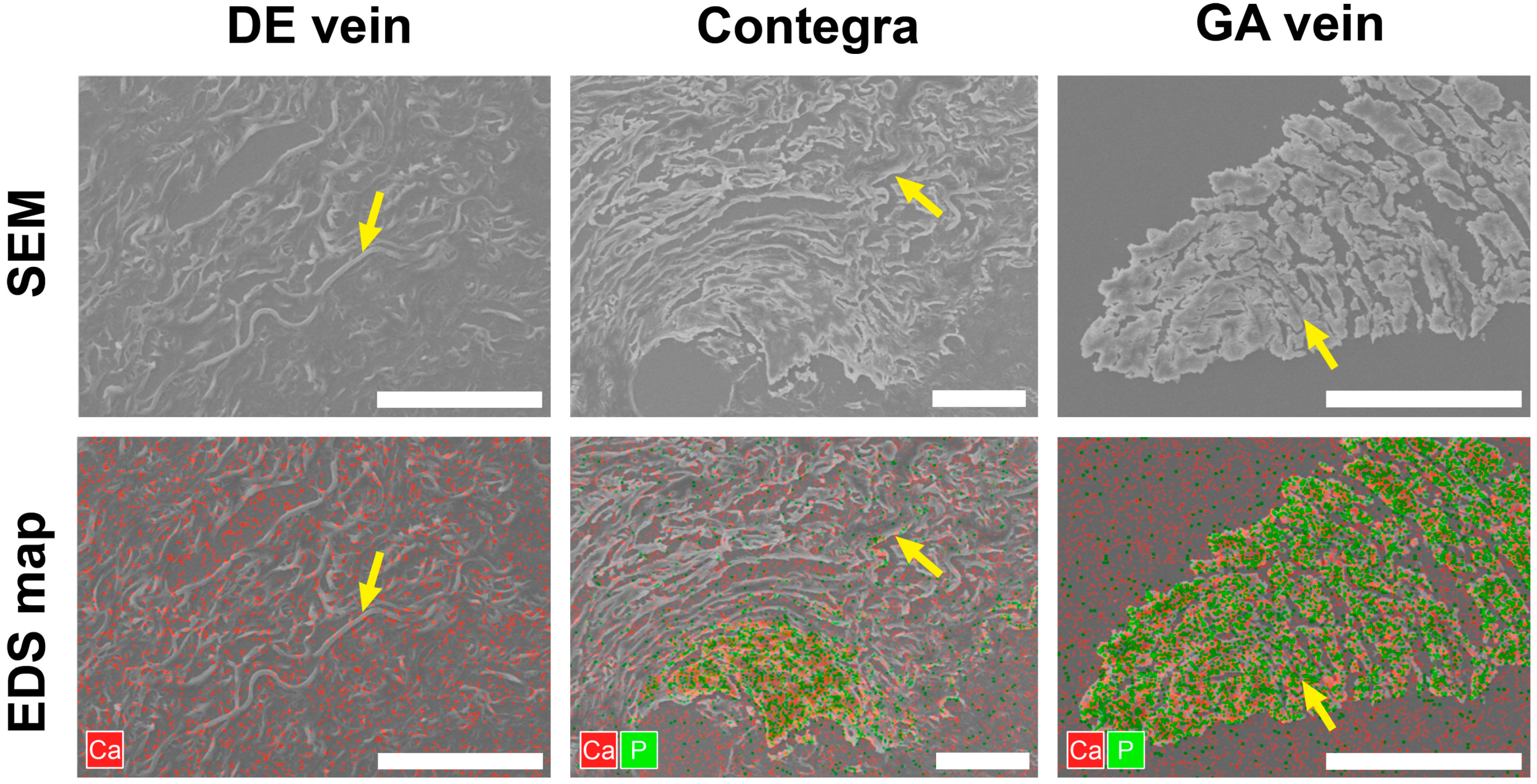
3.3. Scanning Electron Microscopy
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yong, M.S.; Yim, D.; d’Udekem, Y.; Brizard, C.P.; Robertson, T.; Galati, J.C.; Konstantinov, I.E. Medium-term outcomes of bovine jugular vein graft and homograft conduits in children. ANZ J. Surg. 2015, 85, 381–385. [Google Scholar] [CrossRef] [PubMed]
- Sandica, E.; Boethig, D.; Blanz, U.; Goerg, R.; Haas, N.A.; Laser, K.T.; Kececioglu, D.; Bertram, H.; Sarikouch, S.; Westhoff-Bleck, M.; et al. Bovine Jugular Veins versus Homografts in the Pulmonary Position: An Analysis across Two Centers and 711 Patients-Conventional Comparisons and Time Status Graphs as a New Approach. Thorac. Cardiovasc. Surg. 2016, 64, 25–35. [Google Scholar] [CrossRef]
- Falchetti, A.; Demanet, H.; Dessy, H.; Melot, C.; Pierrakos, C.; Wauthy, P. Contegra versus pulmonary homograft for right ventricular outflow tract reconstruction in newborns. Cardiol. Young 2019, 29, 505–510. [Google Scholar] [CrossRef]
- Urso, S.; Rega, F.; Meuris, B.; Gewillig, M.; Eyskens, B.; Daenen, W.; Heying, R.; Meyns, B. The Contegra conduit in the right ventricular outflow tract is an independent risk factor for graft replacement. Eur. J. Cardiothorac. Surg. 2011, 40, 603–609. [Google Scholar] [CrossRef]
- Patel, P.M.; Tan, C.; Srivastava, N.; Herrmann, J.L.; Rodefeld, M.D.; Turrentine, M.W.; Brown, J.W. Bovine Jugular Vein Conduit: A Mid- to Long-Term Institutional Review. World J. Pediatr. Congenit. Heart Surg. 2018, 9, 489–495. [Google Scholar] [CrossRef] [PubMed]
- Meyns, B.; Van Garsse, L.; Boshoff, D.; Eyskens, B.; Mertens, L.; Gewillig, M.; Fieuws, S.; Verbeken, E.; Daenen, W. The Contegra conduit in the right ventricular outflow tract induces supravalvular stenosis. J. Thorac. Cardiovasc. Surg. 2004, 128, 834–840. [Google Scholar] [CrossRef]
- Gist, K.M.; Mitchell, M.B.; Jaggers, J.; Campbell, D.N.; Yu, J.A.; Landeck, B.F., II. Assessment of the relationship between Contegra conduit size and early valvar insufficiency. Ann. Thorac. Surg. 2012, 93, 856–861. [Google Scholar] [CrossRef] [PubMed]
- Mery, C.M.; Guzmán-Pruneda, F.A.; De León, L.E.; Zhang, W.; Terwelp, M.D.; Bocchini, C.E.; Adachi, I.; Heinle, J.S.; McKenzie, E.D.; Fraser, C.D., Jr. Risk factors for development of endocarditis and reintervention in patients undergoing right ventricle to pulmonary artery valved conduit placement. J. Thorac. Cardiovasc. Surg. 2016, 151, 432–439. [Google Scholar] [CrossRef]
- Gröning, M.; Tahri, N.B.; Søndergaard, L.; Helvind, M.; Ersbøll, M.K.; Ørbæk Andersen, H. Infective endocarditis in right ventricular outflow tract conduits: A register-based comparison of homografts, Contegra grafts and Melody transcatheter valves. Eur. J. Cardiothorac. Surg. 2019, 56, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Imamura, E.; Sawatani, O.; Koyanagi, H.; Noishiki, Y.; Miyata, T. Epoxy compounds as a new cross-linking agent for porcine aortic leaflets: Subcutaneous implant studies in rats. J. Card. Surg. 1989, 4, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Umashankar, P.R.; Mohanan, P.V.; Kumari, T.V. Glutaraldehyde treatment elicits toxic response compared to decellularization in bovine pericardium. Toxicol. Int. 2012, 19, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Grabenwöger, M.; Sider, J.; Fitzal, F.; Zelenka, C.; Windberger, U.; Grimm, M.; Moritz, A.; Böck, P.; Wolner, E. Impact of glutaraldehyde on calcification of pericardial bioprosthetic heart valve material. Ann. Thorac. Surg. 1996, 62, 772–777. [Google Scholar] [CrossRef] [PubMed]
- Eybl, E.; Griesmacher, A.; Grimm, M.; Wolner, E. Toxic effects of aldehydes released from fixed pericardium on bovine aortic endothelial cells. J. Biomed. Mater. Res. 1989, 23, 1355–1365. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Moya, M.; Melgar-Lesmes, P.; Kolandaivelu, K.; de la Torre Hernández, J.M.; Edelman, E.R.; Balcells, M. Optimizing Glutaraldehyde-Fixed Tissue Heart Valves with Chondroitin Sulfate Hydrogel for Endothelialization and Shielding against Deterioration. Biomacromolecules 2018, 19, 1234–1244. [Google Scholar] [CrossRef]
- Zhuravleva, I.Y.; Nichay, N.R.; Kulyabin, Y.Y.; Timchenko, T.P.; Korobeinikov, A.A.; Polienko, Y.F.; Shatskaya, S.S.; Kuznetsova, E.V.; Voitov, A.V.; Bogachev-Prokophiev, A.V.; et al. In search of the best xenogeneic material for a paediatric conduit: An experimental study. Interact. Cardiovasc. Thorac. Surg. 2018, 26, 738–744. [Google Scholar] [CrossRef]
- Xi, T.; Ma, J.; Tian, W.; Lei, X.; Long, S.; Xi, B. Prevention of tissue calcification on bioprosthetic heart valve by using epoxy compounds: A study of calcification tests in vitro and in vivo. J. Biomed. Mater. Res. 1992, 26, 1241–1251. [Google Scholar] [CrossRef]
- Chang, Y.; Sung, H.; Chiu, Y.; Lu, J. Assessment of an epoxy-fixed pericardial patch with or without ionically bound heparin in a canine model. Int. J. Artif. Organs 1997, 20, 332–340. [Google Scholar] [CrossRef] [PubMed]
- Zhuravleva, I.Y.; Karpova, E.V.; Dokuchaeva, A.A.; Kuznetsova, E.V.; Vladimirov, S.V.; Ksenofontov, A.L.; Nichay, N.R. Bovine jugular vein conduit: What affects its elastomechanical properties and thermostability? J. Biomed. Mater. Res. A 2022, 110, 394–408. [Google Scholar] [CrossRef] [PubMed]
- Nishi, C.; Nakajima, N.; Ikada, Y. In vitro evaluation of cytotoxicity of diepoxy compounds used for biomaterial modification. J. Biomed. Mater. Res. 1995, 29, 829–834. [Google Scholar] [CrossRef] [PubMed]
- Karaskov, A.; Sharifulin, R.; Zheleznev, S.; Demin, I.; Lenko, E.; Bogachev-Prokophiev, A. Results of the Ross procedure in adults: A single-centre experience of 741 operations. Eur. J. Cardiothorac. Surg. 2016, 49, e97–e104. [Google Scholar] [CrossRef]
- Karaskov, A.; Bogachev-Prokophiev, A.; Sharifulin, R.; Zheleznev, S.; Demin, I.; Pivkin, A.; Zhuravleva, I. Right Ventricular Outflow Tract Replacement with Xenografts in Ross Patients Older than 60 Years. Ann. Thorac. Surg. 2016, 101, 2252–2259. [Google Scholar] [CrossRef]
- Nichay, N.R.; Zhuravleva, I.Y.; Kulyabin, Y.Y.; Zubritskiy, A.V.; Voitov, A.V.; Soynov, I.A.; Gorbatykh, A.V.; Bogachev-Prokophiev, A.V.; Karaskov, A.M. Diepoxy-Versus Glutaraldehyde-Treated Xenografts: Outcomes of Right Ventricular Outflow Tract Reconstruction in Children. World J. Pediatr. Congenit. Heart Surg. 2020, 11, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Nikitin, S.V.; Knyazev, S.P.; Shatokhin, K.S. Miniature pigs of ICG as a model object for morphogenetic research. Russ. J. Genet. Appl. Res. 2014, 4, 511–522. [Google Scholar] [CrossRef]
- Zhuravleva, I.Y. Biocidal Composition for Aseptic Storage of Preserved Prosthetic Material from Tissues of Animal Origin. Patent RU2580621C1, 10 April 2016. Available online: https://patents.google.com/patent/RU2580621C1/en (accessed on 3 October 2023).
- Bondarenko, N.A.; Surovtseva, M.A.; Lykov, A.P.; Kim, I.I.; Zhuravleva, I.Y.; Poveschenko, O.V. Cytotoxicity of Xenogeneic Pericardium Preserved by Epoxy Cross-Linking Agents. Sovrem. Tekhnologii. Med. 2021, 13, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.W.; Huang, R.N.; Huang, L.L.; Tsai, C.C. In vitro evaluation of cytotoxicity of a naturally occurring cross-linking reagent for biological tissue fixation. J. Biomater. Sci. Polym. Ed. 1999, 10, 63–78. [Google Scholar] [CrossRef]
- Schoenhoff, F.S.; Loup, O.; Gahl, B.; Banz, Y.; Pavlovic, M.; Pfammatter, J.P.; Carrel, T.P.; Kadner, A. The Contegra bovine jugular vein graft versus the Shelhigh pulmonic porcine graft for reconstruction of the right ventricular outflow tract: A comparative study. J. Thorac. Cardiovasc. Surg. 2011, 141, 654–661. [Google Scholar] [CrossRef] [PubMed]
- Wiebe, D.; Megerman, J.; L’Italien, G.J.; Abbott, W.M. Glutaraldehyde release from vascular prostheses of biologic origin. Surgery 1988, 104, 26–33. [Google Scholar] [PubMed]
- Grimm, M.; Eybl, E.; Grabenwöger, M.; Griesmacher, A.; Losert, U.; Böck, P.; Müller, M.M.; Wolner, E. Biocompatibility of aldehyde-fixed bovine pericardium. An in vitro and in vivo approach toward improvement of bioprosthetic heart valves. J. Thorac. Cardiovasc. Surg. 1991, 102, 195–201. [Google Scholar] [CrossRef]
- Ichikawa, Y.; Noishiki, Y.; Kosuge, T.; Yamamoto, K.; Kondo, J.; Matsumoto, A. Use of a bovine jugular vein graft with natural valve for right ventricular outflow tract reconstruction: A one-year animal study. J. Thorac. Cardiovasc. Surg. 1997, 114, 224–233. [Google Scholar] [CrossRef]
- Noishiki, Y.; Hata, C.; Tu, R.; Shen, S.H.; Lin, D.; Sung, H.W.; Witzel, T.; Wang, E.; Thyagarajan, K.; Tomizawa, Y.; et al. Development and evaluation of a pliable biological valved conduit. Part I: Preparation, biochemical properties, and histological findings. Int. J. Artif. Organs 1993, 16, 192–198. [Google Scholar] [CrossRef]
- Patel, S.D.; Waltham, M.; Wadoodi, A.; Burnand, K.G.; Smith, A. The role of endothelial cells and their progenitors in intimal hyperplasia. Ther. Adv. Cardiovasc. Dis. 2010, 4, 129–141. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.; Liang, H.C.; Wei, H.J.; Chu, C.P.; Sung, H.W. Tissue regeneration patterns in acellular bovine pericardia implanted in a canine model as a vascular patch. J. Biomed. Mater. Res. A 2004, 69, 323–333. [Google Scholar] [CrossRef] [PubMed]
- Lemson, M.S.; Tordoir, J.H.; Daemen, M.J.; Kitslaar, P.J. Intimal hyperplasia in vascular grafts. Eur. J. Vasc. Endovasc. Surg. 2000, 19, 336–350. [Google Scholar] [CrossRef]
- Berdajs, D.; Mosbahi, S.; Vos, J.; Charbonnier, D.; Hullin, R.; von Segesser, L.K. Fluid dynamics simulation of right ventricular outflow tract oversizing. Interact. Cardiovasc. Thorac. Surg. 2015, 21, 176–182. [Google Scholar] [CrossRef] [PubMed]
- Peivandi, A.D.; Seiler, M.; Mueller, K.M.; Martens, S.; Malec, E.; Asfour, B.; Lueck, S. Elastica degeneration and intimal hyperplasia lead to Contegra® conduit failure. Eur. J. Cardiothorac. Surg. 2019, 56, 1154–1161. [Google Scholar] [CrossRef] [PubMed]
- Herijgers, P.; Ozaki, S.; Verbeken, E.; Van Lommel, A.; Meuris, B.; Lesaffre, E.; Daenen, W.; Flameng, W. Valved jugular vein segments for right ventricular outflow tract reconstruction in young sheep. J. Thorac. Cardiovasc. Surg. 2002, 124, 798–805. [Google Scholar] [CrossRef]
- Attmann, T.; Quaden, R.; Freistedt, A.; König, C.; Cremer, J.; Lutter, G. Percutaneous heart valve replacement: Histology and calcification characteristics of biological valved stents in juvenile sheep. Cardiovasc. Pathol. 2007, 16, 165–170. [Google Scholar] [CrossRef]
- Vyavahare, N.R.; Hirsch, D.; Lerner, E.; Baskin, J.Z.; Zand, R.; Schoen, F.J.; Levy, R.J. Prevention of calcification of glutaraldehyde-crosslinked porcine aortic cusps by ethanol preincubation: Mechanistic studies of protein structure and water-biomaterial relationships. J. Biomed. Mater. Res. 1998, 40, 577–585. [Google Scholar] [CrossRef]
- Scavo, V.A., Jr.; Turrentine, M.W.; Aufiero, T.X.; Sharp, T.G.; Brown, J.W. Valved bovine jugular venous conduits for right ventricular to pulmonary artery reconstruction. ASAIO J. 1999, 45, 482–487. [Google Scholar] [CrossRef]
- Sung, H.W.; Shen, S.H.; Tu, R.; Lin, D.; Hata, C.; Noishiki, Y.; Tomizawa, Y.; Quijano, R.C. Comparison of the cross-linking characteristics of porcine heart valves fixed with glutaraldehyde or epoxy compounds. ASAIO J. 1993, 39, M532–M536. [Google Scholar]
- Köstering, H.; Mast, W.P.; Kaethner, T.; Nebendahl, K.; Holtz, W.H. Blood coagulation studies in domestic pigs (Hanover breed) and minipigs (Goettingen breed). Lab. Anim. 1983, 17, 346–349. [Google Scholar] [CrossRef] [PubMed]
- Sondeen, J.L.; de Guzman, R.; Amy Polykratis, I.; Dale Prince, M.; Hernandez, O.; Cap, A.P.; Dubick, M.A. Comparison between human and porcine thromboelastograph parameters in response to ex-vivo changes to platelets, plasma, and red blood cells. Blood Coagul. Fibrinolysis 2013, 24, 818–829. [Google Scholar] [CrossRef] [PubMed]
- Sinha, R.; Kumar, T.; Singh, A.; Swati. Primary Ovarian Lipoleiomyoma with Chondroid Metaplasia: An Original Case Report with Literature Review. SN Compr. Clin. Med. 2023, 5, 97. [Google Scholar] [CrossRef]
- Papatheodoridi, A.; Papamattheou, E.; Marinopoulos, S.; Ntanasis-Stathopoulos, I.; Dimitrakakis, C.; Giannos, A.; Kaparelou, M.; Liontos, M.; Dimopoulos, M.A.; Zagouri, F. Metaplastic Carcinoma of the Breast: Case Series of a Single Institute and Review of the Literature. Med. Sci. 2023, 11, 35. [Google Scholar] [CrossRef]
- Nagase, M.; Araki, A.; Ishikawa, N.; Nagano, N.; Fujimoto, M.; Biyajima, K.; Yamagami, N.; Yamamoto, S.; Maruyama, R. Tenosynovial Giant Cell Tumor, Localized Type with Extensive Chondroid Metaplasia: A Case Report with Immunohistochemical and Molecular Genetic Analysis. Int. J. Surg. Pathol. 2020, 28, 447–453. [Google Scholar] [CrossRef]
- Santibáñez-Salgado, J.A.; Olmos-Zúñiga, J.R.; Pérez-López, M.; Aboitiz-Rivera, C.; Gaxiola-Gaxiola, M.; Jasso-Victoria, R.; Sotres-Vega, A.; Baltazares-Lipp, M.; Pérez-Covarrubias, D.; Villalba-Caloca, J. Lyophilized glutaraldehyde-preserved bovine pericardium for experimental atrial septal defect closure. Eur. Cell Mater. 2010, 19, 158–165. [Google Scholar] [CrossRef]
- Mathieu, P.; Roussel, J.C.; Dagenais, F.; Anegon, I. Cartilaginous metaplasia and calcification in aortic allograft is associated with transforming growth factor beta 1 expression. J. Thorac. Cardiovasc. Surg. 2003, 126, 1449–1454. [Google Scholar] [CrossRef]
- Mohler, E.R., III; Gannon, F.; Reynolds, C.; Zimmerman, R.; Keane, M.G.; Kaplan, F.S. Bone formation and inflammation in cardiac valves. Circulation 2001, 103, 1522–1528. [Google Scholar] [CrossRef]
- Steiner, I.; Kasparová, P.; Kohout, A.; Dominik, J. Bone formation in cardiac valves: A histopathological study of 128 cases. Virchows Arch. 2007, 450, 653–657. [Google Scholar] [CrossRef]
- Yin, A.; Zhuang, W.; Liu, G.; Lan, X.; Tang, Z.; Deng, Y.; Wang, Y. Performance of PEGylated chitosan and poly (L-lactic acid-co-ε-caprolactone) bilayer vascular grafts in a canine femoral artery model. Colloids Surf. B Biointerfaces 2020, 188, 110806. [Google Scholar] [CrossRef]
- Zhao, L.; Li, X.; Yang, L.; Sun, L.; Mu, S.; Zong, H.; Li, Q.; Wang, F.; Song, S.; Yang, C.; et al. Evaluation of remodeling and regeneration of electrospun PCL/fibrin vascular grafts in vivo. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 118, 111441. [Google Scholar] [CrossRef] [PubMed]
- Dokuchaeva, A.A.; Mochalova, A.B.; Timchenko, T.P.; Podolskaya, K.S.; Pashkovskaya, O.A.; Karpova, E.V.; Ivanov, I.A.; Filatova, N.A.; Zhuravleva, I.Y. In Vivo Evaluation of PCL Vascular Grafts Implanted in Rat Abdominal Aorta. Polymers 2022, 14, 3313. [Google Scholar] [CrossRef]
- Fuery, M.A.; Liang, L.; Kaplan, F.S.; Mohler, E.R., III. Vascular ossification: Pathology, mechanisms, and clinical implications. Bone 2018, 109, 28–34. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Wang, X.; Lin, H. The hypoxic microenvironment: A driving force for heterotopic ossification progression. Cell Commun. Signal. 2020, 18, 20. [Google Scholar] [CrossRef] [PubMed]
- Gychka, S.G.; Shults, N.V.; Nikolaienko, S.I.; Marcocci, L.; Sariipek, N.E.; Rybka, V.; Malysheva, T.A.; Dibrova, V.A.; Suzuki, Y.J.; Gavrish, A.S. Vasa Vasorum Lumen Narrowing in Brain Vascular Hyalinosis in Systemic Hypertension Patients Who Died of Ischemic Stroke. Int. J. Mol. Sci. 2020, 21, 9611. [Google Scholar] [CrossRef] [PubMed]
- Flameng, W.; Hermans, H.; Verbeken, E.; Meuris, B. A randomized assessment of an advanced tissue preservation technology in the juvenile sheep model. J. Thorac. Cardiovasc. Surg. 2015, 149, 340–345. [Google Scholar] [CrossRef]
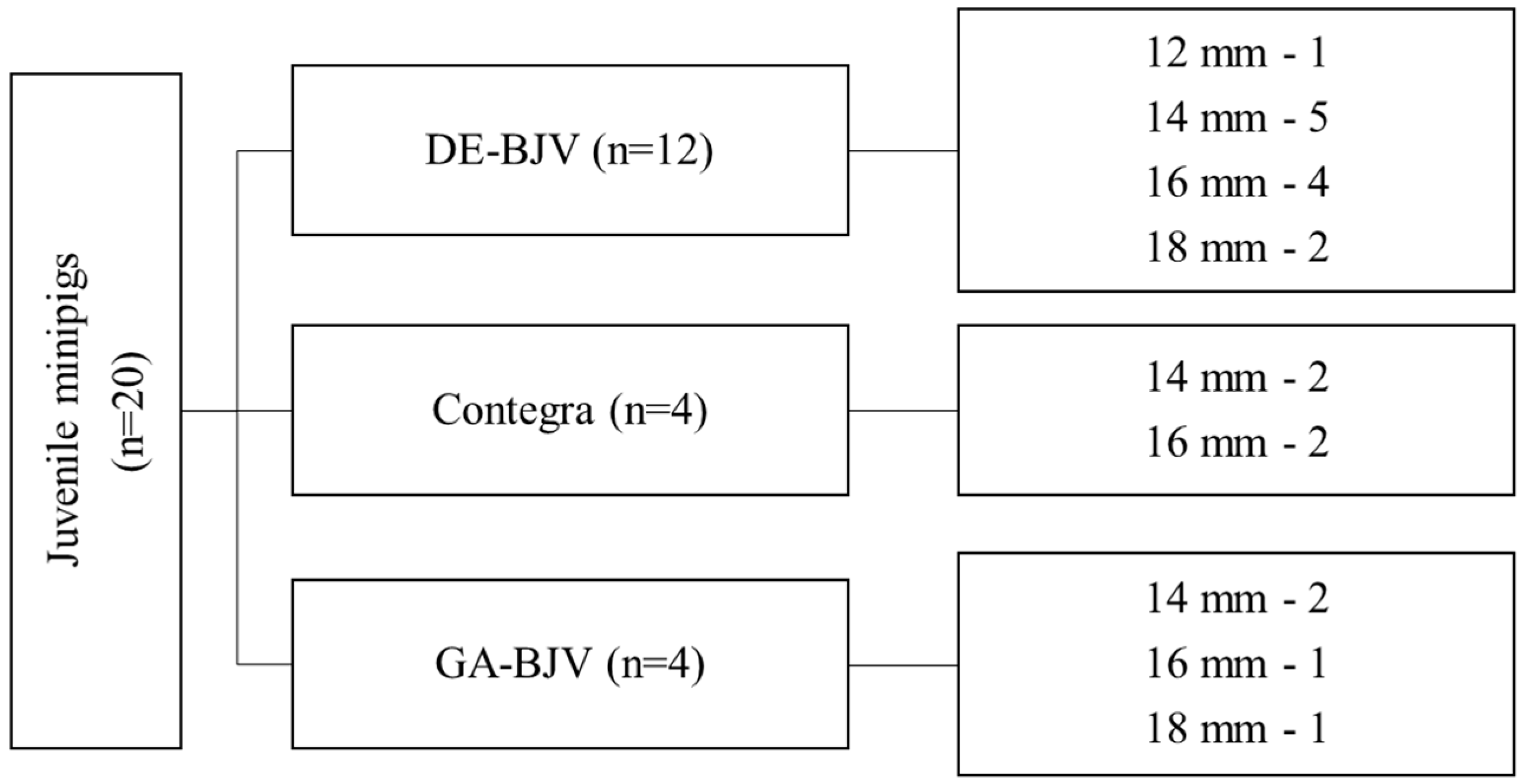


| Parameter | Me (IQR) |
|---|---|
| Animal gender, ♂/♀, n (%) | 9/11 (45.0%/55.0%) |
| Weight at surgery, kg | 46 (37.1–58.5) |
| Age at surgery, year | 1 (0.83–1) |
| BJV diameter, n (%): | |
| 12 mm | 1 (5.0%) |
| 14 mm | 9 (45.0%) |
| 16 mm | 7 (35.0%) |
| 18 mm | 3 (15.0%) |
| Type of cannulation: | |
| central, n (%) | 12 (60.0%) |
| peripheral, n (%) | 8 (40.0%) |
| CPB time, min | 60 (45–65) |
| Mechanical ventilation, h | 5.5 (4.0–5.5) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nichay, N.R.; Dokuchaeva, A.A.; Kulyabin, Y.Y.; Boyarkin, E.V.; Kuznetsova, E.V.; Rusakova, Y.L.; Murashov, I.S.; Vaver, A.A.; Bogachev-Prokophiev, A.V.; Zhuravleva, I.Y. Epoxy- versus Glutaraldehyde-Treated Bovine Jugular Vein Conduit for Pulmonary Valve Replacement: A Comparison of Morphological Changes in a Pig Model. Biomedicines 2023, 11, 3101. https://doi.org/10.3390/biomedicines11113101
Nichay NR, Dokuchaeva AA, Kulyabin YY, Boyarkin EV, Kuznetsova EV, Rusakova YL, Murashov IS, Vaver AA, Bogachev-Prokophiev AV, Zhuravleva IY. Epoxy- versus Glutaraldehyde-Treated Bovine Jugular Vein Conduit for Pulmonary Valve Replacement: A Comparison of Morphological Changes in a Pig Model. Biomedicines. 2023; 11(11):3101. https://doi.org/10.3390/biomedicines11113101
Chicago/Turabian StyleNichay, Nataliya R., Anna A. Dokuchaeva, Yuriy Yu. Kulyabin, Evgeniy V. Boyarkin, Elena V. Kuznetsova, Yanina L. Rusakova, Ivan S. Murashov, Andrey A. Vaver, Alexander V. Bogachev-Prokophiev, and Irina Yu. Zhuravleva. 2023. "Epoxy- versus Glutaraldehyde-Treated Bovine Jugular Vein Conduit for Pulmonary Valve Replacement: A Comparison of Morphological Changes in a Pig Model" Biomedicines 11, no. 11: 3101. https://doi.org/10.3390/biomedicines11113101




