A Systematic Study of Anti-Osteosarcoma Mechanism of pH-Sensitive Charge-Conversion Cinnamaldehyde Polymeric Prodrug Micelles In Vitro
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cells and Materials
2.2. Preparation of mPEG-b-P(C7-co-CA) and mPEG-b-PC7A Micelles
2.3. Morphology, Particle Size and Zeta Potential of mPEG-b-P(C7-co-CA) Micelles
2.4. Cellular Uptake Assay
2.5. Cell Cytotoxicity Assay
2.6. Cell Counting Kit-8 (CCK-8) Assay
2.7. EdU Staining Assay
2.8. Cell-Cycle Analysis by Flow Cytometry
2.9. Wound-Healing Assay
2.10. Transwell Assays
2.11. Western Blotting (WB)
2.12. Statistical Analysis
3. Results and Discussion
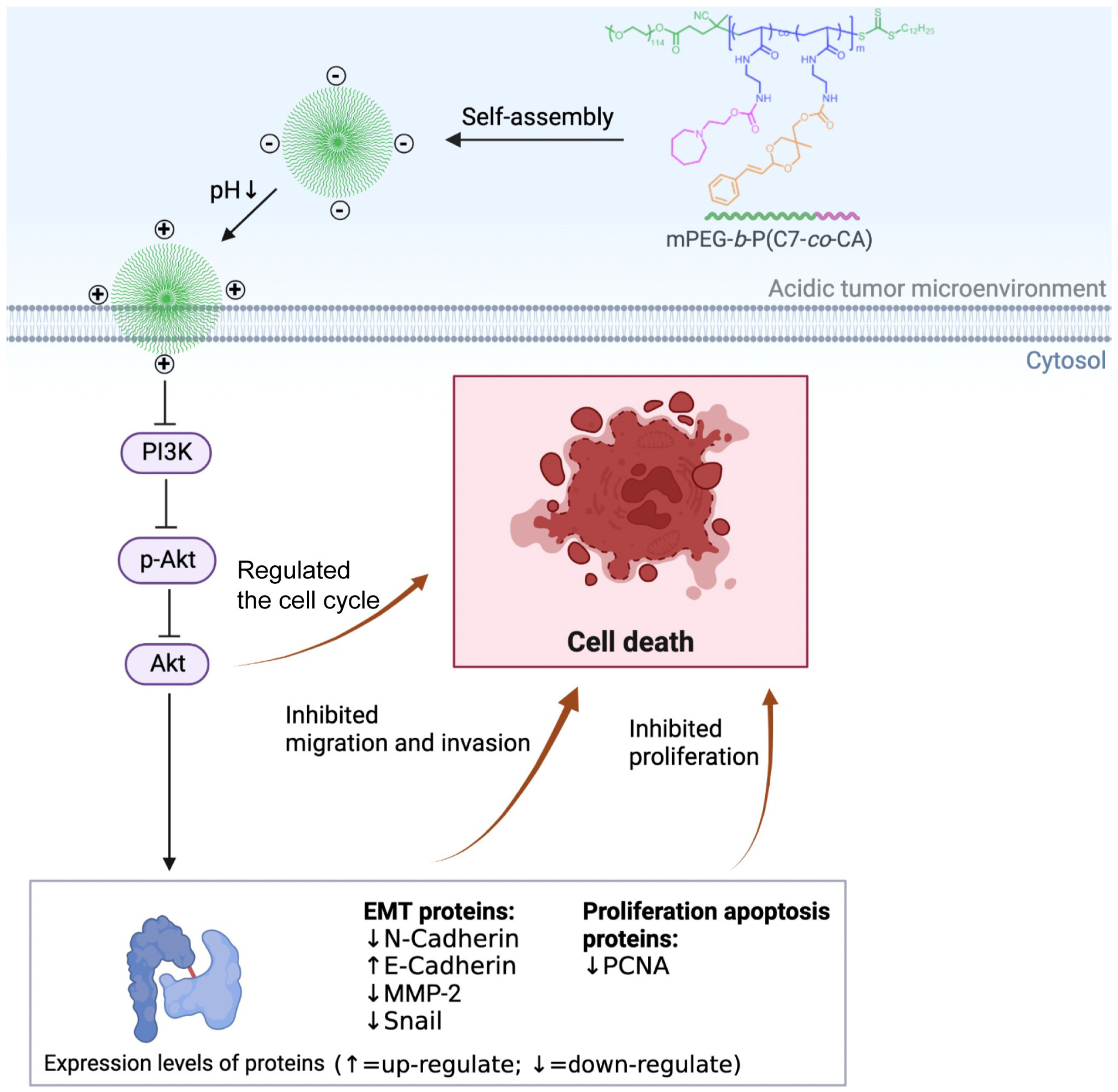
3.1. Characterization of mPEG-b-P(C7-co-CA) Micelles
3.2. Effects of mPEG-b-P(C7-co-CA) Micelles on the Proliferation of 143B Cells
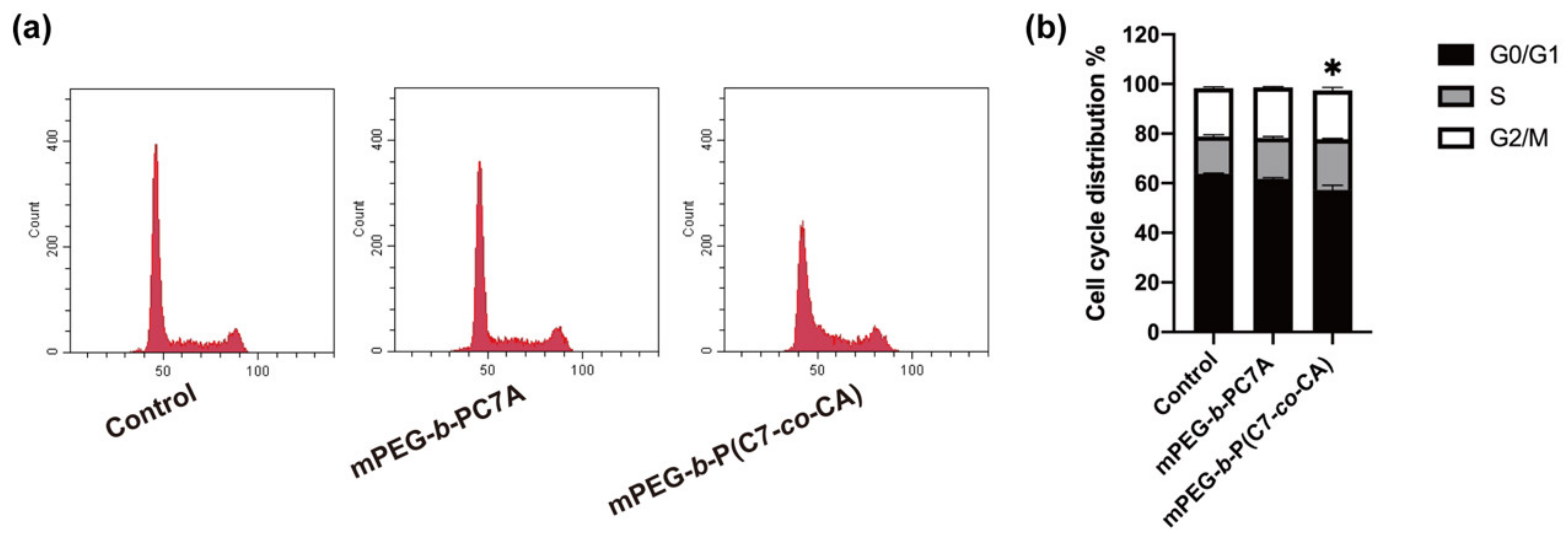
3.3. Effects of mPEG-b-P(C7-co-CA) Micelles on 143B Cell Cycle
3.4. Effects of mPEG-b-P(C7-co-CA) Micelles on Migration and Invasion of 143B Cells
3.5. mPEG-b-P(C7-co-CA) Micelles Downregulated PI3K/Akt Signaling Pathway in 143B Cells
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ling, C.Q.; Yue, X.Q.; Ling, C. Three advantages of using traditional Chinese medicine to prevent and treat tumor. J. Integr. Med. 2014, 12, 331–335. [Google Scholar] [CrossRef]
- Sim, J.X.F.; Khazandi, M.; Pi, H.; Venter, H.; Trott, D.J.; Deo, P. Antimicrobial effects of cinnamon essential oil and cinnamaldehyde combined with EDTA against canine otitis externa pathogens. J. Appl. Microbiol. 2019, 127, 99–108. [Google Scholar] [CrossRef] [PubMed]
- Kostrzewa, T.; Przychodzen, P.; Gorska-Ponikowska, M.; Kuban-Jankowska, A. Curcumin and Cinnamaldehyde as PTP1B Inhibitors with Antidiabetic and Anticancer Potential. Anticancer. Res. 2019, 39, 745–749. [Google Scholar] [CrossRef] [PubMed]
- Gulec Peker, E.G.; Kaltalioglu, K. Cinnamaldehyde and eugenol protect against LPS-stimulated oxidative stress and inflammation in Raw 264.7 cells. J. Food Biochem. 2021, 45, e13980. [Google Scholar] [CrossRef]
- Ka, H.; Park, H.J.; Jung, H.J.; Choi, J.W.; Cho, K.S.; Ha, J.; Lee, K.T. Cinnamaldehyde induces apoptosis by ROS-mediated mitochondrial permeability transition in human promyelocytic leukemia HL-60 cells. Cancer Lett. 2003, 196, 143–152. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Robinson, J.T.; Sun, X.; Dai, H. PEGylated nanographene oxide for delivery of water-insoluble cancer drugs. J. Am. Chem. Soc. 2008, 130, 10876–10877. [Google Scholar] [CrossRef] [Green Version]
- Lee, K.; Kwon, B.M.; Kim, K.; Ryu, J.; Oh, S.J.; Lee, K.S.; Kwon, M.G.; Park, S.K.; Kang, J.S.; Lee, C.W.; et al. Plasma pharmacokinetics and metabolism of the antitumour drug candidate 2′-benzoyloxycinnamaldehyde in rats. Xenobiotica 2009, 39, 255–265. [Google Scholar] [CrossRef]
- Olsen, R.V.; Andersen, H.H.; Møller, H.G.; Eskelund, P.W.; Arendt-Nielsen, L. Somatosensory and vasomotor manifestations of individual and combined stimulation of TRPM8 and TRPA1 using topical L-menthol and trans-cinnamaldehyde in healthy volunteers. Eur. J. Pain. 2014, 18, 1333–1342. [Google Scholar] [CrossRef]
- Ferrari, M. Cancer nanotechnology: Opportunities and challenges. Nat. Rev. Cancer 2005, 5, 161–171. [Google Scholar] [CrossRef]
- Pérez-Herrero, E.; Fernández-Medarde, A. Advanced targeted therapies in cancer: Drug nanocarriers, the future of chemotherapy. Eur. J. Pharm. Biopharm. 2015, 93, 52–79. [Google Scholar] [CrossRef] [Green Version]
- Liu, Q.; Ding, X.; Xu, X.; Lai, H.; Zeng, Z.; Shan, T.; Zhang, T.; Chen, M.; Huang, Y.; Huang, Z.; et al. Tumor-targeted hyaluronic acid-based oxidative stress nanoamplifier with ROS generation and GSH depletion for antitumor therapy. Int. J. Biol. Macromol. 2022, 207, 771–783. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Yao, J.; Guan, Z.; Wu, H.; Cheng, H.; Yan, G.; Tang, R. pH-triggered small molecule nano-prodrugs emulsified from tryptamine-cinnamaldehyde twin drug for targeted synergistic glioma therapy. Colloids Surf. B Biointerfaces 2021, 207, 112052. [Google Scholar] [CrossRef] [PubMed]
- Wani, K.D.; Kadu, B.S.; Mansara, P.; Gupta, P.; Deore, A.V.; Chikate, R.C.; Poddar, P.; Dhole, S.D.; Kaul-Ghanekar, R. Synthesis, characterization and in vitro study of biocompatible cinnamaldehyde functionalized magnetite nanoparticles (CPGF Nps) for hyperthermia and drug delivery applications in breast cancer. PLoS ONE 2014, 9, e107315. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.A.; Lim, J.; Jin, H.Y.; Park, M.; Park, H.J.; Park, J.W.; Kim, J.H.; Kang, H.G.; Won, Y.-J. Osteosarcoma in adolescents and young adults. Cells 2021, 10, 2684. [Google Scholar] [CrossRef] [PubMed]
- Chu, S.-C.; Hsieh, Y.-S.; Hsu, L.-S.; Lin, C.-Y.; Lai, Y.-A.; Chen, P.-N. Cinnamaldehyde decreases the invasion and u-PA expression of osteosarcoma by down-regulating the FAK signalling pathway. Food Funct. 2022, 13, 6574–6582. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Yu, X.-H.; Yan, Y.-G.; Wang, C.; Wang, W.-J. PI3K/Akt signaling in osteosarcoma. Clin. Chim. Acta 2015, 444, 182–192. [Google Scholar] [CrossRef] [PubMed]
- Arvizo, R.R.; Miranda, O.R.; Thompson, M.A.; Pabelick, C.M.; Bhattacharya, R.; Robertson, J.D.; Rotello, V.M.; Prakash, Y.; Mukherjee, P. Effect of nanoparticle surface charge at the plasma membrane and beyond. Nano Lett. 2010, 10, 2543–2548. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fang, Z.; Pan, S.; Gao, P.; Sheng, H.; Li, L.; Shi, L.; Zhang, Y.; Cai, X. Stimuli-responsive charge-reversal nano drug delivery system: The promising targeted carriers for tumor therapy. Int. J. Pharm. 2020, 575, 118841. [Google Scholar] [CrossRef]
- Liu, L.; Hitchens, T.K.; Ye, Q.; Wu, Y.; Barbe, B.; Prior, D.E.; Li, W.F.; Yeh, F.-C.; Foley, L.M.; Bain, D.J. Decreased reticuloendothelial system clearance and increased blood half-life and immune cell labeling for nano-and micron-sized superparamagnetic iron-oxide particles upon pre-treatment with Intralipid. Biochim. Biophys. Acta Gen. Subj. 2013, 1830, 3447–3453. [Google Scholar] [CrossRef] [Green Version]
- Bernkop-Schnürch, A. Strategies to overcome the polycation dilemma in drug delivery. Adv. Drug Deliv. Rev. 2018, 136, 62–72. [Google Scholar] [CrossRef]
- Wu, W.; Luo, L.; Wang, Y.; Wu, Q.; Dai, H.-B.; Li, J.-S.; Durkan, C.; Wang, N.; Wang, G.-X. Endogenous pH-responsive nanoparticles with programmable size changes for targeted tumor therapy and imaging applications. Theranostics 2018, 8, 3038. [Google Scholar] [CrossRef] [PubMed]
- Deng, J.; Liu, S.; Li, G.; Zheng, Y.; Zhang, W.; Lin, J.; Yu, F.; Weng, J.; Liu, P.; Hui, Z. pH-sensitive charge-conversion cinnamaldehyde polymeric prodrug micelles for effective targeted chemotherapy of osteosarcoma in vitro. Front. Chem. 2023, 11, 1190596. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Zhang, M.; Xu, F.; Jiang, S. Wnt signaling in breast cancer: Biological mechanisms, challenges and opportunities. Mol. Cancer 2020, 19, 165. [Google Scholar] [CrossRef] [PubMed]
- Jayaraman, S.; Pazhani, J.; PriyaVeeraraghavan, V.; Raj, A.T.; Somasundaram, D.B.; Patil, S. PCNA and Ki67: Prognostic proliferation markers for oral cancer. Oral Oncol. 2022, 130, 105943. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Chen, J.; Yang, S.; Tan, T.; Wang, N.; Wang, Y.; Zhang, L.; Yang, C.; Huang, H.; Luo, J.; et al. Cinnamaldehyde Inhibits the Function of Osteosarcoma by Suppressing the Wnt/β-Catenin and PI3K/Akt Signaling Pathways. Drug Des. Dev. Ther. 2020, 14, 4625–4637. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.Y.; Hsieh, Y.S.; Chu, S.C.; Hsu, L.S.; Huang, S.C.; Chen, P.N. Reduction of invasion and cell stemness and induction of apoptotic cell death by Cinnamomum cassia extracts on human osteosarcoma cells. Environ. Toxicol. 2022, 37, 1261–1274. [Google Scholar] [CrossRef]
- Wei, H.; Chen, J.; Wang, S.; Fu, F.; Zhu, X.; Wu, C.; Liu, Z.; Zhong, G.; Lin, J. A Nanodrug Consisting Of Doxorubicin And Exosome Derived From Mesenchymal Stem Cells For Osteosarcoma Treatment In Vitro. Int. J. Nanomed. 2019, 14, 8603–8610. [Google Scholar] [CrossRef] [Green Version]
- Howe, L.R.; Brown, A.M. Wnt signaling and breast cancer. Cancer Biol. Ther. 2004, 3, 36–41. [Google Scholar] [CrossRef] [Green Version]
- Brown, J.A.; Yonekubo, Y.; Hanson, N.; Sastre-Perona, A.; Basin, A.; Rytlewski, J.A.; Dolgalev, I.; Meehan, S.; Tsirigos, A.; Beronja, S.; et al. TGF-β-Induced Quiescence Mediates Chemoresistance of Tumor-Propagating Cells in Squamous Cell Carcinoma. Cell Stem Cell 2017, 21, 650–664.e658. [Google Scholar] [CrossRef]
- Yang, C.; Tian, Y.; Zhao, F.; Chen, Z.; Su, P.; Li, Y.; Qian, A. Bone microenvironment and osteosarcoma metastasis. Int. J. Mol. Sci. 2020, 21, 6985. [Google Scholar] [CrossRef]
- Tsai, J.H.; Yang, J. Epithelial-mesenchymal plasticity in carcinoma metastasis. Genes Dev. 2013, 27, 2192–2206. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abd ElMoneim, H.M.; Zaghloul, N.M. Expression of E-cadherin, N-cadherin and snail and their correlation with clinicopathological variants: An immunohistochemical study of 132 invasive ductal breast carcinomas in Egypt. Clinics 2011, 66, 1765–1771. [Google Scholar]
- Papiewska-Pająk, I.; Kowalska, M.A.; Boncela, J. Expression and activity of SNAIL transcription factor during Epithelial to Mesenchymal Transition (EMT) in cancer progression. Adv. Hyg. Exp. Med. 2016, 70, 968–980. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Zhang, J.; Liu, X.; Huo, P.; Zhang, Y.; Chen, H.; Tian, Q.; Zhang, N. MMP-2-responsive gelatin nanoparticles for synergistic tumor therapy. Pharm. Dev. Technol. 2019, 24, 1002–1013. [Google Scholar] [CrossRef]
- Xu, Z.; Han, X.; Ou, D.; Liu, T.; Li, Z.; Jiang, G.; Liu, J.; Zhang, J. Targeting PI3K/AKT/mTOR-mediated autophagy for tumor therapy. Appl. Microbiol. Biotechnol. 2020, 104, 575–587. [Google Scholar] [CrossRef]
- Liu, F.; Xu, J.; Yang, R.; Liu, S.; Hu, S.; Yan, M.; Han, F. New light on treatment of cervical cancer: Chinese medicine monomers can be effective for cervical cancer by inhibiting the PI3K/Akt signaling pathway. Biomed. Pharmacother. 2023, 157, 114084. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deng, J.; Wang, Q.; Xu, H.; Li, G.; Liu, S.; Chen, Y.; Yu, F.; Yan, W.; Zeng, H.; Liu, P. A Systematic Study of Anti-Osteosarcoma Mechanism of pH-Sensitive Charge-Conversion Cinnamaldehyde Polymeric Prodrug Micelles In Vitro. Biomedicines 2023, 11, 1524. https://doi.org/10.3390/biomedicines11061524
Deng J, Wang Q, Xu H, Li G, Liu S, Chen Y, Yu F, Yan W, Zeng H, Liu P. A Systematic Study of Anti-Osteosarcoma Mechanism of pH-Sensitive Charge-Conversion Cinnamaldehyde Polymeric Prodrug Micelles In Vitro. Biomedicines. 2023; 11(6):1524. https://doi.org/10.3390/biomedicines11061524
Chicago/Turabian StyleDeng, Jiapeng, Qichang Wang, Huihui Xu, Guoqing Li, Su Liu, Yixiao Chen, Fei Yu, Weiqiang Yan, Hui Zeng, and Peng Liu. 2023. "A Systematic Study of Anti-Osteosarcoma Mechanism of pH-Sensitive Charge-Conversion Cinnamaldehyde Polymeric Prodrug Micelles In Vitro" Biomedicines 11, no. 6: 1524. https://doi.org/10.3390/biomedicines11061524





