Anti-Osteoarthritis Mechanism of the Nrf2 Signaling Pathway
Abstract
:1. Introduction
2. Correlation between Oxidative Stress and Inflammation in Osteoarthritis
3. Role of the Nrf2 Signaling Pathway in Osteoarthritis
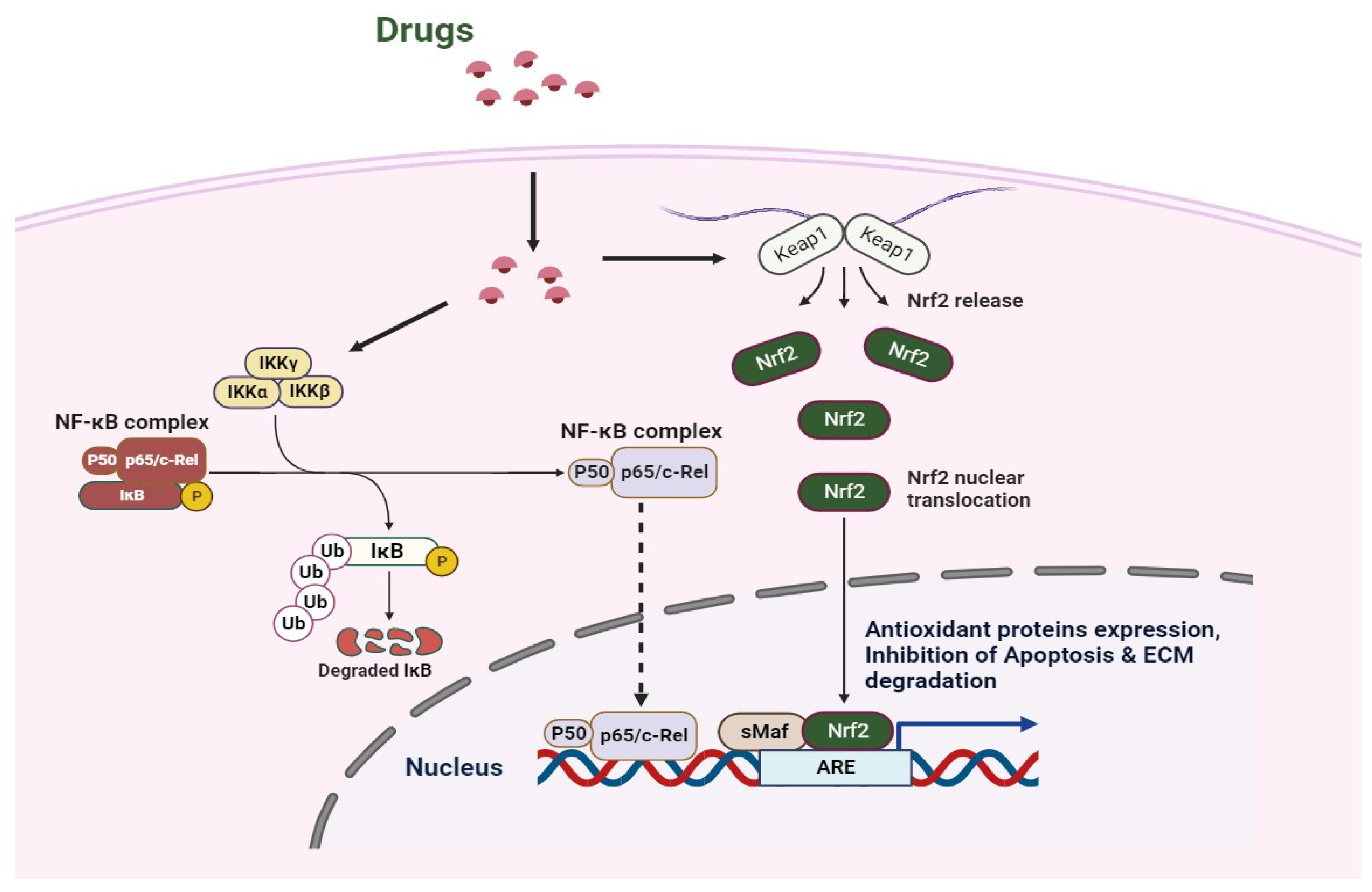
4. Crosstalk of Nrf2 and NF-κB Signaling in Osteoarthritis
5. Potential Therapeutic Agents of Osteoarthritis via the Nrf2 Signaling Pathway
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hunter, D.J.; Bierma-Zeinstra, S. Osteoarthritis. Lancet 2019, 393, 1745–1759. [Google Scholar] [CrossRef]
- Geiger, B.C.; Wang, S.; Padera, R.F., Jr.; Grodzinsky, A.J.; Hammond, P.T. Cartilage-penetrating nanocarriers improve delivery and efficacy of growth factor treatment of osteoarthritis. Sci. Transl. Med. 2018, 10, eaat8800. [Google Scholar] [CrossRef]
- Bruyère, O.; Honvo, G.; Veronese, N.; Arden, N.K.; Branco, J.; Curtis, E.M.; Al-Daghri, N.M.; Herrero-Beaumont, G.; Martel-Pelletier, J.; Pelletier, J.P.; et al. An updated algorithm recommendation for the management of knee osteoarthritis from the European Society for Clinical and Economic Aspects of Osteoporosis, Osteoarthritis and Musculoskeletal Diseases (ESCEO). Semin Arth. Rheum. 2019, 49, 337–350. [Google Scholar] [CrossRef]
- Sokolove, J.; Lepus, C.M. Role of inflammation in the pathogenesis of osteoarthritis: Latest findings and interpretations. Ther. Adv. Musculoskelet. Dis. 2013, 5, 77–94. [Google Scholar] [CrossRef]
- Tong, L.; Yu, H.; Huang, X.; Shen, J.; Xiao, G.; Chen, L.; Wang, H.; Xing, L.; Chen, D. Current understanding of osteoarthritis pathogenesis and relevant new approaches. Bone Res. 2022, 10, 60. [Google Scholar] [CrossRef]
- Cao, M.; Ong, M.T.Y.; Yung, P.S.H.; Tuan, R.S.; Jiang, Y. Role of synovial lymphatic function in osteoarthritis. Osteoarthr. Cart. 2022, 30, 1186–1197. [Google Scholar] [CrossRef]
- Kou, L.; Xiao, S.; Sun, R.; Bao, S.; Yao, Q.; Chen, R. Biomaterial-engineered intra-articular drug delivery systems for osteoarthritis therapy. Drug Deliv. 2019, 26, 870–885. [Google Scholar] [CrossRef]
- Karsdal, M.A.; Michaelis, M.; Ladel, C.; Siebuhr, A.S.; Bihlet, A.R.; Andersen, J.R.; Guehring, H.; Christiansen, C.; Bay-Jensen, A.C.; Kraus, V.B. Disease-modifying treatments for osteoarthritis (DMOADs) of the knee and hip: Lessons learned from failures and opportunities for the future. Osteoarthr. Cartil. 2016, 24, 2013–2021. [Google Scholar] [CrossRef]
- Ghanem, C.I.; Pérez, M.J.; Manautou, J.E.; Mottino, A.D. Acetaminophen from liver to brain: New insights into drug pharmacological action and toxicity. Pharmacol. Res. 2016, 109, 119–131. [Google Scholar] [CrossRef]
- Bolduc, J.A.; Collins, J.A.; Loeser, R.F. Reactive oxygen species, aging and articular cartilage homeostasis. Free Radic. Biol. Med. 2019, 132, 73–82. [Google Scholar] [CrossRef]
- Mobasheri, A.; Batt, M. An update on the pathophysiology of osteoarthritis. Ann. Phys. Rehabil. Med. 2016, 59, 333–339. [Google Scholar] [CrossRef]
- Martin, J.A.; Buckwalter, J.A. Aging, articular cartilage chondrocyte senescence and osteoarthritis. Biogerontology 2002, 3, 257–264. [Google Scholar] [CrossRef]
- Pan, X.X.; Chen, T.T.; Zhang, Z.J.; Chen, X.W.; Chen, C.S.; Chen, L.; Wang, X.Y.; Ying, X.Z. Activation of Nrf2/HO-1 signal with Myricetin for attenuating ECM degradation in human chondrocytes and ameliorating the murine osteoarthritis. Int. Immunopharmacol. 2019, 75, 105742. [Google Scholar] [CrossRef]
- Chen, P.; Ruan, A.M.; Zhou, J.; Huang, L.W.; Zhang, X.Z.; Ma, Y.F.; Wang, Q.F. Cinnamic Aldehyde Inhibits Lipopolysaccharide-Induced Chondrocyte Inflammation and Reduces Cartilage Degeneration by Blocking the Nuclear Factor-Kappa B Signaling Pathway. Front. Pharmacol. 2020, 11, 949. [Google Scholar] [CrossRef]
- Wu, J.; Zhang, X.Y.; Hu, S.Q.; Pan, S.H.; Wang, C.L. Polygonatum sibiricum polysaccharide inhibits IL-1 beta-induced inflammation in human chondrocytes. Food Sci. Technol. 2022, 42, e40421. [Google Scholar] [CrossRef]
- Saha, S.; Buttari, B.; Panieri, E.; Profumo, E.; Saso, L. An Overview of Nrf2 signaling pathway and its role in inflammation. Molecules 2020, 25, 5474. [Google Scholar] [CrossRef]
- Hayes, J.D.; Dinkova-Kostova, A.T.; Tew, K.D. Oxidative Stress in Cancer. Cancer Cell 2020, 38, 167–197. [Google Scholar] [CrossRef]
- Trachootham, D.; Lu, W.; Ogasawara, M.A.; Nilsa, R.D.; Huang, P. Redox regulation of cell survival. Antioxid. Redox Signal 2008, 10, 1343–1374. [Google Scholar] [CrossRef]
- Lismont, C.; Nordgren, M.; Van Veldhoven, P.P.; Fransen, M. Redox interplay between mitochondria and peroxisomes. Front. Cell Dev. Biol. 2015, 3, 35. [Google Scholar] [CrossRef]
- Grange, L.; Nguyen, M.V.; Lardy, B.; Derouazi, M.; Campion, Y.; Trocme, C.; Paclet, M.H.; Gaudin, P.; Morel, F. NAD(P)H oxidase activity of Nox4 in chondrocytes is both inducible and involved in collagenase expression. Antioxid. Redox Signal 2006, 8, 1485–1496. [Google Scholar] [CrossRef] [PubMed]
- van Lent, P.L.; Nabbe, K.C.; Blom, A.B.; Sloetjes, A.; Holthuysen, A.E.; Kolls, J.; Van De Loo, F.A.; Holland, S.M.; Van Den Berg, W.B. NADPH-oxidase-driven oxygen radical production determines chondrocyte death and partly regulates metalloproteinase-mediated cartilage matrix degradation during interferon-gamma-stimulated immune complex arthritis. Arthritis Res. Ther. 2005, 7, R885–R895. [Google Scholar] [CrossRef] [PubMed]
- Altay, M.A.; Erturk, C.; Bilge, A.; Yapti, M.; Levent, A.; Aksoy, N. Evaluation of prolidase activity and oxidative status in patients with knee osteoarthritis: Relationships with radiographic severity and clinical parameters. Rheumatol. Int. 2015, 35, 1725–1731. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Deng, Y.; Tan, Y.; Qin, J.; Chen, L.B. Association between severity of knee osteoarthritis and serum and synovial fluid interleukin 17 concentrations. J. Int. Med. Res. 2014, 42, 138–144. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Ahmed, S.; Islam, N.; Goldberg, V.M.; Haqqi, T.M. Epigallocatechin-3-gallate inhibits interleukin-1beta-induced expression of nitric oxide synthase and production of nitric oxide in human chondrocytes: Suppression of nuclear factor kappaB activation by degradation of the inhibitor of nuclear factor kappaB. Arthritis Rheum. 2002, 46, 2079–2086. [Google Scholar] [CrossRef] [PubMed]
- Ansari, M.Y.; Khan, N.M.; Ahmad, I.; Haqqi, T.M. Parkin clearance of dysfunctional mitochondria regulates ROS levels and increases survival of human chondrocytes. Osteoarthr. Cartil. 2018, 26, 1087–1097. [Google Scholar] [CrossRef]
- Altindag, O.; Erel, O.; Aksoy, N.; Selek, S.; Celik, H.; Karaoglanoglu, M. Increased oxidative stress and its relation with collagen metabolism in knee osteoarthritis. Rheumatol. Int. 2007, 27, 339–344. [Google Scholar] [CrossRef]
- Loeser, R.F.; Collins, J.A.; Diekman, B.O. Ageing and the pathogenesis of osteoarthritis. Nat. Rev. Rheumatol. 2016, 12, 412–420. [Google Scholar] [CrossRef]
- Afonso, V.; Champy, R.; Mitrovic, D.; Collin, P.; Lomri, A. Reactive oxygen species and superoxide dismutases: Role in joint diseases, Joint, bone spine: Revue du rhumatisme. Joint Bone Spine. 2007, 74, 324–329. [Google Scholar] [CrossRef]
- Lepetsos, P.; Papavassiliou, A.G. ROS/oxidative stress signaling in osteoarthritis. Biochim. Biophys. Acta 2016, 1862, 576–591. [Google Scholar] [CrossRef]
- Häuselmann, H.J.; Stefanovic-Racic, M.; Michel, B.A.; Evans, C.H. Differences in nitric oxide production by superficial and deep human articular chondrocytes: Implications for proteoglycan turnover in inflammatory joint diseases. J. Immunol. 1998, 160, 1444–1448. [Google Scholar] [CrossRef]
- Collins, J.A.; Wood, S.T.; Nelson, K.J.; Rowe, M.A.; Carlson, C.S.; Chubinskaya, S.; Poole, L.B.; Furdui, C.M.; Loeser, R.F. Oxidative stress promotes peroxiredoxin hyperoxidation and attenuates pro-survival signaling in aging chondrocytes. J. Biol. Chem. 2016, 291, 6641–6654. [Google Scholar] [CrossRef]
- Englund, M.; Guermazi, A.; Lohmander, L.S. The meniscus in knee osteoarthritis. Rheum. Dis. Clin. North Am. 2009, 35, 579–590. [Google Scholar] [CrossRef]
- Ioan-Facsinay, A.; Kloppenburg, M. An emerging player in knee osteoarthritis: The infrapatellar fat pad. Arthritis Res. Ther. 2013, 15, 225. [Google Scholar] [CrossRef]
- Luo, J.; Mills, K.; le Cessie, S.; Noordam, R.; van Heemst, D. Ageing, age-related diseases and oxidative stress: What to do next? Ageing Res. Rev. 2020, 57, 100982. [Google Scholar] [CrossRef]
- Haubruck, P.; Pinto, M.M.; Moradi, B.; Little, C.B.; Gentek, R. Monocytes, macrophages, and their potential niches in synovial joints-therapeutic targets in post-traumatic osteoarthritis? Front. Immunol. 2021, 12, 763702. [Google Scholar] [CrossRef]
- Zhang, H.; Lin, C.; Zeng, C.; Wang, Z.; Wang, H.; Lu, J.; Bai, X. Synovial macrophage M1 polarisation exacerbates experimental osteoarthritis partially through r-spondin-2. Ann. Rheum. Dis. 2018, 77, 1524–1534. [Google Scholar] [CrossRef]
- Cutolo, M.; Campitiello, R.; Gotelli, E.; Soldano, S. The role of M1/M2 macrophage polarization in rheumatoid arthritis synovitis. Front. Immunol. 2022, 13, 867260. [Google Scholar] [CrossRef]
- Lin, J.B.; Poh, S.; Panitch, A. Controlled release of anti-inflammatory peptides from reducible thermosensitive nanoparticles suppresses cartilage inflammation. Nanomed. 2016, 12, 2095–2100. [Google Scholar] [CrossRef]
- Daghestani, H.N.; Pieper, C.F.; Kraus, V.B. Soluble macrophage biomarkers indicate inflammatory phenotypes in patients with knee osteoarthritis. Arthritis Rheumatol. 2015, 67, 956–965. [Google Scholar] [CrossRef]
- Kawai, T.; Akira, S. The role of pattern-recognition receptors in innate immunity: Update on Toll-like receptors. Nat. Immunol. 2010, 11, 373–384. [Google Scholar] [CrossRef]
- Rosenthal, A.K. Crystals, inflammation, and osteoarthritis. Curr. Opin. Rheumatol. 2011, 23, 170–173. [Google Scholar] [CrossRef]
- Khan, N.M.; Haseeb, A.; Ansari, M.Y.; Devarapalli, P.; Haynie, S.; Haqqi, T.M. Wogonin, a plant derived small molecule, exerts potent anti-inflammatory and chondroprotective effects through the activation of ROS/ERK/Nrf2 signaling pathways in human Osteoarthritis chondrocytes. Free Radic. Biol. Med. 2017, 106, 288–301. [Google Scholar] [CrossRef]
- Kapoor, M.; Martel-Pelletier, J.; Lajeunesse, D.; Pelletier, J.P.; Fahmi, H. Role of proinflammatory cytokines in the pathophysiology of osteoarthritis. Nature Rev. Rheumatol. 2011, 7, 33–42. [Google Scholar] [CrossRef]
- Blom, A.B.; Van Lent, P.L.; Libregts, S.; Holthuysen, A.E.; Van Der Kraan, P.M.; Van Rooijen, N.; Van Den Berg, W.B. Crucial role of macrophages in matrix metalloproteinase-mediated cartilage destruction during experimental osteoarthritis: Involvement of matrix metalloproteinase 3. Arthritis Rheum. 2007, 56, 147–157. [Google Scholar] [CrossRef]
- Daheshia, M.; Yao, J.Q. The interleukin 1beta pathway in the pathogenesis of osteoarthritis. J. Rheumatol. 2008, 35, 2306–2312. [Google Scholar] [CrossRef]
- Haseeb, A.; Chen, D.; Haqqi, T.M. Delphinidin inhibits IL-1beta-induced activation of NF-kappaB by modulating the phosphorylation of IRAK-1(Ser376) in human articular chondrocytes. Rheumatol. 2013, 52, 998–1008. [Google Scholar] [CrossRef]
- Fushimi, K.; Troeberg, L.; Nakamura, H.; Lim, N.H.; Nagase, H. Functional differences of the catalytic and non-catalytic domains in human ADAMTS-4 and ADAMTS-5 in aggrecanolytic activity. J. Biol. Chem. 2008, 283, 6706–6716. [Google Scholar] [CrossRef]
- Tabeian, H.; Betti, B.F.; Dos Santos Cirqueira, C.; de Vries, T.J.; Lobbezoo, F.; Ter Linde, A.V.; Zandieh-Doulabi, B.; Koenders, M.I.; Everts, V.; Bakker, A.D. IL-1beta damages fibrocartilage and upregulates MMP-13 expression in Fibrochondrocytes in the condyle of the temporomandibular joint. Int. J. Mol. Sci. 2019, 20, 2260. [Google Scholar] [CrossRef]
- Thomas, D.P.P.; King, B.; Stephens, T.; Dingle, J.T. In vivo studies of cartilage regeneration after damage induced by catabolin/interleukin-1. Ann. Rheum. Dis. 1991, 50, 75–80. [Google Scholar] [CrossRef]
- Ryu, J.H.; Yang, S.; Shin, Y.; Rhee, J.; Chun, C.H.; Chun, J.S. Interleukin-6 plays an essential role in hypoxia-inducible factor 2alpha-induced experimental osteoarthritic cartilage destruction in mice. Arthritis Rheum. 2011, 63, 2732–2743. [Google Scholar] [CrossRef]
- de Hooge, A.S.; van de Loo, F.A.; Bennink, M.B.; Arntz, O.J.; de Hooge, P.; van den Berg, W.B. Male IL-6 gene knock out mice developed more advanced osteoarthritis upon aging. Osteoarthr. Cartil. 2005, 13, 66–73. [Google Scholar] [CrossRef]
- Akhtar, N.; Haqqi, T.M. Epigallocatechin-3-gallate suppresses the global interleukin-1beta-induced inflammatory response in human chondrocytes. Arthritis Res. Ther. 2011, 13, R93. [Google Scholar] [CrossRef]
- Saklatvala, J. Inflammatory signaling in cartilage: MAPK and NF-kappaB pathways in chondrocytes and the use of inhibitors for research into pathogenesis and therapy of osteoarthritis. Curr. Drug Targets. 2007, 8, 305–313. [Google Scholar] [CrossRef]
- Yin, W.; Park, J.I.; Loeser, R.F. Oxidative stress inhibits insulin-like growth factor-I induction of chondrocyte proteoglycan synthesis through differential regulation of phosphatidylinositol 3-Kinase-Akt and MEK-ERK MAPK signaling pathways. J. Biol. Chem. 2009, 284, 31972–31981. [Google Scholar] [CrossRef]
- Lo, Y.Y.; Cruz, T.F. Involvement of reactive oxygen species in cytokine and growth factor induction of c-fos expression in chondrocytes. J. Biol. Chem. 1995, 270, 11727–11730. [Google Scholar] [CrossRef]
- Lo, Y.Y.; Conquer, J.A.; Grinstein, S.; Cruz, T.F. Interleukin-1 beta induction of c-fos and collagenase expression in articular chondrocytes: Involvement of reactive oxygen species. J. Cell Biochem. 1998, 69, 19–29. [Google Scholar] [CrossRef]
- Latourte, A.; Cherifi, C.; Maillet, J.; Ea, H.K.; Bouaziz, W.; Funck-rentano, T.; Cohen-Solal, M.; Hay, E.; Richette, P. Systemic inhibition of IL-6/Stat3 signalling protects against experimental osteoarthritis. Ann. Rheum. Dis. 2017, 76, 748–755. [Google Scholar] [CrossRef]
- Yammani, R.R. S100 proteins in cartilage: Role in arthritis. Biochim. Biophys. Acta 2012, 1822, 600–606. [Google Scholar] [CrossRef]
- Li, H.; Wang, D.; Yuan, Y.; Min, J. New insights on the MMP-13 regulatory network in the pathogenesis of early osteoarthritis. Arthritis Res. Ther. 2017, 19, 248. [Google Scholar] [CrossRef]
- Amin, A.R.; Islam, A.B. Genomic analysis and differential expression of HMG and S100A family in human arthritis: Upregulated expression of chemokines, IL-8 and nitric oxide by HMGB1. DNA Cell Biol. 2014, 33, 550–565. [Google Scholar] [CrossRef]
- Wang, L.C.; Zhang, H.Y.; Shao, L.; Chen, L.; Liu, Z.H.; He, X.; Gong, W.X. S100A12 levels in synovial fluid may reflect clinical severity in patients with primary knee osteoarthritis. Biomarkers 2013, 18, 216–220. [Google Scholar] [CrossRef]
- van Lent, P.L.; Blom, A.B.; Schelbergen, R.F.; Slöetjes, A.; Lafeber, F.P.; Lems, W.F.; van den Berg, W.B. Active involvement of alarmins S100A8 and S100A9 in the regulation of synovial activation and joint destruction during mouse and human osteoarthritis. Arthritis Rheum. 2012, 64, 1466–1476. [Google Scholar] [CrossRef]
- Cremers, N.A.; van den Bosch, M.H.; van Dalen, S.; Di Ceglie, I.; Ascone, G.; van de Loo, F.; van Lent, P.L. S100A8/ A9 increases the mobilization of pro-inflammatory Ly6C(high) monocytes to the synovium during experimental osteoarthritis. Arthritis Res. Ther. 2017, 19, 217. [Google Scholar] [CrossRef]
- van den Bosch, M.H.; Blom, A.B.; Schelbergen, R.F.; Koenders, M.I.; van de Loo, F.A.; van den Berg, W.B.; van Lent, P.L. Alarmin S100A9 induces proinflammatory and catabolic effects predominantly in the M1 macrophages of human osteoarthritic synovium. J. Rheumatol. 2016, 43, 1874–1884. [Google Scholar] [CrossRef]
- van den Bosch, M.H.; Blom, A.B.; Schelbergen, R.F.; Vogl, T.; Roth, J.P.; Slöetjes, A.W.; van Lent, P.L. Induction of canonical Wnt signaling by the alarmins S100A8/A9 in murine knee joints: Implications for osteoarthritis. Arthritis Rheumatol. 2016, 68, 152–163. [Google Scholar] [CrossRef]
- Kansanen, E.; Kuosmanen, S.M.; Leinonen, H.; Levonen, A. The Keap1-Nrf2pathway: Mechanisms of activation and dysregulation in cancer. Redox Biol. 2013, 1, 45–49. [Google Scholar] [CrossRef]
- Ogura, T.; Tong, K.I.; Mio, K.; Maruyama, Y.; Kurokawa, H.; Sato, C.; Yamamoto, M. Keap1 is a forked-stem dimer structure with two large spheres enclosing the intervening, double glycine repeat, and C-terminal domains. Proc. Natl. Acad. Sci. USA 2010, 107, 2842–2847. [Google Scholar] [CrossRef]
- Krajka-Ku’zniak, V.; Paluszczak, J.; Baer-Dubowska, W. The Nrf2-ARE signaling pathway: An update on its regulation and possible role in cancer prevention and treatment. Pharmacol. Rep. 2017, 69, 393–402. [Google Scholar] [CrossRef]
- Sun, Z.; Chin, Y.E.; Zhang, D.D. Acetylation of Nrf2 by p300/CBP augments promoter-specific DNA binding of Nrf2 during the antioxidant response. Mol. Cell Biol. 2009, 29, 2658–2672. [Google Scholar] [CrossRef]
- Nioi, P.; Nguyen, T.; Sherratt, P.J.; Pickett, C.B. The carboxy-terminal Neh3 domain of Nrf2 is required for transcriptional activation. Mol. Cell Biol. 2005, 25, 10895–10906. [Google Scholar] [CrossRef]
- Rada, P.; Rojo, A.I.; Evrard-Todeschi, N.; Innamorato, N.G.; Cotte, A.; Jaworski, T.; Tobon-Velasco, J.C.; Devijver, H.; Garcia-Mayoral, M.F.; Van Leuven, F.; et al. Structural and functional characterization of Nrf2 degradation by the glycogen synthase kinase 3/beta-TrCP axis. Mol. Cell Biol. 2012, 32, 3486–3499. [Google Scholar] [CrossRef]
- Wang, H.; Liu, K.; Geng, M.; Gao, P.; Wu, X.; Hai, Y.; Li, Y.; Li, Y.; Luo, L.; Hayes, J.D.; et al. RXRalpha inhibits the NRF2-ARE signaling pathway through a direct interaction with the Neh7 domain of NRF2. Cancer Res. 2013, 73, 3097–3108. [Google Scholar] [CrossRef]
- Furukawa, M.; Xiong, Y. BTB protein Keap1 targets antioxidant transcription factor Nrf2 for ubiquitination by the Cullin 3-Roc1 ligase. Mol. Cell Biol. 2005, 25, 162–171. [Google Scholar] [CrossRef]
- Panieri, E.; Pinho, S.A.; Afonso, G.J.M.; Oliveira, P.J.; Cunha-Oliveira, T.; Saso, L. NRF2 and Mitochondrial Function in Cancer and Cancer Stem Cells. Cells 2022, 11, 2401. [Google Scholar] [CrossRef]
- Hayes, J.D.; Dinkova-Kostova, A.T. The Nrf2 regulatory network provides an interface between redox and intermediary metabolism. Trends Biochem. Sci. 2014, 39, 199–218. [Google Scholar] [CrossRef]
- Boutten, A.; Goven, D.; Artaud-Macari, E.; Bonay, M. Protective role of Nrf2 in the lungs against oxidative airway diseases. Med. Sci. 2011, 27, 966–972. [Google Scholar]
- Gan, L.; Johnson, J.A. Oxidative damage and the Nrf2-ARE pathway in neurodegenerative diseases. Biochim. Biophys. Acta 2014, 1842, 1208–1218. [Google Scholar] [CrossRef]
- Howden, R. Nrf2 and cardiovascular defense. Oxid. Med. Cell Longev. 2013, 2013, 104308. [Google Scholar] [CrossRef]
- Brüne, B.; Dehne, N.; Grossmann, N.; Jung, M.; Namgaladze, D.; Schmid, T.; Weigert, A. Redox control of inflammation in macrophages. Antioxid. Redox Signal 2013, 19, 595–637. [Google Scholar] [CrossRef]
- Robledinos-Anton, N.; Fernandez-Gines, R.; Manda, G.; Cuadrado, A. Activators and inhibitors of NRF2: A review of their potential for clinical development. Oxid. Med. Cell Longev. 2019, 2019, 9372182. [Google Scholar] [CrossRef]
- Satoh, T.; Lipton, S. Recent advances in understanding NRF2 as a druggable target: Development of pro-electrophilic and non-covalent NRF2 activators to overcome systemic side effects of electrophilic drugs like dimethyl fumarate. F1000Res 2017, 6, 2138. [Google Scholar] [CrossRef]
- Jiang, Z.Y.; Lu, M.C.; You, Q.D. Discovery and development of Kelch-like ECH-associated protein 1. Nuclear factor erythroid 2-related factor 2 (KEAP1:NRF2) protein-protein interaction inhibitors: Achievements, challenges, and future directions. J. Med. Chem. 2016, 59, 10837–10858. [Google Scholar] [CrossRef] [PubMed]
- Kwon, J.S.; Joung, H.; Kim, Y.S.; Shim, Y.S.; Ahn, Y.; Jeong, M.H.; Kee, H.J. Sulforaphane inhibits restenosis by suppressing inflammation and the proliferation of vascular smooth muscle cells. Atherosclerosis. 2016, 225, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.M.U.; Luo, L.; Namani, A.; Wang, X.J.; Tang, X. Nrf2 signaling pathway: Pivotal roles in inflammation. Biochim. Biophys. Acta (BBA) Mol. Basis Dis. 2017, 1863, 585–597. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.G.; Zhang, Y.Q.; Wu, Z.Z.; Hsieh, C.W.; Chu, C.S.; Wung, B.S. Peanut arachidin-1 enhances Nrf2-mediated protective mechanisms against TNF-α induced ICAM-1 expression and NF-kB activation in endothelial cells. Int. J. Mol. Med. 2018, 41, 541–547. [Google Scholar] [PubMed]
- Koliaraki, V.; Kollias, G. A new role for myeloid HO-1 in the innate to adaptive crosstalk and immune homeostasis. Adv. Exp. Med. Biol. 2011, 780, 101–111. [Google Scholar] [PubMed]
- Kim, S.L.; Choi, H.S.; Ko, Y.C.; Yun, B.S.; Lee, D.S. 5-hydroxymaltol derived from beetroot juice through lactobacillus fermentation suppresses inflammatory effect and oxidant stress via regulating NF-κB, MAPKs pathway and NRF2/HO-1 expression. Antioxidants 2021, 10, 1324. [Google Scholar] [CrossRef]
- Hwang, J.; Jin, J.; Jeon, S.; Moon, S.H.; Park, M.Y.; Yum, D.Y.; Oh, G.T. SOD1 suppresses pro-inflammatory immune responses by protecting against oxidative stress in colitis. Redox Biol. 2020, 37, 101760. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, X.; Ding, Y.; Zhou, W.; Tao, L.; Lu, P.; Hu, R. Nuclear factor E2-related factor-2 negatively regulates NLRP3 inflammasome activity by inhibiting reactive oxygen species-induced NLRP3 priming. Antioxid. Red. Signal 2017, 26, 28–43. [Google Scholar] [CrossRef]
- Kim, M.J.; Jeon, J.H. Recent advances in understanding Nrf2 agonism and its potential clinical application to metabolic and inflammatory diseases. Int. J. Mol. Sci. 2022, 23, 2846. [Google Scholar] [CrossRef]
- Yu, M.; Li, H.; Liu, Q.; Liu, F.; Tang, L.; Li, C.; Yang, X. Nuclear factor p65 interacts with Keap1 to repress the Nrf2-ARE pathway. Cell Sig. 2011, 23, 883–892. [Google Scholar] [CrossRef] [PubMed]
- Cuadrado, A.; Martn-Moldes, Z.; Ye, J.; Lastres-Becker, I. Transcription factors NRF2 and NF-kB are coordinated effectors of the rho family, GTP-binding protein RAC1 during inflammation. J. Biol. Chem. 2014, 289, 15244–15258. [Google Scholar] [CrossRef] [PubMed]
- Ni, L.; Lin, Z.; Hu, S.; Shi, Y.; Jiang, Z.; Zhao, J.; Wang, X. Itaconate attenuates osteoarthritis by inhibiting STING/NF-kB axis in chondrocytes and promoting M2 polarization in macrophages. Biochem. Pharmacol. 2022, 198, 114935. [Google Scholar] [CrossRef] [PubMed]
- Lv, Z.; Xu, X.; Sun, Z.; Yang, Y.X.; Guo, H.; Li, J.; Shi, D. TRPV1 alleviates osteoarthritis by inhibiting M1 macrophage polarization via Ca(2+)/CaMKII/Nrf2 signaling pathway. Cell Death Dis. 2021, 12, 504. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liu, T.; Yang, H.; He, F.; Zhu, X. Melatonin: A novel candidate for the treatment of osteoarthritis. Ageing Res. Rev. 2022, 78, 101635. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Tang, J.; Hu, Y. Dimethyl fumarate protection against collagen II degradation. Biochem. Biophys. Res. Commun. 2014, 454, 257–261. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Zhang, F.; Sun, F.; Gu, K.; Dong, S.; He, D. Dimethyl fumarate for multiple sclerosis. Cochrane Database Syst. Rev. 2015, 4, CD011076. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.J.; Li, D.Y.; Liu, D.Q.; Sun, J.; Zhang, L.Q.; Wu, J.Y.; Song, F.H.; Zhou, Y.Q.; Mei, W. Dimethyl Fumarate Attenuates Pain Behaviors in Osteoarthritis Rats via Induction of Nrf2-Mediated Mitochondrial Biogenesis. Mol. Pain. 2022, 18, 17448069221124920. [Google Scholar] [CrossRef]
- Chen, H.; Qin, J.; Shi, H.; Li, Q.; Zhou, S.; Chen, L. Rhoifolin ameliorates osteoarthritis via the Nrf2/NF-κB axis: In vitro and in vivo experiments. Osteoarthr. Cartil. 2022, 30, 735–745. [Google Scholar] [CrossRef]
- Du, F.; Huang, H.; Cao, Y.; Ran, Y.; Wu, Q.; Chen, B. Notoginsenoside R1 Protects Against High Glucose-Induced Cell Injury Through AMPK/Nrf2 and Downstream HO-1 Signaling. Front. Cell Dev. Biol. 2021, 9, 791643. [Google Scholar] [CrossRef]
- Jiang, C.; Luo, P.; Li, X.; Liu, P.; Li, Y.; Xu, J. Nrf2/ARE is a key pathway for curcumin-mediated protection of TMJ chondrocytes from oxidative stress and inflammation. Cell Stress Chap. 2020, 25, 395–406. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Wang, Y.; Tang, Y.; Lu, H.; Qi, Y.; Li, G.; Sun, H. Quercetin alleviates osteoarthritis progression in rats by suppressing inflammation and apoptosis via inhibition of IRAK1/NLRP3 signaling. J. Inflam. Res. 2021, 14, 3393–3403. [Google Scholar] [CrossRef] [PubMed]
- Qiu, L.; Luo, Y.; Chen, X. Quercetin attenuates mitochondrial dysfunction and biogenesis via upregulated AMPK/SIRT1 signaling pathway in OA rats. BioMed. Pharmacother. 2018, 103, 1585–1591. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Gui, Z.; Zhou, Y.; Xia, L.; Lin, K.; Xu, Y. Quercetin alleviates rat osteoarthritis by inhibiting inflammation and apoptosis of chondrocytes, modulating synovial macrophages polarization to M2 macrophages. Free. Radic. Biol. Med. 2019, 145, 146–160. [Google Scholar] [CrossRef]
- Qu, Y.; Wang, C.; Liu, N.; Gao, C.; Liu, F. Morin Exhibits Anti-Inflammatory Effects on IL-1β-Stimulated Human Osteoarthritis Chondrocytes by Activating the Nrf2 Signaling Pathway. Cell Physiol. Biochem. 2018, 51, 1830–1838. [Google Scholar] [CrossRef]
- Sun, K.; Luo, J.; Jing, X.; Guo, J.; Yao, X.; Hao, X.; Ye, Y.; Liang, S.; Lin, J.; Wang, G.; et al. Astaxanthin protects against osteoarthritis via Nrf2: A guardian of cartilage homeostasis. Aging 2019, 11, 10513–10531. [Google Scholar] [CrossRef]
- Kühn, K.; D’Lima, D.D.; Hashimoto, S.; Lotz, M. Cell death in cartilage. Osteoarthr. Cart. 2004, 12, 1–16. [Google Scholar] [CrossRef]
- Roman-Blas, J.A.; Jimenez, S.A. NF-kappa B as a potential therapeutic target in osteoarthritis and rheumatoid arthritis. Osteoarthr. Cartil. 2006, 14, 839–848. [Google Scholar] [CrossRef]
- Busa, P.; Lee, S.O.; Huang, N.; Kuthati, Y.; Wong, C.S. Carnosine Alleviates Knee Osteoarthritis and Promotes Synoviocyte Protection via Activating the Nrf2/HO-1 Signaling Pathway: An In-Vivo and In-Vitro Study. Antioxidants 2022, 11, 1209. [Google Scholar] [CrossRef]
- Zhang, P.; Wang, X.; Peng, Q.; Jin, Y.; Shi, G.; Fan, Z.; Zhou, Z. Four-octyl itaconate protects chondrocytes against H(2)O(2)-induced oxidative injury and attenuates osteoarthritis progression by activating Nrf2 signaling. Oxid. Med. Cell Longev. 2022, 2022, 2206167. [Google Scholar] [CrossRef]
- Liu, S.C.; Tsai, C.H.; Wang, Y.H.; Su, C.M.; Wu, H.C.; Fong, Y.C.; Tang, C.H. Melatonin abolished proinflammatory factor expression and antagonized osteoarthritis progression in vivo. Cell Death Dis. 2022, 13, 215. [Google Scholar] [CrossRef] [PubMed]
- Mingbao, G.; Jie, J.; Chenghao, R.; Ximiao, C.; Weiyang, G.; Xiangyang, W.; Yaosen, W.; Naifeng, T.; Zongyou, P.; Wu, A.; et al. Akebia Saponin D suppresses inflammation in chondrocytes via the NRF2/HO-1/NF-κB axis and ameliorates osteoarthritis in mice. Food Funct. 2020, 11, 10852–10863. [Google Scholar]
- Zhang, P.; Jin, Y.; Xia, W.; Wang, X.; Zhou, Z. Phillygenin inhibits inflammation in chondrocytes via the Nrf2/NF-κB axis and ameliorates osteoarthritis in mice. J. Orthop. Translat. 2023, 41, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Xie, W.; Huang, D.; Cui, Y.; Yue, J.; He, Q.; Jiang, L.; Xiong, J.; Sun, W.; Yi, Q. Caffeic acid phenethyl ester attenuates osteoarthritis progression by activating NRF2/HO-1 and inhibiting the NF-κB signaling pathway. Int. J. Mol. Med. 2022, 50, 134. [Google Scholar] [CrossRef] [PubMed]
- Elmali, N.; Ayan, I.; Turkoz, Y.; Mizrak, B.; Germen, B.; Bora, A. Effect of caffeic acid phenethyl ester on cartilage in experimental osteoarthritis. Rheumatol. Int. 2002, 22, 222–226. [Google Scholar] [CrossRef]
- Yan, Z.; Qi, W.; Zhan, J.; Lin, Z.; Lin, J.; Xue, X.; Pan, X.; Zhou, Y. Activating Nrf2 signalling alleviates osteoarthritis development by inhibiting inflammasome activation. J. Cell Mol. Med. 2020, 24, 13046–13057. [Google Scholar] [CrossRef]
- Jia, T.; Qiao, J.; Guan, D.; Chen, T. Anti-inflammatory effects of licochalcone A on IL-1beta-stimulated human osteoarthritis chondrocytes. Inflammation 2017, 40, 1894–1902. [Google Scholar] [CrossRef]
- Kong, P.; Chen, G.; Jiang, A.; Wang, Y.; Song, C.; Zhuang, J.; Xi, C.; Wang, G.; Ji, Y.; Yan, J. Sesamin inhibits IL-1β-stimulated inflammatory response in human osteoarthritis chondrocytes by activating Nrf2 signaling pathway. Oncotarget 2016, 7, 83720–83726. [Google Scholar] [CrossRef]
- Xue, E.X.; Lin, J.P.; Zhang, Y.; Sheng, S.R.; Liu, H.X.; Zhou, Y.L.; Xu, H. Pterostilbene inhibits inflammation and ROS production in chondrocytes by activating Nrf2 pathway. Oncotarget 2017, 8, 41988–42000. [Google Scholar] [CrossRef]
- Qiao, Y.Q.; Jiang, P.F.; Gao, Y.Z. Lutein prevents osteoarthritis through Nrf2 activation and downregulation of inflammation. Arch. Med. Sci. 2018, 14, 617–624. [Google Scholar] [CrossRef]
- Yang, G.; Sun, S.; Wang, J.; Li, W.; Wang, X.; Yuan, L.; Li, S. S-Allylmercaptocysteine Targets Nrf2 in Osteoarthritis Treatment Through NOX4/NF-κB Pathway. Drug Des. Devel. Ther. 2020, 14, 4533–4546. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Li, H.; Hu, F.; Luo, P. Stevioside attenuates osteoarthritis via regulating Nrf2/HO-1/NF-κB pathway. J Orthop Translat. 2022, 38, 190–202. [Google Scholar] [CrossRef] [PubMed]
- Dinkova-Kostova, A.T.; Kostov, R.V.; Kazantsev, A.G. The role of Nrf2 signaling in counteracting neurodegenerative diseases. Febs, J. 2018, 285, 3576–3590. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Zhong, H.; Wei, J.; Lin, S.; Zong, Z.; Gong, F.; Huang, X.; Sun, J.; Li, P.; Lin, H.; et al. Inhibition of Nrf2/HO-1 signaling leads to increased activation of the NLRP3 inflammasome in osteoarthritis. Arthritis Res. Ther. 2019, 21, 300. [Google Scholar] [CrossRef] [PubMed]
- Hou, M.; Zhang, Y.; Zhou, X.; Liu, T.; Yang, H.; Chen, X.; He, F.; Zhu, X. Kartogenin prevents cartilage degradation and alleviates osteoarthritis progression in mice via the miR-146a/NRF2 axis. Cell Death Dis. 2021, 12, 483. [Google Scholar] [CrossRef] [PubMed]
- Cheleschi, S.; De Palma, A.; Pascarelli, N.A.; Giordano, N.; Galeazzi, M.; Tenti, S.; Fioravanti, A. Could oxidative stress regulate the expression of MicroRNA-146a and MicroRNA-34a in human osteoarthritic chondrocyte cultures? Int. J. Mol. Sci. 2017, 18, 2660. [Google Scholar] [CrossRef]
- Kwan, J.Y.Y.; Psarianos, P.; Bruce, J.P.; Yip, K.W.; Liu, F.F. The complexity of microRNAs in human cancer. J. Rad. Res. 2016, 57, i106–i111. [Google Scholar] [CrossRef]
- Tao, L.; Zeng, Y.; Wang, J.; Liu, Z.; Shen, B.; Ge, J.; Liu, Y.; Guo, Y.; Qiu, J. Differential microRNA expression in aristolochic acid-induced upper urothelial tract cancers ex vivo. Mol. Med. Rep. 2015, 12, 6533–6546. [Google Scholar] [CrossRef]
- Zhen, Y.; Xinghui, Z.; Chao, W.; Yi, Z.; Jinwen, C.; Ruifang, G.; Chao, Z.; Min, Z.; Chunlei, G.; Yan, F.; et al. Several microRNAs could predict survival in patients with hepatitis B-related liver cancer. Sci. Rep. 2017, 7, 45195. [Google Scholar] [CrossRef]
- Miyaki, S.; Sato, T.; Inoue, A.; Otsuki, S.; Ito, Y.; Yokoyama, S.; Kato, Y.; Takemoto, F.; Nakasa, T.; Yamashita, S.; et al. MicroRNA-140 plays dual roles in both cartilage development and homeostasis. Gene. Dev. 2010, 24, 1173–1185. [Google Scholar] [CrossRef]
- Cheng, Y.; Xiao-Peng, G.; Teng, Z.; Hong, H.; Jie, B. MiR-182-5p Regulates Chondrocyte Inflammation and Oxidative Stress to Promote Osteoarthritis through Targeting the SIRT1/Nrf2 Signaling Pathway. J. Biol. Reg. Homeost. Ag. 2023, 37, 3197–3206. [Google Scholar]
- Deng, Z.; Li, Y.; Liu, H.; Xiao, S.; Li, L.; Tian, J.; Zhang, F. The role of sirtuin 1 and its activator, resveratrol in osteoarthritis. Biosci. Rep. 2019, 39, BSR20190189. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, A.M.; Cheung, W.Y.; Gómez-Aristizábal, A.; Sharma, A.; Nakamura, S.; Chaboureau, A.; Viswanathan, S. Iron nanoparticle-labeled murine mesenchymal stromal cells in an osteoarthritic model persists and suggests anti-inflammatory mechanism of action. PLoS ONE 2019, 14, e0214107. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Huang, X.; Lin, H.; Zhao, M.; Liu, W.; Li, W.; Han, L.; Ma, Q.; Dong, C.; Li, Y.; et al. Adipose mesenchymal stem cell-derived antioxidative extracellular vesicles exhibit anti-oxidative stress and immunomodulatory effects under PM(2.5) exposure. Toxicology 2021, 447, 152627. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saha, S.; Rebouh, N.Y. Anti-Osteoarthritis Mechanism of the Nrf2 Signaling Pathway. Biomedicines 2023, 11, 3176. https://doi.org/10.3390/biomedicines11123176
Saha S, Rebouh NY. Anti-Osteoarthritis Mechanism of the Nrf2 Signaling Pathway. Biomedicines. 2023; 11(12):3176. https://doi.org/10.3390/biomedicines11123176
Chicago/Turabian StyleSaha, Sarmistha, and Nazih Y. Rebouh. 2023. "Anti-Osteoarthritis Mechanism of the Nrf2 Signaling Pathway" Biomedicines 11, no. 12: 3176. https://doi.org/10.3390/biomedicines11123176







