Targeting CD24 in Cancer Immunotherapy
Abstract
:1. Introduction
2. Structure
3. Expression Pattern of CD24
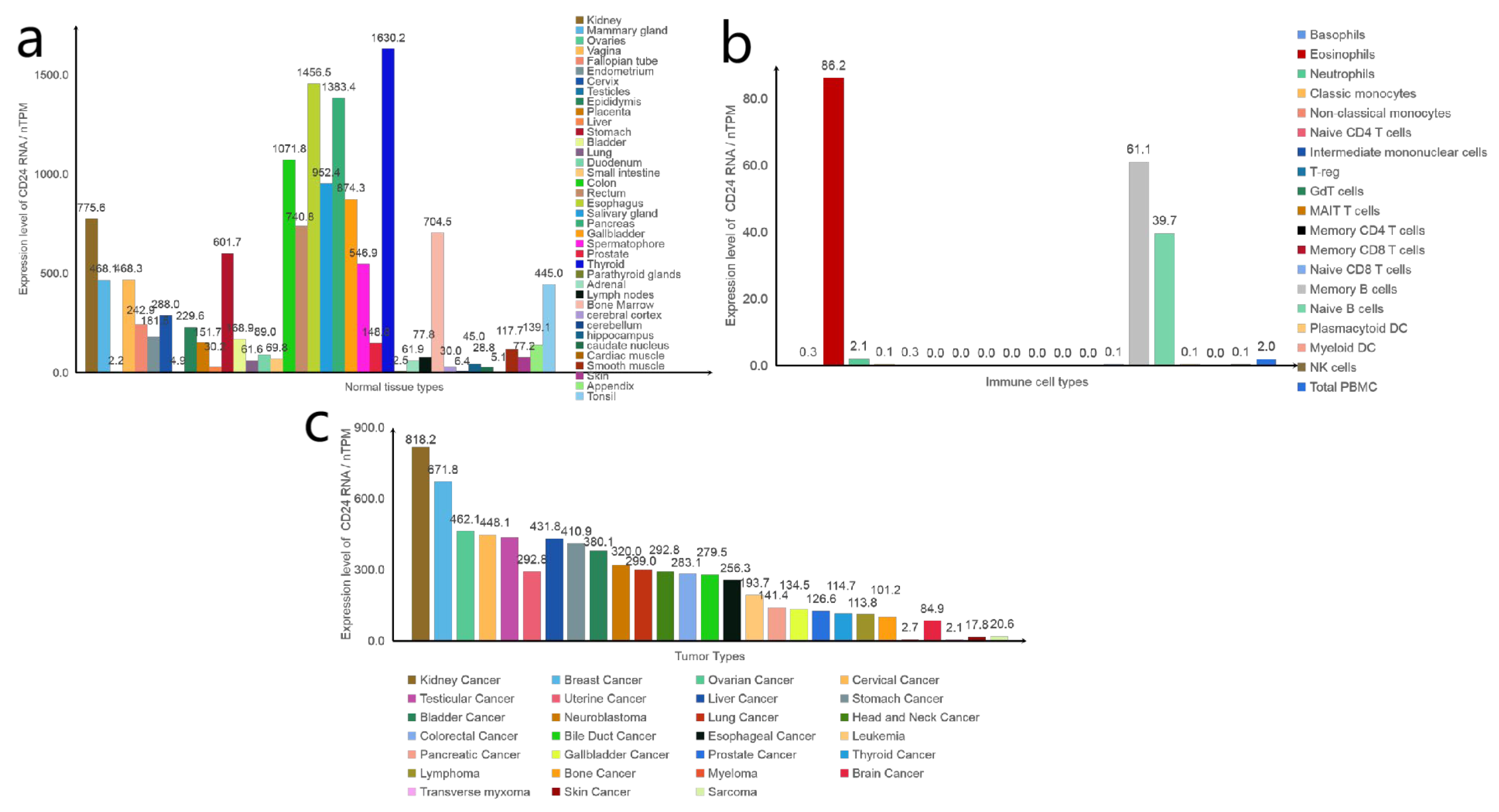
3.1. CD24 Is Widely Expressed in Cancer
3.2. CD24 Is Closely Related to Cancer Stem Cells
4. The Function of CD24 in Cancer Progression
4.1. Signalling Pathways Associated with CD24 and Cancer
4.1.1. Src-Mediated Signalling Pathway
4.1.2. Epidermal-Growth-Factor-Receptor-Mediated Signalling Pathway
4.1.3. Ras/Ral-GTPases-Mediated Signalling Pathways
4.1.4. Mitogen-Activated-Protein-Kinase-Mediated Signalling Pathway
4.2. CD24 Affects the Function of Other Molecules
4.2.1. P-Selectin
4.2.2. CXCR4
4.2.3. G3BP
4.2.4. p53
4.3. The Role of CD24 on Immune Cells
4.3.1. Macrophages
4.3.2. T Cells
4.3.3. B Cells
4.3.4. NK Cells
5. Current Drug Therapy Targeting CD24
5.1. Monoclonal Antibodies
5.1.1. ALB9 Antibody
5.1.2. SWA11 Antibody
5.1.3. Other Monoclonal Antibodies
5.2. Antibody–Drug Conjugates
5.2.1. SWA11-Ricin A Chain Couplers
5.2.2. SWA11-Pseudomonas Exotoxin Derivative (PE38)
5.2.3. hG7-BM3-VcMMAE
5.2.4. G7mAb-DOX
5.2.5. HN-01
5.3. CAR-T
5.4. CAR-NK
5.5. Clinical Research
6. Risks and Opportunities of Anti-CD24 Drugs in Cancer Treatment Process
6.1. CD24 Plays a Role in Tumour Drug Resistance
6.2. CD24 Prevents Excessive Inflammatory Response
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Kristiansen, G.; Sammar, M.; Altevogt, P. Tumour biological aspects of CD24, a mucin-like adhesion molecule. J. Mol. Histol. 2004, 35, 255–262. [Google Scholar] [CrossRef]
- Springer, T.; Galfrè, G.; Secher, D.S.; Milstein, C. Monoclonal xenogeneic antibodies to murine cell surface antigens: Identification of novel leukocyte differentiation antigens. Eur. J. Immunol. 1978, 8, 539–551. [Google Scholar] [CrossRef]
- Barkal, A.A.; Brewer, R.E.; Markovic, M.; Kowarsky, M.; Barkal, S.A.; Zaro, B.W.; Krishnan, V.; Hatakeyama, J.; Dorigo, O.; Barkal, L.J.; et al. CD24 signalling through macrophage Siglec-10 is a target for cancer immunotherapy. Nature 2019, 572, 392–396. [Google Scholar] [CrossRef]
- Wenger, R.H.; Rochelle, J.M.; Seldin, M.F.; Köhler, G.; Nielsen, P.J. The heat stable antigen (mouse CD24) gene is differentially regulated but has a housekeeping promoter. J. Biol. Chem. 1993, 268, 23345–23352. [Google Scholar] [CrossRef]
- Hough, M.R.; Rosten, P.M.; Sexton, T.L.; Kay, R.; Humphries, R.K. Mapping of CD24 and homologous sequences to multiple chromosomal loci. Genomics 1994, 22, 154–161. [Google Scholar] [CrossRef] [PubMed]
- Kay, R.; Takei, F.; Humphries, R.K. Expression cloning of a cDNA encoding M1/69-J11d heat-stable antigens. J. Immunol. 1990, 145, 1952–1959. [Google Scholar] [CrossRef] [PubMed]
- Kay, R.; Rosten, P.M.; Humphries, R.K. CD24, a signal transducer modulating B cell activation responses, is a very short peptide with a glycosyl phosphatidylinositol membrane anchor. J. Immunol. 1991, 147, 1412–1416. [Google Scholar] [CrossRef] [PubMed]
- Ohl, C.; Albach, C.; Altevogt, P.; Schmitz, B. N-glycosylation patterns of HSA/CD24 from different cell lines and brain homogenates: A comparison. Biochimie 2003, 85, 565–573. [Google Scholar] [CrossRef] [PubMed]
- Motari, E.; Zheng, X.; Su, X.; Liu, Y.; Kvaratskhelia, M.; Freitas, M.; Wang, P.G. Analysis of Recombinant CD24 Glycans by MALDI-TOF-MS Reveals Prevalence of Sialyl-T Antigen. Am. J. Biomed. Sci. 2009, 1, 1–11. [Google Scholar] [CrossRef]
- Hunte, B.E.; Capone, M.; Zlotnik, A.; Rennick, D.; Moore, T.A. Acquisition of CD24 expression by Lin-CD43+B220(low)ckit(hi) cells coincides with commitment to the B cell lineage. Eur. J. Immunol. 1998, 28, 3850–3856. [Google Scholar] [CrossRef]
- Liu, W.; Zhang, Y.; Wei, S.; Bae, S.; Yang, W.H.; Smith, G.J.; Mohler, J.L.; Fontham, E.T.H.; Bensen, J.T.; Sonpavde, G.P.; et al. A CD24-p53 axis contributes to African American prostate cancer disparities. Prostate 2020, 80, 609–618. [Google Scholar] [CrossRef]
- Ahmed, M.A.; Al-Attar, A.; Kim, J.; Watson, N.F.S.; Scholefield, J.H.; Durrant, L.G.; Ilyas, M. CD24 shows early upregulation and nuclear expression but is not a prognostic marker in colorectal cancer. J. Clin. Pathol. 2009, 62, 1117–1122. [Google Scholar] [CrossRef]
- Li, B.; Shao, Q.; Ji, D.; Li, F.; Guo, X.; Chen, G. Combined aberrant expression of N-Myc downstream-regulated gene 2 and CD24 is associated with disease-free survival and over-all survival in patients with hepatocellular carcinoma. Diagn. Pathol. 2014, 9, 209. [Google Scholar] [CrossRef]
- Taniuchi, K.; Nishimori, I.; Hollingsworth, M.A. Intracellular CD24 Inhibits Cell Invasion by Posttranscriptional Regulation of BART through Interaction with G3BP. Cancer Res. 2011, 71, 895–905. [Google Scholar] [CrossRef]
- Overdevest, J.B.; Thomas, S.; Kristiansen, G.; Hansel, D.E.; Smith, S.C.; Theodorescu, D. CD24 offers a therapeutic target for control of bladder cancer metastasis based on a requirement for lung colonization. Cancer Res. 2011, 71, 3802–3811. [Google Scholar] [CrossRef] [PubMed]
- Duex, J.E.; Owens, C.; Chauca-Diaz, A.; Dancik, G.M.; Vanderlinden, L.A.; Ghosh, D.; Leivo, M.Z.; Hansel, D.E.; Theodorescu, D. Nuclear CD24 Drives Tumor Growth and Is Predictive of Poor Patient Prognosis. Cancer Res. 2017, 77, 4858–4867. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Liu, R.; Ye, P.; Wong, C.; Chen, G.-Y.; Zhou, P.; Sakabe, K.; Zheng, X.; Wu, W.; Zhang, P.; et al. Intracellular CD24 disrupts the ARF–NPM interaction and enables mutational and viral oncogene-mediated p53 inactivation. Nat. Commun. 2015, 6, 5909. [Google Scholar] [CrossRef] [PubMed]
- Ishiwata, T.; Matsuda, Y.; Yoshimura, H.; Sasaki, N.; Ishiwata, S.; Ishikawa, N.; Takubo, K.; Arai, T.; Aida, J. Pancreatic cancer stem cells: Features and detection methods. Pathol. Oncol. Res. 2018, 24, 797–805. [Google Scholar] [CrossRef]
- Palomeras, S.; Ruiz-Martínez, S.; Puig, T. Targeting Breast Cancer Stem Cells to Overcome Treatment Resistance. Molecules 2018, 23, 2193. [Google Scholar] [CrossRef] [PubMed]
- Ischenko, I.; Seeliger, H.; Kleespies, A.; Angele, M.K.; Eichhorn, M.E.; Jauch, K.-W.; Bruns, C.J. Pancreatic cancer stem cells: New understanding of tumorigenesis, clinical implications. Langenbecks Arch. Surg. 2010, 395, 1–10. [Google Scholar] [CrossRef]
- Altevogt, P.; Sammar, M.; Hüser, L.; Kristiansen, G. Novel insights into the function of CD24: A driving force in cancer. Int. J. Cancer 2021, 148, 546–559. [Google Scholar] [CrossRef] [PubMed]
- Al-Hajj, M.; Wicha, M.S.; Benito-Hernandez, A.; Morrison, S.J.; Clarke, M.F. Prospective identification of tumorigenic breast cancer cells. Proc. Natl. Acad. Sci. USA 2003, 100, 3983–3988. [Google Scholar] [CrossRef] [PubMed]
- Jaggupilli, A.; Elkord, E. Significance of CD44 and CD24 as Cancer Stem Cell Markers: An Enduring Ambiguity. Clin. Dev. Immunol. 2012, 2012, 708036. [Google Scholar] [CrossRef] [PubMed]
- Lyu, C.; Bhimani, A.K.; Draus, W.T.; Weigel, R.; Chen, S. Active Gαi/o mutants accelerate breast tumor metastasis via the c-Src pathway. bioRxiv 2023. [Google Scholar] [CrossRef]
- Nandi, I.; Smith, H.W.; Sanguin-Gendreau, V.; Ji, L.; Pacis, A.; Papavasiliou, V.; Zuo, D.; Nam, S.; Attalla, S.S.; Kim, S.H.; et al. Coordinated activation of c-Src and FOXM1 drives tumor cell proliferation and breast cancer progression. J. Clin. Investig. 2023, 133, e162324. [Google Scholar] [CrossRef] [PubMed]
- Bretz, N.P.; Salnikov, A.V.; Perne, C.; Keller, S.; Wang, X.; Mierke, C.T.; Fogel, M.; Erbe-Hofmann, N.; Schlange, T.; Moldenhauer, G.; et al. CD24 controls Src/STAT3 activity in human tumors. Cell. Mol. Life Sci. 2012, 69, 3863–3879. [Google Scholar] [CrossRef] [PubMed]
- Sammar, M.; Aigner, S.; Hubbe, M.; Schirrmacher, V.; Schachner, M.; Vestweber, D.; Altevogt, P. Heat-stable antigen (CD24) as ligand for mouse P-selectin. Int. Immunol. 1994, 6, 1027–1036. [Google Scholar] [CrossRef]
- Baumann, P.; Cremers, N.; Kroese, F.; Orend, G.; Chiquet-Ehrismann, R.; Uede, T.; Yagita, H.; Sleeman, J.P. CD24 Expression Causes the Acquisition of Multiple Cellular Properties Associated with Tumor Growth and Metastasis. Cancer Res. 2005, 65, 10783–10793. [Google Scholar] [CrossRef] [PubMed]
- Mierke, C.T.; Bretz, N.; Altevogt, P. Contractile forces contribute to increased glycosylphosphatidylinositol-anchored receptor CD24-facilitated cancer cell invasion. J. Biol. Chem. 2011, 286, 34858–34871. [Google Scholar] [CrossRef]
- Baumann, P.; Thiele, W.; Cremers, N.; Muppala, S.; Krachulec, J.; Diefenbacher, M.; Kassel, O.; Mudduluru, G.; Allgayer, H.; Frame, M.; et al. CD24 interacts with and promotes the activity of c-src within lipid rafts in breast cancer cells, thereby increasing integrin-dependent adhesion. Cell. Mol. Life Sci. 2012, 69, 435–448. [Google Scholar] [CrossRef]
- Aigner, S.; Sthoeger, Z.M.; Fogel, M.; Weber, E.; Zarn, J.; Ruppert, M.; Zeller, Y.; Vestweber, D.; Stahel, R.; Sammar, M.; et al. CD24, a Mucin-Type Glycoprotein, Is a Ligand for P-Selectin on Human Tumor Cells. Blood 1997, 89, 3385–3395. [Google Scholar] [CrossRef] [PubMed]
- Aigner, S.; Ramos, C.L.; Hafezi-Moghadam, A.; Lawrence, M.B.; Friederichs, J.; Altevogt, P.; Ley, K. CD24 mediates rolling of breast carcinoma cells on P-selectin. FASEB J. 1998, 12, 1241–1251. [Google Scholar] [CrossRef] [PubMed]
- Carroll, M.J.; Fogg, K.C.; Patel, H.A.; Krause, H.B.; Mancha, A.-S.; Patankar, M.S.; Weisman, P.S.; Barroilhet, L.; Kreeger, P.K. Alternatively-Activated Macrophages Upregulate Mesothelial Expression of P-Selectin to Enhance Adhesion of Ovarian Cancer Cells. Cancer Res. 2018, 78, 3560–3573. [Google Scholar] [CrossRef]
- Lee, K.-m.; Ju, J.-h.; Jang, K.; Yang, W.; Yi, J.Y.; Noh, D.Y.; Shin, I. CD24 regulates cell proliferation and transforming growth factor β-induced epithelial to mesenchymal transition through modulation of integrin β1 stability. Cell. Signal. 2012, 24, 2132–2142. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.L.; Oshi, M.; Endo, I.; Takabe, K. Clinical relevance of stem cell surface markers CD133, CD24, and CD44 in colorectal cancer. Am. J. Cancer Res. 2021, 11, 5141–5154. [Google Scholar] [PubMed]
- Deng, W.; Gu, L.; Li, X.; Zheng, J.; Zhang, Y.; Duan, B.; Cui, J.; Dong, J.; Du, J. CD24 associates with EGFR and supports EGF/EGFR signaling via RhoA in gastric cancer cells. J. Transl. Med. 2016, 14, 32. [Google Scholar] [CrossRef]
- Atmaca, A.; Werner, D.; Pauligk, C.; Steinmetz, K.; Wirtz, R.; Altmannsberger, H.-M.; Jäger, E.; Al-Batran, S.-E. The prognostic impact of epidermal growth factor receptor in patients with metastatic gastric cancer. BMC Cancer 2012, 12, 524. [Google Scholar] [CrossRef]
- Press, M.F.; Lenz, H.-J. EGFR, HER2 and VEGF Pathways. Drugs 2007, 67, 2045–2075. [Google Scholar] [CrossRef]
- Yip, W.K.; Seow, H.F. Activation of phosphatidylinositol 3-kinase/Akt signaling by EGF downregulates membranous E-cadherin and β-catenin and enhances invasion in nasopharyngeal carcinoma cells. Cancer Lett. 2012, 318, 162–172. [Google Scholar] [CrossRef]
- Li, Q.; Mattingly, R.R. Mattingly, Restoration of E-cadherin cell-cell junctions requires both expression of E-cadherin and suppression of ERK MAP ki-nase activation in Ras-transformed breast epithelial cells. Neoplasia 2008, 10, 1444–1458. [Google Scholar] [CrossRef]
- Veluchamy, J.P.; Spanholtz, J.; Tordoir, M.; Thijssen, V.L.; Heideman, D.A.M.; Verheul, H.M.W.; de Gruijl, T.D.; van der Vliet, H.J. Combination of NK Cells and Cetuximab to Enhance Anti-Tumor Responses in RAS Mutant Metastatic Colorectal Cancer. PLoS ONE 2016, 11, e0157830. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.-H.; An, M.; Bao, B.-L.; Ren, F.; Xia, P. Nicotine inhibits CD24 expression in Lewis lung carcinoma cells by upregulation of RAS expression. Int. J. Oncol. 2018, 53, 815–822. [Google Scholar] [CrossRef] [PubMed]
- Chien, Y.; White, M.A. RAL GTPases are linchpin modulators of human tumour-cell proliferation and survival. EMBO Rep. 2003, 4, 800–806. [Google Scholar] [CrossRef]
- Smith, S.C.; Oxford, G.; Wu, Z.; Nitz, M.D.; Conaway, M.; Frierson, H.F.; Hampton, G.; Theodorescu, D. The metastasis-associated gene CD24 is regulated by Ral GTPase and is a mediator of cell proliferation and survival in human cancer. Cancer Res. 2006, 66, 1917–1922. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Wang, X.; Peng, L.; Deng, Q.; Liang, Y.; Qing, H.; Jiang, B. CD24-dependent MAPK pathway activation is required for colorectal cancer cell proliferation. Cancer Sci. 2010, 101, 112–119. [Google Scholar] [CrossRef] [PubMed]
- Friederichs, J.; Zeller, Y.; Hafezi-Moghadam, A.; Gröne, H.J.; Ley, K.; Altevogt, P. The CD24/P-selectin binding pathway initiates lung arrest of human A125 adenocarcinoma cells. Cancer Res. 2000, 60, 6714–6722. [Google Scholar] [PubMed]
- Runz, S.; Mierke, C.T.; Joumaa, S.; Behrens, J.; Fabry, B.; Altevogt, P. CD24 induces localization of β1 integrin to lipid raft domains. Biochem. Biophys. Res. Commun. 2008, 365, 35–41. [Google Scholar] [CrossRef]
- Guo, W.; Huai, Q.; Zhou, B.; Guo, L.; Sun, L.; Xue, X.; Tan, F.; Xue, Q.; Gao, S.; He, J. Comprehensive analysis of the immunological implication and prognostic value of CXCR4 in non-small cell lung cancer. Cancer Immunol. Immunother. 2023, 72, 1029–1045. [Google Scholar] [CrossRef]
- Dekkers, S.; Caspar, B.; Goulding, J.; Kindon, N.D.; Kilpatrick, L.E.; Stoddart, L.A.; Briddon, S.J.; Kellam, B.; Hill, S.J.; Stocks, M.J. Small-Molecule Fluorescent Ligands for the CXCR4 Chemokine Receptor. J. Med. Chem. 2023, 66, 5208–5222. [Google Scholar] [CrossRef]
- Wysoczynski, M.; Reca, R.; Ratajczak, J.; Kucia, M.; Shirvaikar, N.; Honczarenko, M.; Mills, M.; Wanzeck, J.; Janowska-Wieczorek, A.; Ratajczak, M.Z. Incorporation of CXCR4 into membrane lipid rafts primes homing-related responses of hematopoietic stem/progenitor cells to an SDF-1 gradient. Blood 2005, 105, 40–48. [Google Scholar] [CrossRef]
- Schabath, H.; Runz, S.; Joumaa, S.; Altevogt, P. CD24 affects CXCR4 function in pre-B lymphocytes and breast carcinoma cells. J. Cell Sci. 2006, 119, 314–325. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Yi, B.; Wang, C.; Chen, D.; Bae, S.; Wei, S.; Guo, R.-J.; Lu, C.; Nguyen, L.L.H.; Yang, W.-H.; et al. Silencing of CD24 Enhances the PRIMA-1-Induced Restoration of Mutant p53 in Prostate Cancer Cells. Clin. Cancer Res. 2016, 22, 2545–2554. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, Y.; Yang, Y.; Weng, L.; Wu, Q.; Zhang, J.; Zhao, P.; Fang, L.; Shi, Y.; Wang, P. Emerging phagocytosis checkpoints in cancer immunotherapy. Signal Transduct. Target. Ther. 2023, 8, 104. [Google Scholar] [CrossRef]
- Fang, X.; Zheng, P.; Tang, J.; Liu, Y. CD24: From A to Z. Cell Mol. Immunol. 2010, 7, 100–103. [Google Scholar] [CrossRef]
- Chen, K.; Dai, M.; Luo, Q.; Wang, Y.; Shen, W.; Liao, Y.; Zhou, Y.; Cheng, W. PARP1 controls the transcription of CD24 by ADP-ribosylating the RNA helicase DDX5 in pancreatic cancer. Int. J. Biochem. Cell Biol. 2023, 155, 106358. [Google Scholar] [CrossRef]
- Zou, K.-L.; Lan, Z.; Cui, H.; Zhao, Y.-Y.; Wang, W.-M.; Yu, G.-T. CD24 blockade promotes anti-tumor immunity in oral squamous cell carcinoma. Oral. Dis. 2022. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Pan, X.; Tian, B.; Hu, Y. Polysaccharide immunization and colorectal cancer: A systematic review and network meta-analysis. Front. Nutr. 2022, 9, 961507. [Google Scholar] [CrossRef] [PubMed]
- Ernst, B.; Lee, D.S.; Chang, J.M.; Sprent, J.; Surh, C.D. The peptide ligands mediating positive selection in the thymus control T cell survival and homeostatic proliferation in the periphery. Immunity 1999, 11, 173–181. [Google Scholar] [CrossRef] [PubMed]
- Li, O.; Zheng, P.; Liu, Y. CD24 Expression on T Cells Is Required for Optimal T Cell Proliferation in Lymphopenic Host. J. Exp. Med. 2004, 200, 1083–1089. [Google Scholar] [CrossRef] [PubMed]
- Christian, S.L. CD24 as a Potential Therapeutic Target in Patients with B-Cell Leukemia and Lymphoma: Current Insights. Onco Targets Ther. 2022, 15, 1391–1402. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, A.; Kerr, S.; Jellusova, J.; Zhang, J.; Weisel, F.; Wellmann, U.; Winkler, T.H.; Kneitz, B.; Crocker, P.R.; Nitschke, L. Siglec-G is a B1 cell–inhibitory receptor that controls expansion and calcium signaling of the B1 cell population. Nat. Immunol. 2007, 8, 695–704. [Google Scholar] [CrossRef] [PubMed]
- Chappel, M.S.; Hough, M.R.; Mittel, A.; Takei, F.; Kay, R.; Humphries, R.K. Cross-linking the murine heat-stable antigen induces apoptosis in B cell precursors and suppresses the anti-CD40-induced proliferation of mature resting B lymphocytes. J. Exp. Med. 1996, 184, 1639–1649. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T.; Kiyokawa, N.; Taguchi, T.; Sekino, T.; Katagiri, Y.U.; Fujimoto, J. CD24 induces apoptosis in human B cells via the glycolipid-enriched membrane domains/rafts-mediated signaling system. J. Immunol. 2001, 166, 5567–5577. [Google Scholar] [CrossRef] [PubMed]
- Hough, M.R.; Chappel, M.S.; Sauvageau, G.; Takei, F.; Kay, R.; Humphries, R.K. Reduction of early B lymphocyte precursors in transgenic mice overexpressing the murine heat-stable antigen. J. Immunol. 1996, 156, 479–488. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Lu, X.; Tao, K.; Shi, L.; Li, W.; Wang, G.; Wu, K. Siglec-10 is associated with survival and natural killer cell dysfunction in hepatocellular carcinoma. J. Surg. Res. 2015, 194, 107–113. [Google Scholar] [CrossRef]
- Han, Y.; Sun, F.; Zhang, X.; Wang, T.; Jiang, J.; Cai, J.; Gao, Q.; Hezam, K.; Liu, Y.; Xie, J.; et al. CD24 targeting bi-specific antibody that simultaneously stimulates NKG2D enhances the efficacy of cancer immunotherapy. J. Cancer Res. Clin. Oncol. 2019, 145, 1179–1190. [Google Scholar] [CrossRef]
- Weber, E.; Lehmann, H.P.; Beck-Sickinger, A.G.; Wawrzynczak, E.J.; Waibel, R.; Folkers, G.; Stahel, R.A. Antibodies to the protein core of the small cell lung cancer workshop antigen cluster-w4 and to the leucocyte workshop antigen CD24 recognize the same short protein sequence leucine-alanine-proline. Clin. Exp. Immunol. 1993, 93, 279–285. [Google Scholar] [CrossRef]
- Kristiansen, G.; Machado, E.; Bretz, N.; Rupp, C.; Winzer, K.J.; König, A.K.; Moldenhauer, G.; Marmé, F.; Costa, J.; Altevogt, P. Molecular and clinical dissection of CD24 antibody specificity by a comprehensive comparative analysis. Lab. Investig. 2010, 90, 1102–1116. [Google Scholar] [CrossRef]
- Majores, M.; Schindler, A.; Fuchs, A.; Stein, J.; Heukamp, L.; Altevogt, P.; Kristiansen, G. Membranous CD24 expression as detected by the monoclonal antibody SWA11 is a prognostic marker in non-small cell lung cancer patients. BMC Clin. Pathol. 2015, 15, 19. [Google Scholar] [CrossRef] [PubMed]
- Sagiv, E.; Starr, A.; Rozovski, U.; Khosravi, R.; Altevogt, P.; Wang, T.; Arber, N. Targeting CD24 for Treatment of Colorectal and Pancreatic Cancer by Monoclonal Antibodies or Small Interfering RNA. Cancer Res. 2008, 68, 2803–2812. [Google Scholar] [CrossRef] [PubMed]
- Barash, U.; Spyrou, A.; Liu, P.; Vlodavsky, E.; Zhu, C.; Luo, J.; Su, D.; Ilan, N.; Forsberg-Nilsson, K.; Vlodavsky, I.; et al. Heparanase promotes glioma progression via enhancing CD24 expression. Int. J. Cancer 2019, 145, 1596–1608. [Google Scholar] [CrossRef] [PubMed]
- Sagiv, E.; Memeo, L.; Karin, A.; Kazanov, D.; Jacob-Hirsch, J.; Mansukhani, M.; Rechavi, G.; Hibshoosh, H.; Arber, N. CD24 is a new oncogene, early at the multistep process of colorectal cancer carcinogenesis. Gastroenterology 2006, 131, 630–639. [Google Scholar] [CrossRef]
- Sagiv, E.; Kazanov, D.; Arber, N. CD24 plays an important role in the carcinogenesis process of the pancreas. Biomed. Pharmacother. 2006, 60, 280–284. [Google Scholar] [CrossRef]
- Salnikov, A.V.; Bretz, N.P.; Perne, C.; Hazin, J.; Keller, S.; Fogel, M.; Herr, I.; Schlange, T.; Moldenhauer, G.; Altevogt, P. Antibody targeting of CD24 efficiently retards growth and influences cytokine milieu in experimental carcinomas. Br. J. Cancer 2013, 108, 1449–1459. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Wang, T.; Tu, X.; Xie, W.; He, H.; Wang, M.; Zhang, J. Antibody-based targeting of CD24 enhances antitumor effect of cetuximab via attenuating phosphorylation of Src/STAT3. Biomed. Pharmacother. 2017, 90, 427–436. [Google Scholar] [CrossRef]
- Zangemeister-Wittke, U.; Lehmann, H.P.; Waibel, R.; Wawrzynczak, E.J.; Stahel, R.A. Action of a CD24-specific deglycosylated ricin-A-chain immunotoxin in conventional and novel models of small-cell-lung-cancer xenograft. Int. J. Cancer 1993, 53, 521–528. [Google Scholar] [CrossRef] [PubMed]
- Schnell, R.; Katouzi, A.A.; Linnartz, C.; Schoen, G.; Drillich, S.; Hansmann, M.L.; Schiefer, D.; Barth, S.; Zangemeister-Wittke, U.; Stahel, R.A.; et al. Potent anti-tumor effects of an anti-CD24 ricin A-chain immunotoxin in vitro and in a disseminated human Burkitt’s lymphoma model in SCID mice. Int. J. Cancer 1996, 66, 526–531. [Google Scholar] [CrossRef]
- Shapira, S.; Shapira, A.; Starr, A.; Kazanov, D.; Kraus, S.; Benhar, I.; Arber, N. An immunoconjugate of anti-CD24 and Pseudomonas exotoxin selectively kills human colorectal tumors in mice. Gastroenterology 2011, 140, 935–946. [Google Scholar] [CrossRef] [PubMed]
- Sun, F.; Wang, Y.; Luo, X.; Ma, Z.; Xu, Y.; Zhang, X.; Lv, T.; Zhang, Y.; Wang, M.; Huang, Z.; et al. Anti-CD24 antibody–nitric oxide conjugate selectively and potently suppresses hepatic carcinoma. Cancer Res. 2019, 79, 3395–3405. [Google Scholar] [CrossRef]
- Ma, Z.; He, H.; Sun, F.; Xu, Y.; Huang, X.; Ma, Y.; Zhao, H.; Wang, Y.; Wang, M.; Zhang, J. Selective targeted delivery of doxorubicin via conjugating to anti-CD24 antibody results in enhanced antitumor potency for hepatocellular carcinoma both in vitro and in vivo. J. Cancer Res. Clin. Oncol. 2017, 143, 1929–1940. [Google Scholar] [CrossRef]
- Mocellin, S.; Bronte, V.; Nitti, D. Nitric oxide, a double edged sword in cancer biology: Searching for therapeutic opportunities. Med. Res. Rev. 2007, 27, 317–352. [Google Scholar] [CrossRef] [PubMed]
- Maliar, A.; Servais, C.; Waks, T.; Chmielewski, M.; Lavy, R.; Altevogt, P.; Abken, H.; Eshhar, Z. Redirected T cells that target pancreatic adenocarcinoma antigens eliminate tumors and metastases in mice. Gastroenterology 2012, 143, 1375–1384.e1375. [Google Scholar] [CrossRef] [PubMed]
- Klapdor, R.; Wang, S.; Morgan, M.; Dörk, T.; Hacker, U.; Hillemanns, P.; Büning, H.; Schambach, A. Characterization of a Novel Third-Generation Anti-CD24-CAR against Ovarian Cancer. Int. J. Mol. Sci. 2019, 20, 660. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Mayea, Y.; Benítez-Álvarez, L.; Sánchez-García, A.; Bataller, M.; Companioni, O.; Mir, C.; Benavente, S.; Lorente, J.; Canela, N.; Fernández-Rozadilla, C.; et al. Transcriptomic and Proteomic Profiles for Elucidating Cisplatin Resistance in Head-and-Neck Squamous Cell Carcinoma. Cancers 2022, 14, 5511. [Google Scholar] [CrossRef]
- Zhang, W.; Ke, Y.; Liu, X.; Jin, M.; Huang, G. Drug resistance in NSCLC is associated with tumor micro-environment. Reprod. Biol. 2022, 22, 100680. [Google Scholar] [CrossRef] [PubMed]
- Hüser, L.; Sachindra, S.; Granados, K.; Federico, A.; Larribère, L.; Novak, D.; Umansky, V.; Altevogt, P.; Utikal, J. SOX2-mediated upregulation of CD24 promotes adaptive resistance toward targeted therapy in melanoma. Int. J. Cancer 2018, 143, 3131–3142. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.; Yao, Y.; Xu, G.; Zhou, C.; Zhang, Y.; Sun, J.; Jiang, R.; Shao, Q.; Chen, Y. CD24 regulates sorafenib resistance via activating autophagy in hepatocellular carcinoma. Cell Death Dis. 2018, 9, 646. [Google Scholar] [CrossRef]
- Koh, J.; Lee, S.-b.; Park, H.; Lee, H.J.; Cho, N.H.; Kim, J. Susceptibility of CD24+ ovarian cancer cells to anti-cancer drugs and natural killer cells. Biochem. Biophys. Res. Commun. 2012, 427, 373–378. [Google Scholar] [CrossRef]
- Chen, C.; Zhang, L.; Ruan, Z. GATA3 Encapsulated by Tumor-Associated Macrophage-Derived Extracellular Vesicles Promotes Immune Escape and Chemotherapy Resistance of Ovarian Cancer Cells by Upregulating the CD24/Siglec-10 Axis. Mol. Pharm. 2023, 20, 971–986. [Google Scholar] [CrossRef]
- Jia, Y.; Gu, D.; Wan, J.; Yu, B.; Zhang, X.; Chiorean, E.G.; Wang, Y.; Xie, J. The role of GLI-SOX2 signaling axis for gemcitabine resistance in pancreatic cancer. Oncogene 2019, 38, 1764–1777. [Google Scholar] [CrossRef]
- Pandey, V.; Jung, Y.; Kang, J.; Steiner, M.; Qian, P.X.; Banerjee, A.; Mitchell, M.D.; Wu, Z.S.; Zhu, T.; Liu, D.X.; et al. Artemin Reduces Sensitivity to Doxorubicin and Paclitaxel in Endometrial Carcinoma Cells through Specific Regulation of CD24. Transl. Oncol. 2010, 3, 218–229. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Liu, M.; Zhang, J.; Brown, N.K.; Zhang, P.; Zhang, Y.; Liu, H.; Du, X.; Wu, W.; Devenport, M.; et al. CD24-Siglec axis is an innate immune checkpoint against metaflammation and metabolic disorder. Cell Metab. 2022, 34, 1088–1103.e1086. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.Y.; Tang, J.; Zheng, P.; Liu, Y. CD24 and Siglec-10 selectively repress tissue damage-induced immune responses. Science 2009, 323, 1722–1725. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Chen, G.Y.; Zheng, P. Sialoside-based pattern recognitions discriminating infections from tissue injuries. Curr. Opin. Immunol. 2011, 23, 41–45. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.Y.; Chen, X.; King, S.; Cavassani, K.A.; Cheng, J.; Zheng, X.; Cao, H.; Yu, H.; Qu, J.; Fang, D.; et al. Amelioration of sepsis by inhibiting sialidase-mediated disruption of the CD24-SiglecG interaction. Nat. Biotechnol. 2011, 29, 428–435. [Google Scholar] [CrossRef] [PubMed]



| Name | Function | Location |
|---|---|---|
| P-Selectin | Promotes cell adhesion | Cytoplasmic membrane |
| CXCR4 | Promote cell migration | Raft region |
| G3BP | Inhibit cell migration | Cytoplasm/cell membrane |
| P53 | Oncogenes, mutations that cause cells to become cancerous | Nucleus (possible) |
| ID | Drug Type | Disease Type | Clinical Progress | |
|---|---|---|---|---|
| 1 | NCT04552704 | CD24 Fc | Advanced Solid Tumours | Phase I/II |
| 2 | NCT04060407 | CD24 Fc | Metastatic Melanoma | Phase Ib/II |
| 3 | NCT05985083 | IMM47 | Advanced Solid Tumours | Phase I |
| 4 | NCT06028373 | ATG-031 | Advanced Solid Tumours or B-cell Non-Hodgkin Lymphomas | Phase I |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, W.; Hu, Z.; Guo, Z. Targeting CD24 in Cancer Immunotherapy. Biomedicines 2023, 11, 3159. https://doi.org/10.3390/biomedicines11123159
Chen W, Hu Z, Guo Z. Targeting CD24 in Cancer Immunotherapy. Biomedicines. 2023; 11(12):3159. https://doi.org/10.3390/biomedicines11123159
Chicago/Turabian StyleChen, Wenwen, Zhigang Hu, and Zhigang Guo. 2023. "Targeting CD24 in Cancer Immunotherapy" Biomedicines 11, no. 12: 3159. https://doi.org/10.3390/biomedicines11123159





