Protective Effect of Topiramate against Diabetic Retinopathy and Computational Approach Recognizing the Role of NLRP3/IL-1β/TNF-α Signaling
Abstract
:1. Introduction
2. Materials and Methods
2.1. Computational Study for Potential Target Prediction of Topiramate Role in Treatment of DREN
2.1.1. Topiramate Predicted Targets
2.1.2. Targets (Genes/Proteins) Involved in Diabetic Retinopathy
2.1.3. Data Analysis and Visualization
2.1.4. Enrichment Analysis
2.2. Chemicals and Drugs
2.3. Biological Study in Mice
2.4. Induction of DM
- Group I (Saline control): Mice received intraperitoneal injections of saline (8 mL/kg).
2.5. ELISA Assay of Glutamate, NLRP3, IL-1β and TNF-α in Retinal Homogenates
2.6. Assessment of Lipid Peroxidation and Reduced Glutathione (GSH)
2.7. Western Blotting for NLRP3 and IL-1β in Retinal Homogenates
2.8. Histological Staining by Hematoxylin–Eosin and Image Analysis
2.9. Immunohistochemistry for Retinal BDNF
2.10. Electron Microscopy
2.11. Statistical Analysis of the Data Sets
3. Results
3.1. Topiramate Predicted Targets
3.2. Targets (Genes/Proteins) Involved in DREN
3.3. Data Analysis and Visualization
3.4. Enrichment Analysis
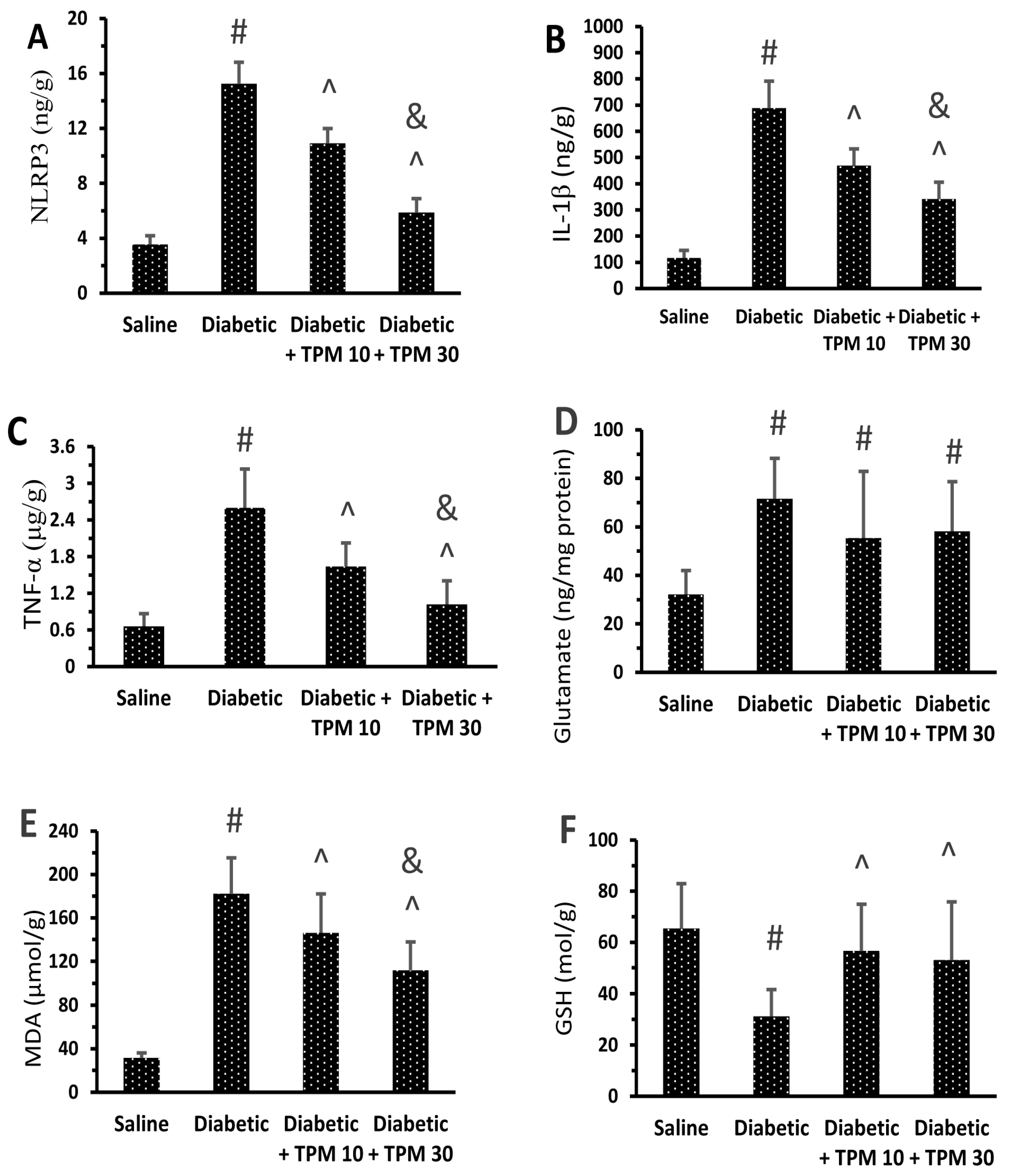
3.5. Topiramate Protected against DREN in Mouse Study
Topiramate Reduced the Level of Retinal Inflammatory Mediators
3.6. Topiramate Protected Retinal Layers and Improved Total Retinal Thickness
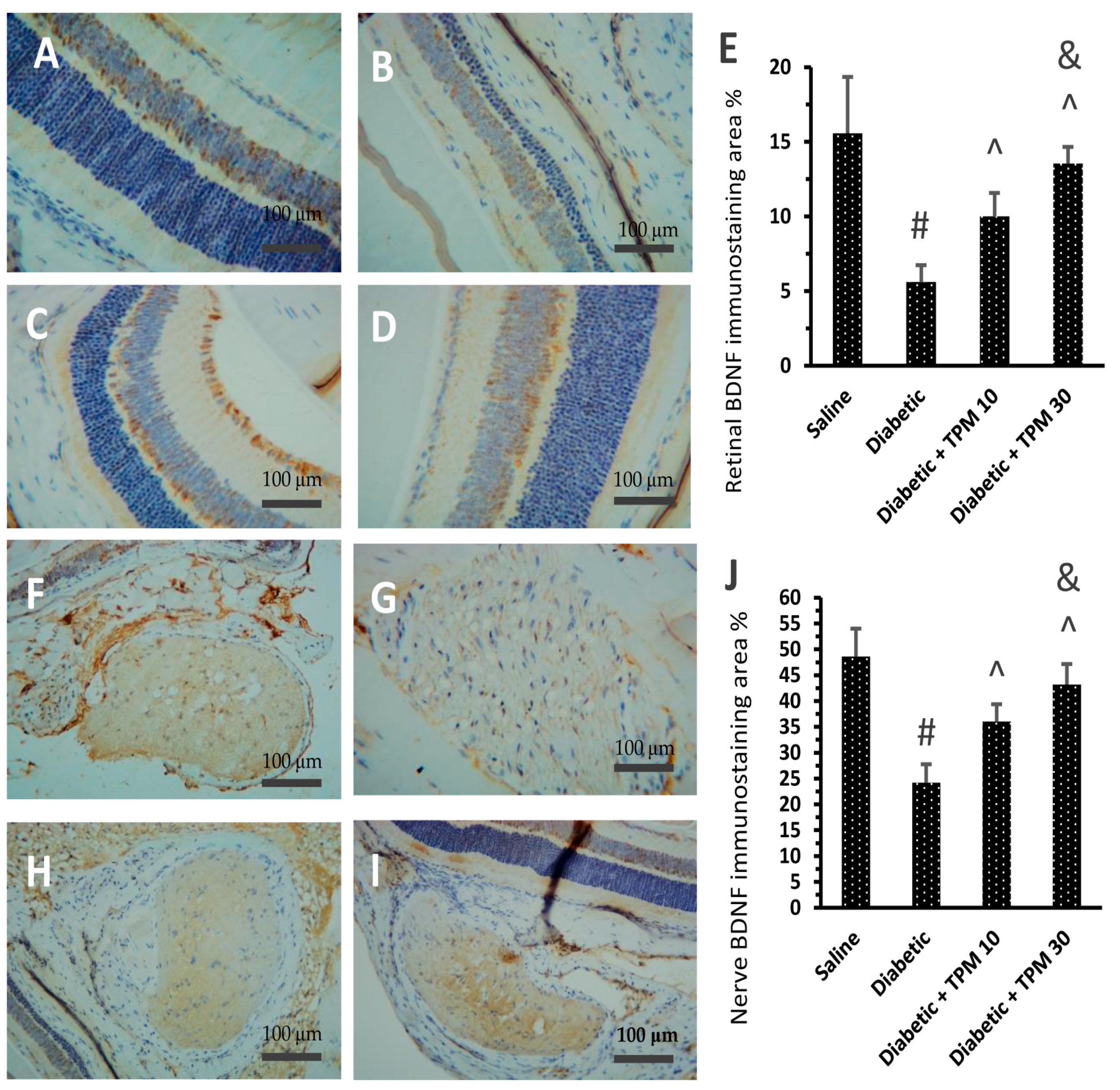
3.7. Topiramate Improved the BDNF Level Diabetic Retinas and Optic Nerves
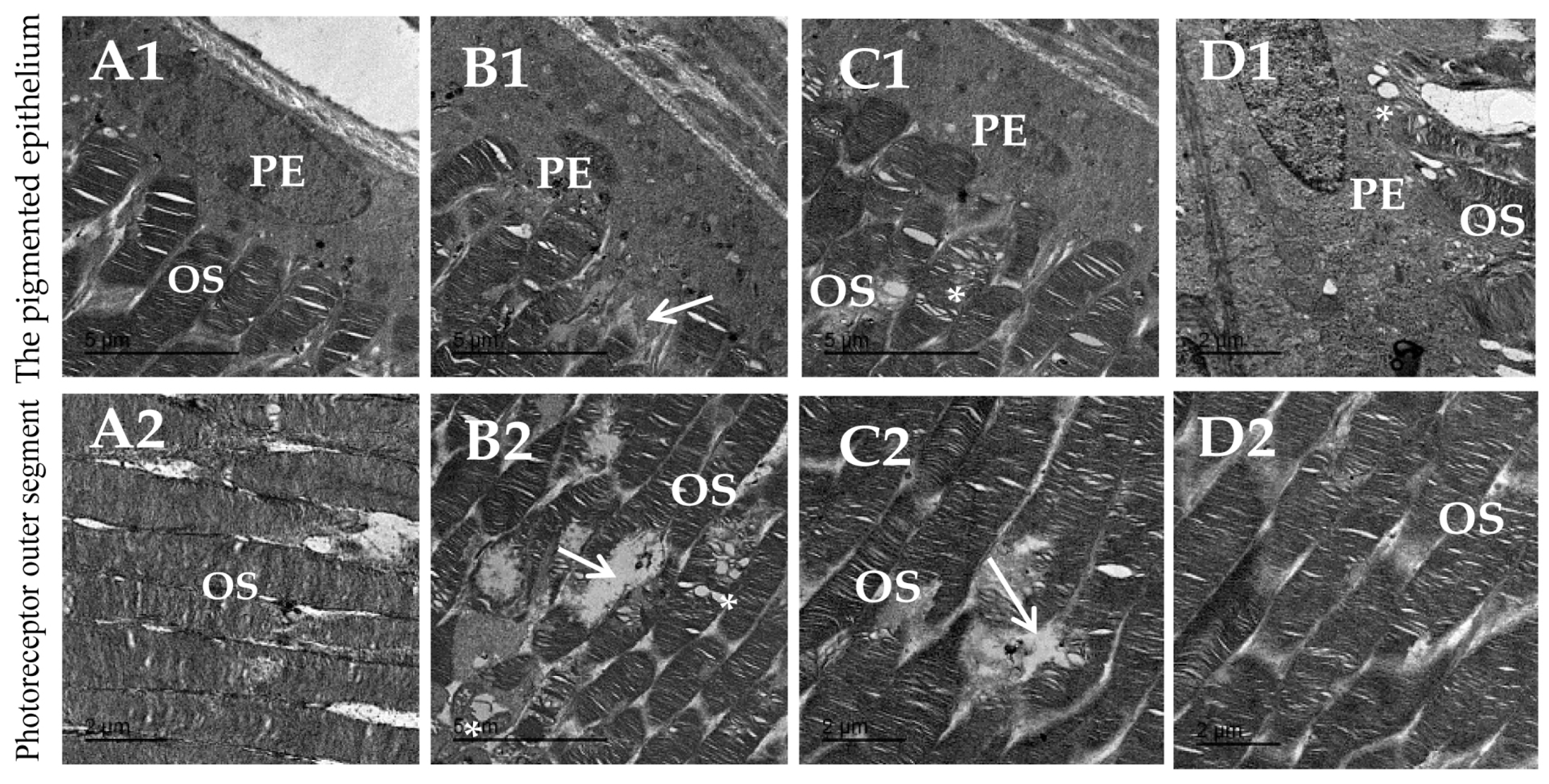
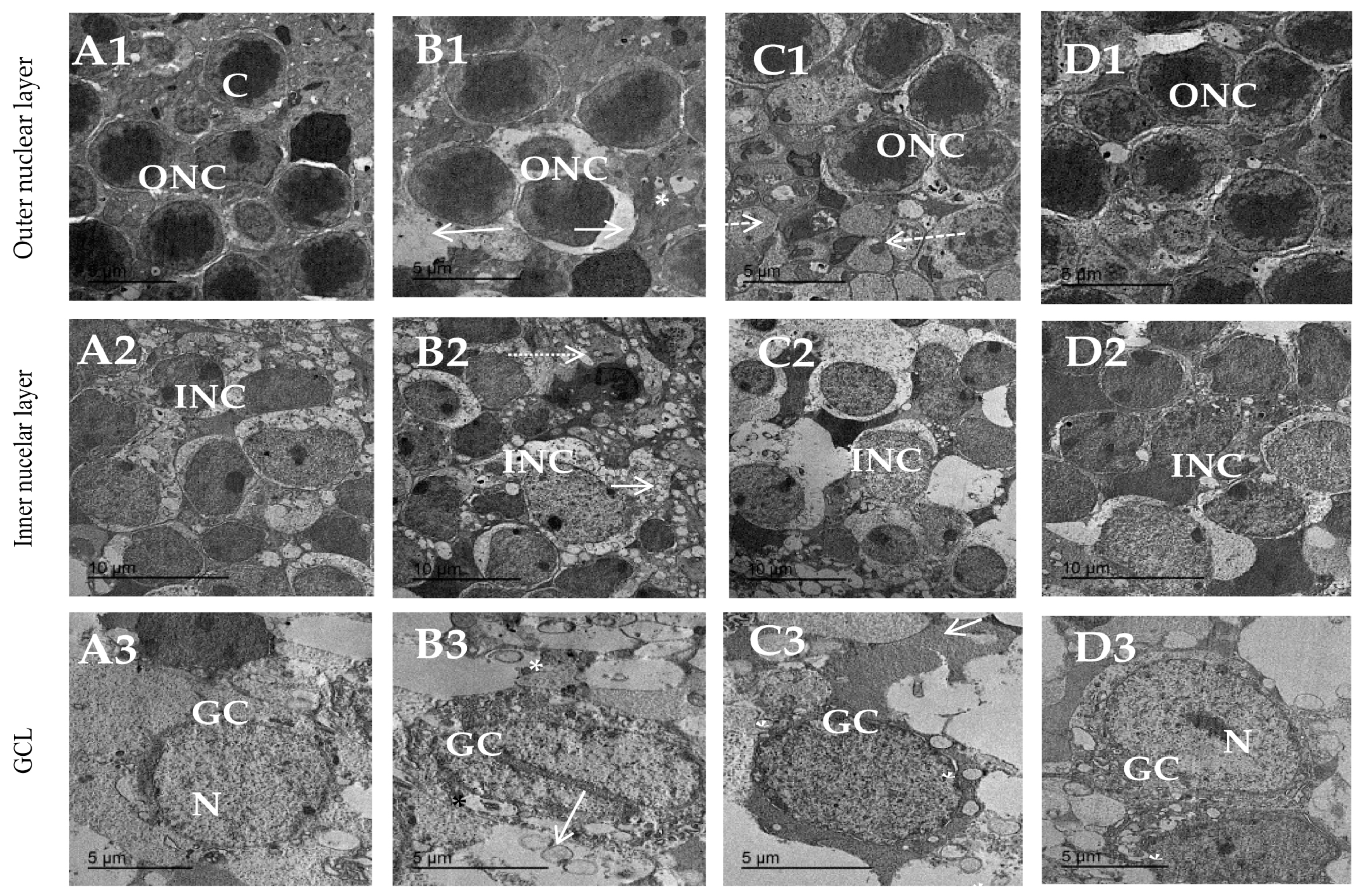
3.8. Topiramate Protected against Ultrastructural Pathology in Diabetic Retinas
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wong, T.Y.; Cheung, C.M.G.; Larsen, M.; Sharma, S.; Simó, R. Diabetic Retinopathy. Nat. Rev. Dis. Primers 2016, 2, 16012. [Google Scholar] [CrossRef]
- Mahajan, N.; Arora, P.; Sandhir, R. Perturbed Biochemical Pathways and Associated Oxidative Stress Lead to Vascular Dysfunctions in Diabetic Retinopathy. Oxidative Med. Cell. Longev. 2019, 2019, 8458472. [Google Scholar] [CrossRef]
- Liu, K.; Gao, X.; Hu, C.; Gui, Y.; Gui, S.; Ni, Q.; Tao, L.; Jiang, Z. Capsaicin Ameliorates Diabetic Retinopathy by Inhibiting Poldip2-Induced Oxidative Stress. Redox Biol. 2022, 56, 102460. [Google Scholar] [CrossRef] [PubMed]
- Price, T.O.; Eranki, V.; Banks, W.A.; Ercal, N.; Shah, G.N. Topiramate Treatment Protects Blood-Brain Barrier Pericytes from Hyperglycemia-Induced Oxidative Damage in Diabetic Mice. Endocrinology 2012, 153, 362–372. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Dong, W.; Lv, Z.; Kong, L.; Ren, X. Thioredoxin-Interacting Protein in Diabetic Retinal Neurodegeneration: A Novel Potential Therapeutic Target for Diabetic Retinopathy. Front. Neurosci. 2022, 16, 957667. [Google Scholar] [CrossRef]
- Yu, X.; Xu, Z.; Mi, M.; Xu, H.; Zhu, J.; Wei, N.; Chen, K.; Zhang, Q.; Zeng, K.; Wang, J.; et al. Dietary Taurine Supplementation Ameliorates Diabetic Retinopathy via Anti-Excitotoxicity of Glutamate in Streptozotocin-Induced Sprague-Dawley Rats. Neurochem. Res. 2008, 33, 500–507. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Dai, J.; Li, L.; Chen, H.; Chai, Y. NLRP3 Inflammasome Expression and Signaling in Human Diabetic Wounds and in High Glucose Induced Macrophages. J. Diabetes Res. 2017, 2017, 5281358. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Ding, S. NLRP3 Inflammasome in Diabetic Cardiomyopathy and Exercise Intervention. Int. J. Mol. Sci. 2021, 22, 13228. [Google Scholar] [CrossRef]
- Murakami, T.; Ockinger, J.; Yu, J.; Byles, V.; McColl, A.; Hofer, A.M.; Horng, T. Critical Role for Calcium Mobilization in Activation of the NLRP3 Inflammasome. Proc. Natl. Acad. Sci. USA. 2012, 109, 11282–11287. [Google Scholar] [CrossRef]
- Weber, K.; Schilling, J.D. Lysosomes Integrate Metabolic-Inflammatory Cross-Talk in Primary Macrophage Inflammasome Activation. J. Biol. Chem. 2014, 289, 9158–9171. [Google Scholar] [CrossRef]
- Kelley, N.; Jeltema, D.; Duan, Y.; He, Y. The NLRP3 Inflammasome: An Overview of Mechanisms of Activation and Regulation. Int. J. Mol. Sci. 2019, 20, 3328. [Google Scholar] [CrossRef]
- Muñoz-Planillo, R.; Kuffa, P.; Martínez-Colón, G.; Smith, B.L.; Rajendiran, T.M.; Núñez, G. K+ Efflux Is the Common Trigger of NLRP3 Inflammasome Activation by Bacterial Toxins and Particulate Matter. Immunity 2013, 38, 1142–1153. [Google Scholar] [CrossRef]
- Cheung, N.; Wong, I.Y.; Wong, T.Y. Ocular Anti-VEGF Therapy for Diabetic Retinopathy: Overview of Clinical Efficacy and Evolving Applications. Diabetes Care 2014, 37, 900–905. [Google Scholar] [CrossRef] [PubMed]
- Fogli, S.; Del Re, M.; Rofi, E.; Posarelli, C.; Figus, M.; Danesi, R. Clinical Pharmacology of Intravitreal Anti-VEGF Drugs. Eye 2018, 32, 1010–1020. [Google Scholar] [CrossRef] [PubMed]
- Santhakumaran, S.; Salimi, A.; Brunetti, V.; Galic, J. Efficacy and Safety of Aflibercept Therapy for Diabetic Macular Edema: A Systematic Review and Meta-Analysis. J. Curr. Ophthalmol. 2022, 34, 133. [Google Scholar] [CrossRef] [PubMed]
- Kiire, C.A.; Morjaria, R.; Rudenko, A.; Fantato, A.; Smith, L.; Smith, A.; Chong, V. Intravitreal Pegaptanib for the Treatment of Ischemic Diabetic Macular Edema. Clin. Ophthalmol. 2015, 9, 2305–2311. [Google Scholar] [CrossRef] [PubMed]
- Devi, T.S.; Yumnamcha, T.; Yao, F.; Somayajulu, M.; Kowluru, R.A.; Singh, L.P. TXNIP Mediates High Glucose-Induced Mitophagic Flux and Lysosome Enlargement in Human Retinal Pigment Epithelial Cells. Biol. Open 2019, 8, bio038521. [Google Scholar] [CrossRef]
- Li, X.; Kover, K.L.; Heruth, D.P.; Watkins, D.J.; Guo, Y.; Moore, W.V.; He, L.G.; Zang, M.; Clements, M.A.; Yan, Y. Thioredoxin-Interacting Protein Promotes High-Glucose-Induced Macrovascular Endothelial Dysfunction. Biochem. Biophys. Res. Commun. 2017, 493, 291–297. [Google Scholar] [CrossRef]
- Abo-Elmatty, D.M.; Zaitone, S.A. Topiramate Induces Weight Loss and Improves Insulin Sensitivity in Dietary Obese Rats: Comparison to Sibutramine. Eur. Rev. Med. Pharmacol. Sci. 2011, 15, 1187–1195. [Google Scholar]
- Cárdenas-Rodríguez, N.; Coballase-Urrutia, E.; Rivera-Espinosa, L.; Romero-Toledo, A.; Sampieri, A.; Ortega-Cuellar, D.; Montesinos-Correa, H.; Floriano-Sánchez, E.; Carmona-Aparicio, L. Modulation of Antioxidant Enzymatic Activities by Certain Antiepileptic Drugs (Valproic Acid, Oxcarbazepine, and Topiramate): Evidence in Humans and Experimental Models. Oxid. Med. Cell. Longev. 2013, 2013, 598493. [Google Scholar] [CrossRef]
- Cárdenas-Rodríguez, N.; Coballase-Urrutia, E.; Huerta-Gertrudis, B.; García-Cruz, M.E.; Pedraza-Chaverri, J.; Coria-Jiménez, R.; Bandala, C.; Ruíz-García, M. Antioxidant Activity of Topiramate: An Antiepileptic Agent. Neurol. Sci. 2013, 34, 741–747. [Google Scholar] [CrossRef] [PubMed]
- Kikumoto, Y.; Sugiyama, H.; Inoue, T.; Morinaga, H.; Takiue, K.; Kitagawa, M.; Fukuoka, N.; Saeki, M.; Maeshima, Y.; Wang, D.-H.; et al. Sensitization to Alloxan-Induced Diabetes and Pancreatic Cell Apoptosis in Acatalasemic Mice. Biochim. Biophys. Acta Mol. Basis Dis. 2010, 1802, 240–246. [Google Scholar] [CrossRef]
- Elsherbiny, N.M.; Abdel-Mottaleb, Y.; Elkazaz, A.Y.; Atef, H.; Lashine, R.M.; Youssef, A.M.; Ezzat, W.; El-Ghaiesh, S.H.; Elshaer, R.E.; El-Shafey, M.; et al. Carbamazepine Alleviates Retinal and Optic Nerve Neural Degeneration in Diabetic Mice via Nerve Growth Factor-Induced PI3K/Akt/mTOR Activation. Front. Neurosci. 2019, 13, 1089. [Google Scholar] [CrossRef]
- Agarwal, N.B.; Agarwal, N.K.; Mediratta, P.K.; Sharma, K.K. Effect of Lamotrigine, Oxcarbazepine and Topiramate on Cognitive Functions and Oxidative Stress in PTZ-Kindled Mice. Seizure 2011, 20, 257–262. [Google Scholar] [CrossRef]
- Liang, Y.; Chen, X.; Osborne, M.; DeCarlo, S.O.; Jetton, T.L.; Demarest, K. Topiramate Ameliorates Hyperglycaemia and Improves Glucose-Stimulated Insulin Release in ZDF Rats and Db/Db Mice. Diabetes Obes. Metab. 2005, 7, 360–369. [Google Scholar] [CrossRef]
- Attia, M.A.; Soliman, N.; Eladl, M.A.; Bilasy, S.E.; El-Abaseri, T.B.; Ali, H.S.; Abbas, F.; Ibrahim, D.; Osman, N.M.S.; Hashish, A.A.; et al. Topiramate Affords Neuroprotection in Diabetic Neuropathy Model via Downregulating Spinal GFAP/Inflammatory Burden and Improving Neurofilament Production. Toxicol. Mech. Methods 2023, 33, 563–577. [Google Scholar] [CrossRef]
- Bari, M.W.; Islam, M.M.; Khatun, M.; Sultana, M.J.; Ahmed, R.; Islam, A.; Hossain, M.I.; Rahman, M.M.; Islam, M.A. Antidiabetic Effect of Wedelia Chinensis Leaf Extract in Alloxan Induced Swiss Albino Diabetic Mice. Clin. Phytosci. 2020, 6, 58. [Google Scholar] [CrossRef]
- Halliwell, B. Reactive Oxygen Species and the Central Nervous System. J. Neurochem. 1992, 59, 1609–1623. [Google Scholar] [CrossRef] [PubMed]
- Ohkawa, H.; Ohishi, N.; Yagi, K. Assay for Lipid Peroxides in Animal Tissues by Thiobarbituric Acid Reaction. Anal. Biochem. 1979, 95, 351–358. [Google Scholar] [CrossRef] [PubMed]
- Beutler, E.; Duron, O.; Kelly, B.M. Improved Method for the Determination of Blood Glutathione. J. Lab. Clin. Med. 1963, 61, 882–888. [Google Scholar]
- Jo, Y.-J.; Sonoda, K.-H.; Oshima, Y.; Takeda, A.; Kohno, R.; Yamada, J.; Hamuro, J.; Yang, Y.; Notomi, S.; Hisatomi, T.; et al. Establishment of a New Animal Model of Focal Subretinal Fibrosis That Resembles Disciform Lesion in Advanced Age-Related Macular Degeneration. Investig. Ophthalmol. Vis. Sci. 2011, 52, 6089. [Google Scholar] [CrossRef]
- Hayat, M.A. Principles and Techniques of Electron Microscopy: Biological Applications, 3rd ed.; CRC Press: Boca Raton, FL, USA, 1989; ISBN 978-0-8493-7111-0. [Google Scholar]
- Hermenean, A.; Trotta, M.C.; Gharbia, S.; Hermenean, A.G.; Peteu, V.E.; Balta, C.; Cotoraci, C.; Gesualdo, C.; Rossi, S.; Gherghiceanu, M.; et al. Changes in Retinal Structure and Ultrastructure in the Aged Mice Correlate With Differences in the Expression of Selected Retinal miRNAs. Front. Pharmacol. 2021, 11, 593514. [Google Scholar] [CrossRef] [PubMed]
- Toplak, H.; Hamann, A.; Moore, R.; Masson, E.; Gorska, M.; Vercruysse, F.; Sun, X.; Fitchet, M.; for the OBDM-002 Study Group. Efficacy and Safety of Topiramate in Combination with Metformin in the Treatment of Obese Subjects with Type 2 Diabetes: A Randomized, Double-Blind, Placebo-Controlled Study. Int. J. Obes. 2007, 31, 138–146. [Google Scholar] [CrossRef]
- Shafik, A.N. Effects of Topiramate on Diabetes Mellitus Induced by Streptozotocin in Rats. Eur. J. Pharmacol. 2012, 684, 161–167. [Google Scholar] [CrossRef] [PubMed]
- Menu, P.; Mayor, A.; Zhou, R.; Tardivel, A.; Ichijo, H.; Mori, K.; Tschopp, J. ER Stress Activates the NLRP3 Inflammasome via an UPR-Independent Pathway. Cell Death Dis. 2012, 3, e261. [Google Scholar] [CrossRef]
- Ali, S.A.; Zaitone, S.A.; Dessouki, A.A.; Ali, A.A. Pregabalin Affords Retinal Neuroprotection in Diabetic Rats: Suppression of Retinal Glutamate, Microglia Cell Expression and Apoptotic Cell Death. Exp. Eye Res. 2019, 184, 78–90. [Google Scholar] [CrossRef] [PubMed]
- Zaitone, S.A.; Alshaman, R.; Alattar, A.; Elsherbiny, N.M.; Abogresha, N.M.; El-Kherbetawy, M.K.; Elaskary, A.A.; Hashish, A.A.; Rashed, L.A.; Ahmed, E. Retinoprotective Effect of Donepezil in Diabetic Mice Involves Mitigation of Excitotoxicity and Activation of PI3K/mTOR/BCl2 Pathway. Life Sci. 2020, 262, 118467. [Google Scholar] [CrossRef]
- ElSayed, M.H.; Elbayoumi, K.S.; Eladl, M.A.; Mohamed, A.A.K.; Hegazy, A.; El-Sherbeeny, N.A.; Attia, M.A.; Hisham, F.A.; Saleh, M.A.K.; Elaskary, A.; et al. Memantine Mitigates ROS/TXNIP/NLRP3 Signaling and Protects against Mouse Diabetic Retinopathy: Histopathologic, Ultrastructural and Bioinformatic Studies. Biomed. Pharmacother. 2023, 163, 114772. [Google Scholar] [CrossRef]
- Yoneda, S.; Tanaka, E.; Goto, W.; Ota, T.; Hara, H. Topiramate Reduces Excitotoxic and Ischemic Injury in the Rat Retina. Brain Res. 2003, 967, 257–266. [Google Scholar] [CrossRef]
- Nguyen, D.; Alavi, M.V.; Kim, K.-Y.; Kang, T.; Scott, R.T.; Noh, Y.H.; Lindsey, J.D.; Wissinger, B.; Ellisman, M.H.; Weinreb, R.N.; et al. A New Vicious Cycle Involving Glutamate Excitotoxicity, Oxidative Stress and Mitochondrial Dynamics. Cell Death Dis. 2011, 2, e240. [Google Scholar] [CrossRef]
- Devi, T.S.; Lee, I.; Hüttemann, M.; Kumar, A.; Nantwi, K.D.; Singh, L.P. TXNIP Links Innate Host Defense Mechanisms to Oxidative Stress and Inflammation in Retinal Muller Glia under Chronic Hyperglycemia: Implications for Diabetic Retinopathy. Exp. Diabetes Res. 2012, 2012, 438238. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Zhao, M.; Zhao, S.; Lu, Q.; Ni, L.; Zou, C.; Lu, L.; Xu, X.; Guan, H.; Zheng, Z.; et al. Activation of the TXNIP/NLRP3 Inflammasome Pathway Contributes to Inflammation in Diabetic Retinopathy: A Novel Inhibitory Effect of Minocycline. Inflamm. Res. 2017, 66, 157–166. [Google Scholar] [CrossRef] [PubMed]
- Pan, W.W.; Lin, F.; Fort, P.E. The Innate Immune System in Diabetic Retinopathy. Prog. Retin. Eye Res. 2021, 84, 100940. [Google Scholar] [CrossRef] [PubMed]
- Raman, K.S.; Matsubara, J.A. Dysregulation of the NLRP3 Inflammasome in Diabetic Retinopathy and Potential Therapeutic Targets. Ocul. Immunol. Inflamm. 2022, 30, 470–478. [Google Scholar] [CrossRef]
- Forrester, J.V.; Kuffova, L.; Delibegovic, M. The Role of Inflammation in Diabetic Retinopathy. Front. Immunol. 2020, 11, 583687. [Google Scholar] [CrossRef]
- Tang, J.; Kern, T.S. Inflammation in Diabetic Retinopathy. Prog. Retin. Eye Res. 2011, 30, 343–358. [Google Scholar] [CrossRef]
- Rübsam, A.; Parikh, S.; Fort, P. Role of Inflammation in Diabetic Retinopathy. Int. J. Mol. Sci. 2018, 19, 942. [Google Scholar] [CrossRef]
- Abu El-Asrar, A. Role of Inflammation in the Pathogenesis of Diabetic Retinopathy. Middle East Afr. J. Ophthalmol. 2012, 19, 70–74. [Google Scholar] [CrossRef]
- dell’Omo, R.; Semeraro, F.; Bamonte, G.; Cifariello, F.; Romano, M.R.; Costagliola, C. Vitreous Mediators in Retinal Hypoxic Diseases. Mediat. Inflamm. 2013, 2013, 935301. [Google Scholar] [CrossRef]
- Andrzejczak, D.; Woldan-Tambor, A.; Bednarska, K.; Zawilska, J.B. The Effects of Topiramate on Lipopolysaccharide (LPS)-Induced Proinflammatory Cytokine Release from Primary Rat Microglial Cell Cultures. Epilepsy Res. 2016, 127, 352–357. [Google Scholar] [CrossRef]
- Motaghinejad, M.; Motevalian, M.; Fatima, S. Mediatory Role of NMDA, AMPA/Kainate, GABA A and Alpha 2 Receptors in Topiramate Neuroprotective Effects against Methylphenidate Induced Neurotoxicity in Rat. Life Sci. 2017, 179, 37–53. [Google Scholar] [CrossRef] [PubMed]
- Narin, F.; Hanalioglu, S.; Ustun, H.; Kilinc, K.; Bilginer, B. Topiramate as a Neuroprotective Agent in a Rat Model of Spinal Cord Injury. Neural Regen. Res. 2017, 12, 2071. [Google Scholar] [CrossRef] [PubMed]
- Jafari, A.; Andishfar, N.; Esmaeilzadeh, Z.; Khezri, M.R.; Ghasemnejad-Berenji, M. Gastroprotective Effect of Topiramate on Indomethacin-induced Peptic Ulcer in Rats: Biochemical and Histological Analyses. Basic Clin. Pharmacol. Toxicol. 2022, 130, 559–568. [Google Scholar] [CrossRef] [PubMed]
- Wal, P.; Dwivedi, J.; Wal, A.; Vig, H.; Singh, Y. Detailed Insight into the Pathophysiology and the Behavioral Complications Associated with the Parkinson’s Disease and Its Medications. Future J. Pharm. Sci. 2022, 8, 33. [Google Scholar] [CrossRef]
- Ishikawa, M. Abnormalities in Glutamate Metabolism and Excitotoxicity in the Retinal Diseases. Scientifica 2013, 2013, 528940. [Google Scholar] [CrossRef]
- Ola, M.S.; Alhomida, A.S.; LaNoue, K.F. Gabapentin Attenuates Oxidative Stress and Apoptosis in the Diabetic Rat Retina. Neurotox. Res. 2019, 36, 81–90. [Google Scholar] [CrossRef] [PubMed]
- Ola, M.; Nawaz, M.; Khan, H.; Alhomida, A. Neurodegeneration and Neuroprotection in Diabetic Retinopathy. Int. J. Mol. Sci. 2013, 14, 2559–2572. [Google Scholar] [CrossRef]
- Whitmire, W.; Al-Gayyar, M.M.; Abdelsaid, M.; Yousufzai, B.K.; El-Remessy, A.B. Alteration of Growth Factors and Neuronal Death in Diabetic Retinopathy: What We Have Learned so Far. Mol. Vis. 2011, 17, 300–308. [Google Scholar]
- Shank, R.P.; Maryanoff, B.E. Molecular Pharmacodynamics, Clinical Therapeutics, and Pharmacokinetics of Topiramate. CNS Neurosci. Ther. 2008, 14, 120–142. [Google Scholar] [CrossRef]
- Gensel, J.C.; Tovar, C.A.; Bresnahan, J.C.; Beattie, M.S. Topiramate Treatment Is Neuroprotective and Reduces Oligodendrocyte Loss after Cervical Spinal Cord Injury. PLoS ONE 2012, 7, e33519. [Google Scholar] [CrossRef]
- Platania, C.B.M.; Maisto, R.; Trotta, M.C.; D’Amico, M.; Rossi, S.; Gesualdo, C.; D’Amico, G.; Balta, C.; Herman, H.; Hermenean, A.; et al. Retinal and Circulating Mi RNA Expression Patterns in Diabetic Retinopathy: An in Silico and in Vivo Approach. Br. J. Pharmacol. 2019, 176, 2179–2194. [Google Scholar] [CrossRef]
- Jun, Y.H.; Kim, S.T. Brain-Derived Neurotrophic Factor in Non-Proliferative Diabetic Retinopathy with Diabetic Macular Edema. Eur. J. Ophthalmol. 2021, 31, 1915–1919. [Google Scholar] [CrossRef] [PubMed]
- Trotta, M.C.; Maisto, R.; Guida, F.; Boccella, S.; Luongo, L.; Balta, C.; D’Amico, G.; Herman, H.; Hermenean, A.; Bucolo, C.; et al. The Activation of Retinal HCA2 Receptors by Systemic Beta-Hydroxybutyrate Inhibits Diabetic Retinal Damage through Reduction of Endoplasmic Reticulum Stress and the NLRP3 Inflammasome. PLoS ONE 2019, 14, e0211005. [Google Scholar] [CrossRef]
- Afarid, M.; Namvar, E.; Sanie-Jahromi, F. Diabetic Retinopathy and BDNF: A Review on Its Molecular Basis and Clinical Applications. J. Ophthalmol. 2020, 2020, 1602739. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Creson, T.K.; Wu, L.-J.; Ren, M.; Gray, N.A.; Falke, C.; Wei, Y.; Wang, Y.; Blumenthal, R.; Machado-Vieira, R.; et al. The Role of Hippocampal GluR1 and GluR2 Receptors in Manic-Like Behavior. J. Neurosci. 2008, 28, 68–79. [Google Scholar] [CrossRef]
- Reagan-Shaw, S.; Nihal, M.; Ahmad, N. Dose Translation from Animal to Human Studies Revisited. FASEB J. 2008, 22, 659–661. [Google Scholar] [CrossRef] [PubMed]
- Kubera, M.; Budziszewska, B.; Jaworska-Feil, L.; Basta-Kaim, A.; Leśkiewicz, M.; Tetich, M.; Maes, M.; Kenis, G.; Marciniak, A.; Czuczwar, S.J.; et al. Effect of Topiramate on the Kainate-Induced Status Epilepticus, Lipid Peroxidation and Immunoreactivity of Rats. Pol. J. Pharmacol. 2004, 56, 553–561. [Google Scholar]
- Nazıroğlu, M.; Kutluhan, S.; Yılmaz, M. Selenium and Topiramate Modulates Brain Microsomal Oxidative Stress Values, Ca2+-ATPase Activity, and EEG Records in Pentylentetrazol-Induced Seizures in Rats. J. Membr. Biol. 2008, 225, 39–49. [Google Scholar] [CrossRef]
- Nazıroğlu, M.; Kutluhan, S.; Uğuz, A.C.; Çelik, Ö.; Bal, R.; Butterworth, P.J. Topiramate and Vitamin E Modulate the Electroencephalographic Records, Brain Microsomal and Blood Antioxidant Redox System in Pentylentetrazol-Induced Seizure of Rats. J. Membr. Biol. 2009, 229, 131–140. [Google Scholar] [CrossRef]
- Shen, H.; Wang, J.; Jiang, D.; Xu, P.; Zhu, X.; Zhang, Y.; Yu, X.; Won, M.-H.; Su, P.Q.; Yan, B.C. Topiramate Improves Neuroblast Differentiation of Hippocampal Dentate Gyrus in the D-Galactose-Induced Aging Mice via Its Antioxidant Effects. Cell Mol. Neurobiol. 2017, 37, 869–877. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohammad, H.M.F.; Eladl, M.A.; Abdelmaogood, A.K.K.; Elshaer, R.E.; Ghanam, W.; Elaskary, A.; Saleh, M.A.K.; Eltrawy, A.H.; Ali, S.K.; Moursi, S.M.M.; et al. Protective Effect of Topiramate against Diabetic Retinopathy and Computational Approach Recognizing the Role of NLRP3/IL-1β/TNF-α Signaling. Biomedicines 2023, 11, 3202. https://doi.org/10.3390/biomedicines11123202
Mohammad HMF, Eladl MA, Abdelmaogood AKK, Elshaer RE, Ghanam W, Elaskary A, Saleh MAK, Eltrawy AH, Ali SK, Moursi SMM, et al. Protective Effect of Topiramate against Diabetic Retinopathy and Computational Approach Recognizing the Role of NLRP3/IL-1β/TNF-α Signaling. Biomedicines. 2023; 11(12):3202. https://doi.org/10.3390/biomedicines11123202
Chicago/Turabian StyleMohammad, Hala M. F., Mohamed Ahmed Eladl, Asmaa K. K. Abdelmaogood, Rabie E. Elshaer, Walaa Ghanam, Abdelhakeem Elaskary, Mohamed A. K. Saleh, Amira H. Eltrawy, Sahar K. Ali, Suzan M. M. Moursi, and et al. 2023. "Protective Effect of Topiramate against Diabetic Retinopathy and Computational Approach Recognizing the Role of NLRP3/IL-1β/TNF-α Signaling" Biomedicines 11, no. 12: 3202. https://doi.org/10.3390/biomedicines11123202






