In Vivo CaV3 Channel Inhibition Promotes Maturation of Glucose-Dependent Ca2+ Signaling in Human iPSC-Islets
Abstract
:1. Introduction
2. Materials and Methods
2.1. In Vitro Cultivation and Differentiation of HiPSCs
2.2. Animals
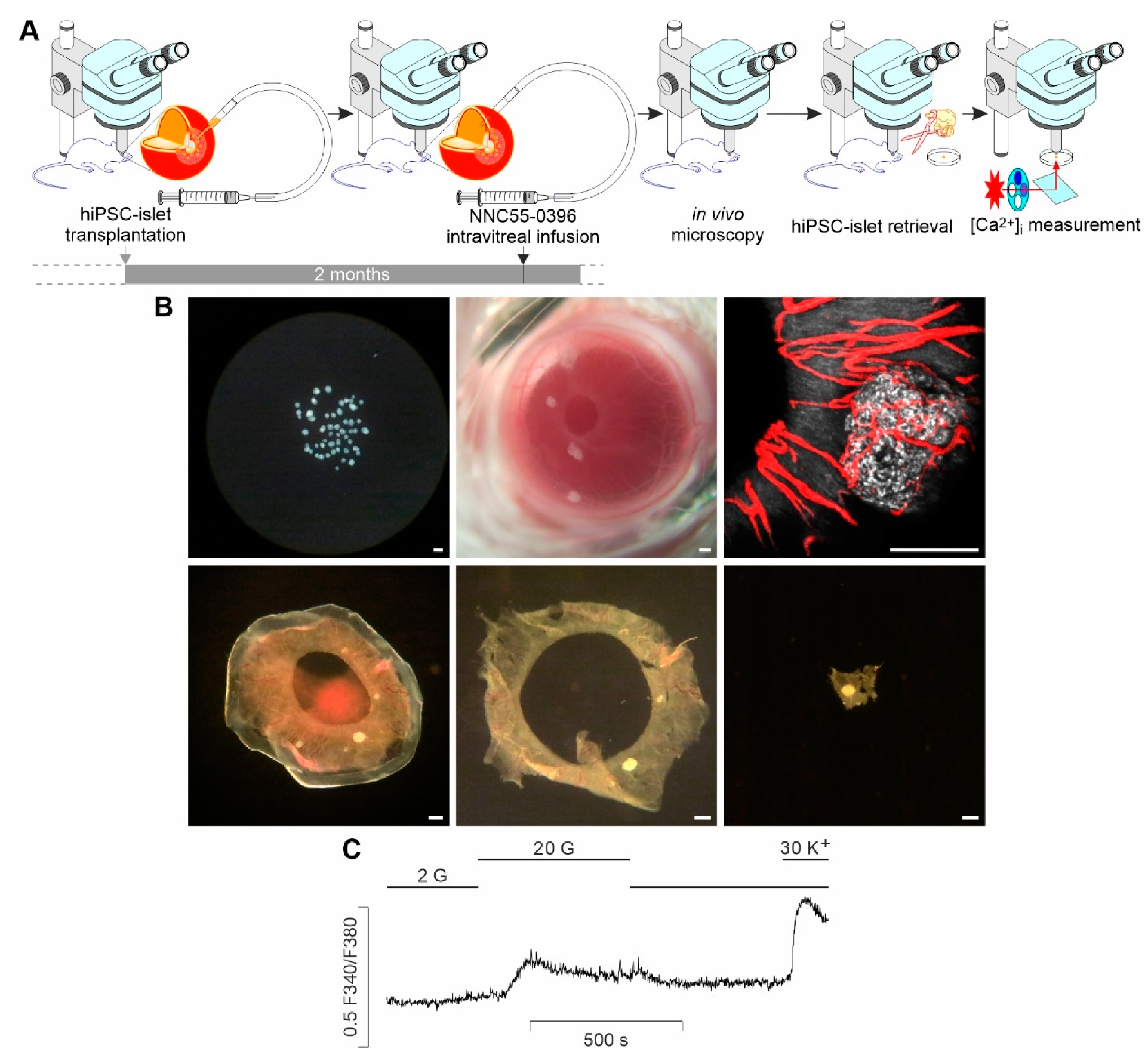
2.3. ACE Transplantation
2.4. Intravitreal Infusion
2.5. In Vivo Confocal Microscopy
2.6. Intraocular HiPSC-Islet Graft Retrieval
2.7. Ex Vivo [Ca2+]i Measurements
2.8. Cultivation of Dispersed Cells of Intraocular HiPSC-Islet Grafts
2.9. Patch Clamp Recordings
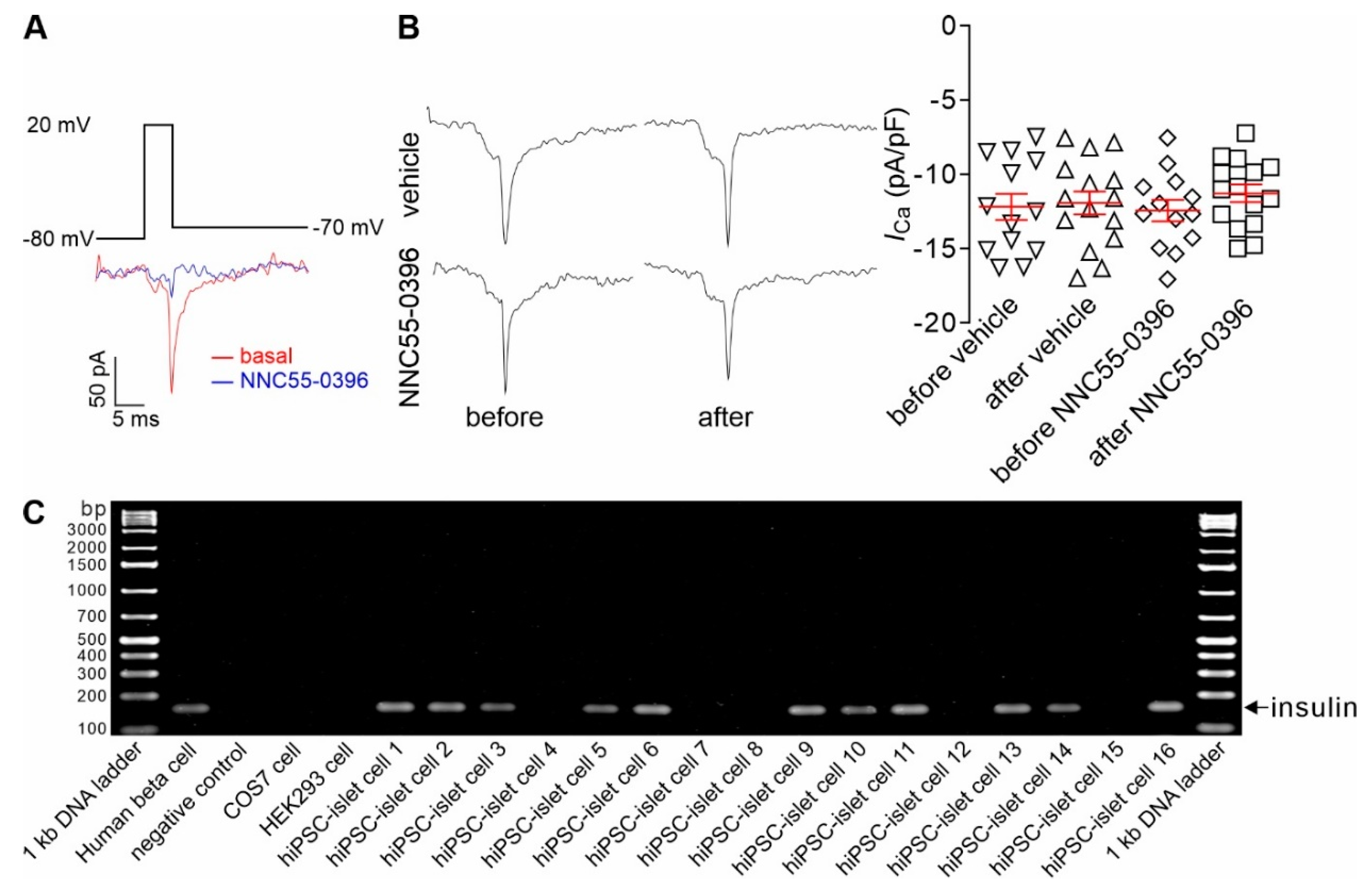
2.10. Single-Cell RT-PCR
2.11. Data Analysis
3. Results
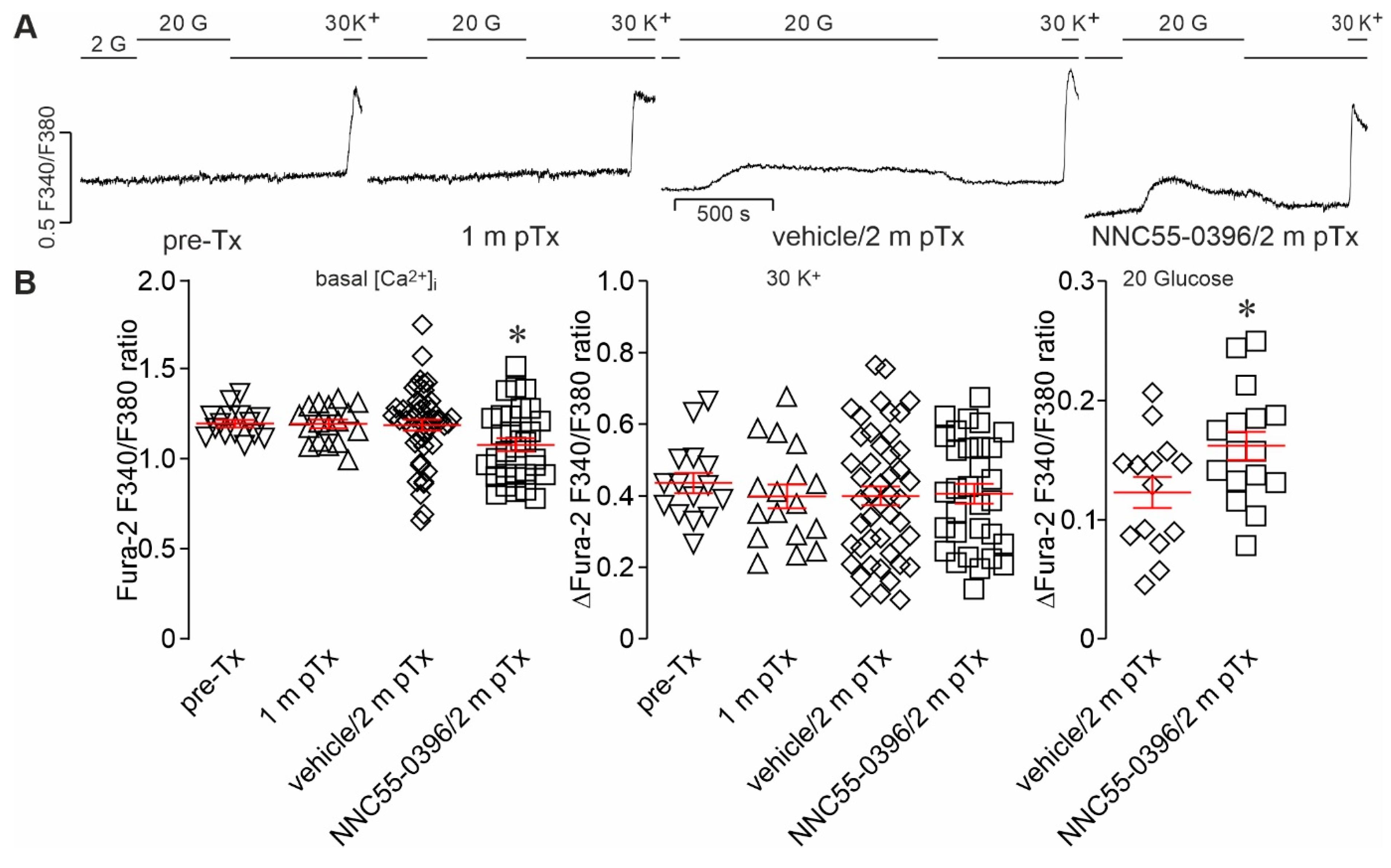
3.1. Ex Vivo Measurements of Glucose-Activated [Ca2+]i Dynamics in Single, Intact HiPSC-Islet Grafts Retrieved from the ACE following In Vivo Local Treatment
3.2. Intravitreal Infusion of NNC55-0396 Promotes Glucose-Activated Ca2+ Signaling in Intact HiPSC-Islet Grafts Harvested from the ACE
3.3. CaV3 Currents Are Present in Insulin-Expressing Cells of Intraocular HiPSC-Islet Grafts and Remain Unaltered after Intravitreal Infusion of NNC55-0396
3.4. Intravitreal Infusion of NNC55-0396 Has No Influence on Vascularization and Light Backscattering of Intraocular HiPSC-Islet Grafts
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pagliuca, F.W.; Millman, J.R.; Gurtler, M.; Segel, M.; Van Dervort, A.; Ryu, J.H.; Peterson, Q.P.; Greiner, D.; Melton, D.A. Generation of functional human pancreatic β cells in vitro. Cell 2014, 159, 428–439. [Google Scholar] [CrossRef] [PubMed]
- Millman, J.R.; Xie, C.; Van Dervort, A.; Gurtler, M.; Pagliuca, F.W.; Melton, D.A. Generation of stem cell-derived β-cells from patients with type 1 diabetes. Nat. Commun. 2016, 7, 11463. [Google Scholar] [CrossRef]
- Rezania, A.; Bruin, J.E.; Arora, P.; Rubin, A.; Batushansky, I.; Asadi, A.; O’Dwyer, S.; Quiskamp, N.; Mojibian, M.; Albrecht, T.; et al. Reversal of diabetes with insulin-producing cells derived in vitro from human pluripotent stem cells. Nat. Biotechnol. 2014, 32, 1121–1133. [Google Scholar] [CrossRef] [PubMed]
- Lanzoni, G.; Ricordi, C. Transplantation of stem cell-derived pancreatic islet cells. Nat. Rev. Endocrinol. 2021, 17, 7–8. [Google Scholar] [CrossRef] [PubMed]
- Zhao, K.; Shi, Y.; Yu, J.; Yu, L.; Mael, A.; Li, Y.; Kolton, A.; Joyce, T.; Odorico, J.; Berggren, P.O.; et al. Intracameral microimaging of maturation of human iPSC derivatives into islet endocrine cells. Cell Transplant. 2022, 31, 9636897211066508. [Google Scholar] [CrossRef] [PubMed]
- Balboa, D.; Barsby, T.; Lithovius, V.; Saarimaki-Vire, J.; Omar-Hmeadi, M.; Dyachok, O.; Montaser, H.; Lund, P.E.; Yang, M.; Ibrahim, H.; et al. Functional, metabolic and transcriptional maturation of human pancreatic islets derived from stem cells. Nat. Biotechnol. 2022, 40, 1042–1055. [Google Scholar] [CrossRef] [PubMed]
- Bruin, J.E.; Rezania, A.; Kieffer, T.J. Replacing and safeguarding pancreatic β cells for diabetes. Sci. Transl. Med. 2015, 7, 316ps23. [Google Scholar] [CrossRef]
- Hrvatin, S.; O’Donnell, C.W.; Deng, F.; Millman, J.R.; Pagliuca, F.W.; DiIorio, P.; Rezania, A.; Gifford, D.K.; Melton, D.A. Differentiated human stem cells resemble fetal, not adult, β cells. Proc. Natl. Acad. Sci. USA 2014, 111, 3038–3043. [Google Scholar] [CrossRef]
- Shahjalal, H.M.; Abdal Dayem, A.; Lim, K.M.; Jeon, T.I.; Cho, S.G. Generation of pancreatic β cells for treatment of diabetes: Advances and challenges. Stem Cell Res. Ther. 2018, 9, 355. [Google Scholar] [CrossRef]
- Dadheech, N.; Shapiro, A.M.J. Human induced pluripotent stem cells in the curative treatment of diabetes and potential impediments ahead. Adv. Exp. Med. Biol. 2019, 1144, 25–35. [Google Scholar]
- Miura, K.; Okada, Y.; Aoi, T.; Okada, A.; Takahashi, K.; Okita, K.; Nakagawa, M.; Koyanagi, M.; Tanabe, K.; Ohnuki, M.; et al. Variation in the safety of induced pluripotent stem cell lines. Nat. Biotechnol. 2009, 27, 743–745. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.N.; Shi, Y.; Yang, G.; Li, Y.; Yu, J.; Berggren, P.O. Ionic mechanisms in pancreatic β cell signaling. Cell. Mol. Life Sci. 2014, 71, 4149–4177. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.N.; Berggren, P.O. β-Cell CaV channel regulation in physiology and pathophysiology. Am. J. Physiol. Endocrinol. Metab. 2005, 288, E16–E28. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.N.; Berggren, P.O. CaV2.3 channel and PKCλ: New players in insulin secretion. J. Clin. Investig. 2005, 115, 16–20. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.N.; Berggren, P.O. The role of voltage-gated calcium channels in pancreatic β-cell physiology and pathophysiology. Endocr. Rev. 2006, 27, 621–676. [Google Scholar] [CrossRef] [PubMed]
- Salinno, C.; Cota, P.; Bastidas-Ponce, A.; Tarquis-Medina, M.; Lickert, H.; Bakhti, M. β-Cell maturation and identity in health and disease. Int. J. Mol. Sci. 2019, 20, 5417. [Google Scholar] [CrossRef]
- Barsby, T.; Otonkoski, T. Maturation of beta cells: Lessons from in vivo and in vitro models. Diabetologia 2022, 65, 917–930. [Google Scholar] [CrossRef]
- Yang, S.N.; Shi, Y.; Zhao, K.; Yang, G.; Yu, J.; Berggren, P.O. Pancreatic β cell CaV channels in health and disease. In Voltage-Gated Calcium Channels; Zamponi, G.W., Weiss, N., Eds.; Springer: Cham, Switzerland, 2022; pp. 425–448. [Google Scholar]
- Zhao, K.; Shi, Y.; Yu, J.; Yu, L.; Mael, A.; Kolton, A.; Joyce, T.; Odorico, J.; Berggren, P.O.; Yang, S.N. Intravital microimaging of human iPSC-derived surrogate islets in the anterior chamber of the eye. Diabetologia 2020, 63, S123. [Google Scholar]
- Inagaki, E.; Arai, E.; Hatou, S.; Sayano, T.; Taniguchi, H.; Negishi, K.; Kanai, Y.; Sato, Y.; Okano, H.; Tsubota, K.; et al. The anterior eye chamber as a visible medium for in vivo tumorigenicity tests. Stem Cells Transl. Med. 2022, 11, 841–849. [Google Scholar] [CrossRef]
- Zhu, W.; Gramlich, O.W.; Laboissonniere, L.; Jain, A.; Sheffield, V.C.; Trimarchi, J.M.; Tucker, B.A.; Kuehn, M.H. Transplantation of iPSC-derived TM cells rescues glaucoma phenotypes in vivo. Proc. Natl Acad. Sci. USA 2016, 113, E3492–E3500. [Google Scholar] [CrossRef]
- Zhu, W.; Jain, A.; Gramlich, O.W.; Tucker, B.A.; Sheffield, V.C.; Kuehn, M.H. Restoration of aqueous humor outflow following transplantation of iPSC-derived trabecular meshwork cells in a transgenic mouse model of glaucoma. Investig. Ophthalmol. Vis. Sci. 2017, 58, 2054–2062. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.J.; Wang, Y.J.; Zhang, M.; Zhang, P.; Liang, H.; Bai, H.J.; Yu, X.J.; Yang, H.T. Functional expression of the Ca2+ signaling machinery in human embryonic stem cells. Acta Pharmacol. Sin. 2017, 38, 1663–1672. [Google Scholar] [CrossRef] [PubMed]
- Lory, P.; Bidaud, I.; Chemin, J. T-type calcium channels in differentiation and proliferation. Cell Calcium 2006, 40, 135–146. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Gomez, J.A.; Levitsky, K.L.; Lopez-Barneo, J. T-type Ca2+ channels in mouse embryonic stem cells: Modulation during cell cycle and contribution to self-renewal. Am. J. Physiol. Cell Physiol. 2012, 302, C494–C504. [Google Scholar] [CrossRef]
- Braun, M.; Ramracheya, R.; Bengtsson, M.; Zhang, Q.; Karanauskaite, J.; Partridge, C.; Johnson, P.R.; Rorsman, P. Voltage-gated ion channels in human pancreatic β-cells: Electrophysiological characterization and role in insulin secretion. Diabetes 2008, 57, 1618–1628. [Google Scholar] [CrossRef]
- Yu, J.; Shi, Y.; Zhao, K.; Yang, G.; Yu, L.; Li, Y.; Andersson, E.M.; Ammala, C.; Yang, S.N.; Berggren, P.O. Enhanced expression of β cell CaV3.1 channels impairs insulin release and glucose homeostasis. Proc. Natl. Acad. Sci. USA 2020, 117, 448–453. [Google Scholar] [CrossRef]
- Zhang, W.; Khan, A.; Ostenson, C.G.; Berggren, P.O.; Efendic, S.; Meister, B. Down-regulated expression of exocytotic proteins in pancreatic islets of diabetic GK rats. Biochem. Biophys. Res. Commun. 2002, 291, 1038–1044. [Google Scholar] [CrossRef]
- Rose, T.; Efendic, S.; Rupnik, M. Ca2+-secretion coupling is impaired in diabetic Goto Kakizaki rats. J. Gen. Physiol. 2007, 129, 493–508. [Google Scholar] [CrossRef]
- Kato, S.; Ishida, H.; Tsuura, Y.; Tsuji, K.; Nishimura, M.; Horie, M.; Taminato, T.; Ikehara, S.; Odaka, H.; Ikeda, H.; et al. Alterations in basal and glucose-stimulated voltage-dependent Ca2+ channel activities in pancreatic β cells of non-insulin-dependent diabetes mellitus GK rats. J. Clin. Investig. 1996, 97, 2417–2425. [Google Scholar] [CrossRef]
- Speier, S.; Nyqvist, D.; Kohler, M.; Caicedo, A.; Leibiger, I.B.; Berggren, P.O. Noninvasive high-resolution in vivo imaging of cell biology in the anterior chamber of the mouse eye. Nat. Protoc. 2008, 3, 1278–1286. [Google Scholar] [CrossRef]
- Perez, V.L.; Caicedo, A.; Berman, D.M.; Arrieta, E.; Abdulreda, M.H.; Rodriguez-Diaz, R.; Pileggi, A.; Hernandez, E.; Dubovy, S.R.; Parel, J.M.; et al. The anterior chamber of the eye as a clinical transplantation site for the treatment of diabetes: A study in a baboon model of diabetes. Diabetologia 2011, 54, 1121–1126. [Google Scholar] [CrossRef]
- Grynkiewicz, G.; Poenie, M.; Tsien, R.Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J. Biol. Chem. 1985, 260, 3440–3450. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Zhao, K.; Yang, G.; Yu, J.; Li, Y.; Kessels, M.M.; Yu, L.; Qualmann, B.; Berggren, P.O.; Yang, S.N. Inositol hexakisphosphate primes syndapin I/PACSIN 1 activation in endocytosis. Cell. Mol. Life Sci. 2022, 79, 286. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Shi, Y.; Yu, J.; Li, Y.; Yu, L.; Welling, A.; Hofmann, F.; Striessnig, J.; Juntti-Berggren, L.; Berggren, P.O.; et al. CaV1.2 and CaV1.3 channel hyperactivation in mouse islet β cells exposed to type 1 diabetic serum. Cell. Mol. Life Sci. 2015, 72, 1197–1207. [Google Scholar] [CrossRef] [PubMed]
- Benninger, R.K.; Piston, D.W. Cellular communication and heterogeneity in pancreatic islet insulin secretion dynamics. Trends Endocrinol. Metab. 2014, 25, 399–406. [Google Scholar] [CrossRef] [PubMed]
- Noguchi, G.M.; Huising, M.O. Integrating the inputs that shape pancreatic islet hormone release. Nat. Metab. 2019, 1, 1189–1201. [Google Scholar] [CrossRef]
- Salem, V.; Silva, L.D.; Suba, K.; Georgiadou, E.; Gharavy, S.N.M.; Akhtar, N.; Martin-Alonso, A.; Gaboriau, D.C.A.; Rothery, S.M.; Stylianides, T.; et al. Leader β-cells coordinate Ca2+ dynamics across pancreatic islets in vivo. Nat. Metab. 2019, 1, 615–629. [Google Scholar] [CrossRef]
- Raikwar, S.P.; Kim, E.M.; Sivitz, W.I.; Allamargot, C.; Thedens, D.R.; Zavazava, N. Human iPS cell-derived insulin producing cells form vascularized organoids under the kidney capsules of diabetic mice. PLoS ONE 2015, 10, e0116582. [Google Scholar] [CrossRef]
- Maxwell, K.G.; Augsornworawat, P.; Velazco-Cruz, L.; Kim, M.H.; Asada, R.; Hogrebe, N.J.; Morikawa, S.; Urano, F.; Millman, J.R. Gene-edited human stem cell-derived β cells from a patient with monogenic diabetes reverse preexisting diabetes in mice. Sci. Transl. Med. 2020, 12, eaax9106. [Google Scholar] [CrossRef]
- Bruin, J.E.; Asadi, A.; Fox, J.K.; Erener, S.; Rezania, A.; Kieffer, T.J. Accelerated maturation of human stem cell-derived pancreatic progenitor cells into insulin-secreting cells in immunodeficient rats relative to mice. Stem Cell Rep. 2015, 5, 1081–1096. [Google Scholar] [CrossRef]
- Yang, S.N.; Berggren, P.O. The eye as a novel imaging site in diabetes research. Pharmacol. Ther. 2019, 197, 103–121. [Google Scholar] [CrossRef] [PubMed]
- Speier, S.; Nyqvist, D.; Cabrera, O.; Yu, J.; Molano, R.D.; Pileggi, A.; Moede, T.; Kohler, M.; Wilbertz, J.; Leibiger, B.; et al. Noninvasive in vivo imaging of pancreatic islet cell biology. Nat. Med. 2008, 14, 574–578. [Google Scholar] [CrossRef] [PubMed]
- Helman, A.; Cangelosi, A.L.; Davis, J.C.; Pham, Q.; Rothman, A.; Faust, A.L.; Straubhaar, J.R.; Sabatini, D.M.; Melton, D.A. A nutrient-sensing transition at birth triggers glucose-responsive insulin secretion. Cell Metab. 2020, 31, 1004–1016.e5. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Dominguez, J.R.; Melton, D.A. Cell maturation: Hallmarks, triggers, and manipulation. Cell 2022, 185, 235–249. [Google Scholar] [CrossRef]
- Bastidas-Ponce, A.; Roscioni, S.S.; Burtscher, I.; Bader, E.; Sterr, M.; Bakhti, M.; Lickert, H. Foxa2 and Pdx1 cooperatively regulate postnatal maturation of pancreatic β-cells. Mol. Metab. 2017, 6, 524–534. [Google Scholar] [CrossRef]
- Thomas, J.; Zimmerlin, L.; Huo, J.S.; Considine, M.; Cope, L.; Zambidis, E.T. Running the full human developmental clock in interspecies chimeras using alternative human stem cells with expanded embryonic potential. NPJ Regen. Med. 2021, 6, 25. [Google Scholar] [CrossRef]
- Antal, L.; Martin-Caraballo, M. T-type calcium channels in cancer. Cancers 2019, 11, 134. [Google Scholar] [CrossRef]
- Bensellam, M.; Jonas, J.C.; Laybutt, D.R. Mechanisms of beta-cell dedifferentiation in diabetes: Recent findings and future research directions. J. Endocrinol. 2018, 236, R109–R143. [Google Scholar] [CrossRef]
- Stancill, J.S.; Cartailler, J.P.; Clayton, H.W.; O’Connor, J.T.; Dickerson, M.T.; Dadi, P.K.; Osipovich, A.B.; Jacobson, D.A.; Magnuson, M.A. Chronic beta-cell depolarization impairs beta-cell identity by disrupting a network of Ca2+-regulated genes. Diabetes 2017, 66, 2175–2187. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, K.; Shi, Y.; Yu, J.; Yu, L.; Köhler, M.; Mael, A.; Kolton, A.; Joyce, T.; Odorico, J.; Berggren, P.-O.; et al. In Vivo CaV3 Channel Inhibition Promotes Maturation of Glucose-Dependent Ca2+ Signaling in Human iPSC-Islets. Biomedicines 2023, 11, 807. https://doi.org/10.3390/biomedicines11030807
Zhao K, Shi Y, Yu J, Yu L, Köhler M, Mael A, Kolton A, Joyce T, Odorico J, Berggren P-O, et al. In Vivo CaV3 Channel Inhibition Promotes Maturation of Glucose-Dependent Ca2+ Signaling in Human iPSC-Islets. Biomedicines. 2023; 11(3):807. https://doi.org/10.3390/biomedicines11030807
Chicago/Turabian StyleZhao, Kaixuan, Yue Shi, Jia Yu, Lina Yu, Martin Köhler, Amber Mael, Anthony Kolton, Thomas Joyce, Jon Odorico, Per-Olof Berggren, and et al. 2023. "In Vivo CaV3 Channel Inhibition Promotes Maturation of Glucose-Dependent Ca2+ Signaling in Human iPSC-Islets" Biomedicines 11, no. 3: 807. https://doi.org/10.3390/biomedicines11030807




