Alteration of Postural Reactions in Rats with Different Levels of Dopamine Depletion
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Surgical Procedure
2.3. Experimental Design
2.4. Analysis and Statistics
3. Results
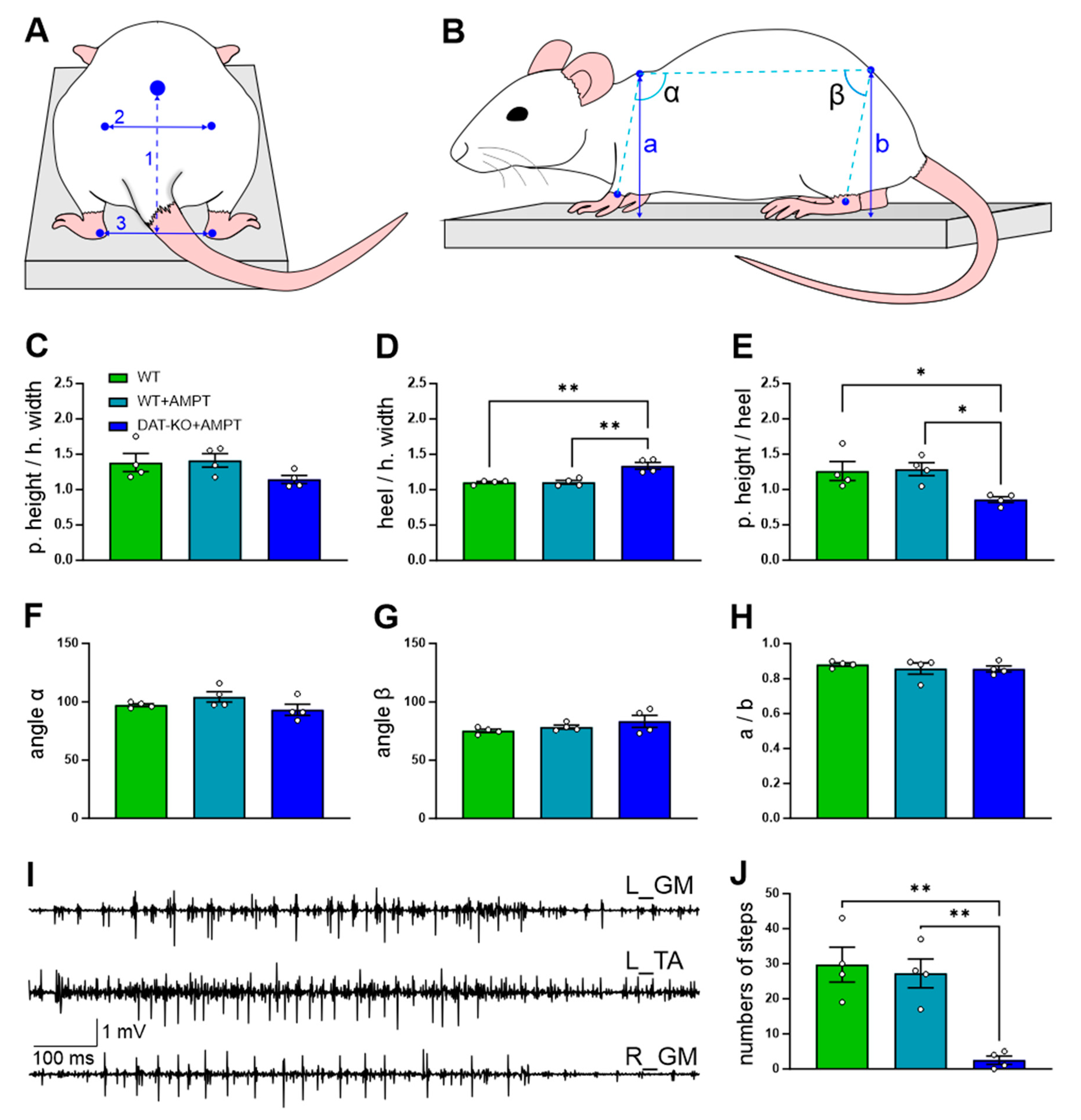
3.1. The General Motor Capacities of the Rats with DA Depletion
3.2. Postural Reactions of WT Rats to Lateral Platform Displacement
3.3. Alteration of Postural Abilities of Rats with Moderate and Severe DA Deficiency
4. Discussion
4.1. The Experimental Model of Progressing DA Depletion
4.2. The Role of Dopamine in the Control of Sensorimotor Behavior
4.3. Specific Alteration of Postural Reactions after Mild and Severe DA Depletion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Horak, F.B. Postural Orientation and Equilibrium: What Do We Need to Know about Neural Control of Balance to Prevent Falls? Age Ageing 2006, 35, ii7–ii11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deliagina, T.G.; Beloozerova, I.N.; Zelenin, P.V.; Orlovsky, G.N. Spinal and Supraspinal Postural Networks. Brain Res. Rev. 2008, 57, 212–221. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Visser, J.E.; Bloem, B.R. Role of the Basal Ganglia in Balance Control. Neural Plast. 2005, 12, 161. [Google Scholar] [CrossRef] [Green Version]
- Matsumoto, N.; Hanakawa, T.; Maki, S.; Graybiel, A.M.; Kimura, M. Nigrostriatal Dopamine System in Learning to Perform Sequential Motor Tasks in a Predictive Manner. J. Neurophysiol. 1999, 82, 978–998. [Google Scholar] [CrossRef] [Green Version]
- Groenewegen, H.J. The Basal Ganglia and Motor Control. Neural Plast. 2003, 10, 107–120. [Google Scholar] [CrossRef] [PubMed]
- Stoker, T.B.; Greenland, J.C. Parkinson’s Disease: Pathogenesis and Clinical Aspects; Codon Publications: Brisbane, QL, Australia, 2018. [Google Scholar]
- Barker, R. Age-Related Dopamine-Dependent Disorders. J. Neurol. Neurosurg. Psychiatry 1995, 59, 344. [Google Scholar] [CrossRef] [Green Version]
- Konnova, E.A.; Swanberg, M. Animal Models of Parkinson’s Disease; Stoker, T.B., Greenland, J.C., Eds.; Codon Publications: Brisbane, QL, Australia, 2018; ISBN 978-0-9944381-6-4. [Google Scholar]
- Lohr, K.M.; Masoud, S.T.; Salahpour, A.; Miller, G.W. Membrane Transporters as Mediators of Synaptic Dopamine Dynamics: Implications for Disease. Eur. J. Neurosci. 2017, 45, 20. [Google Scholar] [CrossRef] [Green Version]
- Sotnikova, T.; Beaulieu, J.-M.; Gainetdinov, R.; Caron, M. Molecular Biology, Pharmacology and Functional Role of the Plasma Membrane Dopamine Transporter. CNS Neurol. Disord. Drug Targets 2006, 5, 45–56. [Google Scholar] [CrossRef]
- Leo, D.; Sukhanov, I.; Zoratto, F.; Illiano, P.; Caffino, L.; Sanna, F.; Messa, G.; Emanuele, M.; Esposito, A.; Dorofeikova, M.; et al. Pronounced Hyperactivity, Cognitive Dysfunctions, and BDNF Dysregulation in Dopamine Transporter Knock-out Rats. J. Neurosci. 2018, 38, 1959–1972. [Google Scholar] [CrossRef] [Green Version]
- Sotnikova, T.D.; Beaulieu, J.M.; Barak, L.S.; Wetsel, W.C.; Caron, M.G.; Gainetdinov, R.R. Dopamine-Independent Locomotor Actions of Amphetamines in a Novel Acute Mouse Model of Parkinson Disease. PLoS Biol. 2005, 3, e271. [Google Scholar] [CrossRef] [Green Version]
- Sukhanov, I.; Leo, D.; Tur, M.A.; Belozertseva, I.V.; Savchenko, A.; Gainetdinov, R.R. Dopamine Transporter Knockout Rats as the New Preclinical Model of Hyper- and Hypo-Dopaminergic Disorders. V.M. Bekhterev Rev. Psychiatry Med. Psychol. 2019, 4, 84–85. [Google Scholar] [CrossRef]
- Sukhanov, I.; Dorotenko, A.; Fesenko, Z.; Savchenko, A.; Efimova, E.V.; Mor, M.S.; Belozertseva, I.V.; Sotnikova, T.D.; Gainetdinov, R.R. Inhibition of PDE10A in a New Rat Model of Severe Dopamine Depletion Suggests New Approach to Non-Dopamine Parkinson’s Disease Therapy. Biomolecules 2022, 13, 9. [Google Scholar] [CrossRef] [PubMed]
- Macpherson, J.M.; Lywood, D.W.; Van Eyken, A. A System for the Analysis of Posture and Stance in Quadrupeds. J. Neurosci. Methods 1987, 20, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Geurts, A.M.; Cost, G.J.; Freyvert, Y.; Zeitler, B.; Miller, J.C.; Choi, V.M.; Jenkins, S.S.; Wood, A.; Cui, X.; Meng, X.; et al. Knockout Rats via Embryo Microinjection of Zinc-Finger Nucleases. Science 2009, 325, 433. [Google Scholar] [CrossRef] [Green Version]
- Brown, A.J.; Fisher, D.A.; Kouranova, E.; McCoy, A.; Forbes, K.; Wu, Y.; Henry, R.; Ji, D.; Chambers, A.; Warren, J.; et al. Whole-Rat Conditional Gene Knockout via Genome Editing. Nat. Methods 2013, 10, 638–640. [Google Scholar] [CrossRef]
- Capogrosso, M.; Wagner, F.B.; Gandar, J.; Moraud, E.M.; Wenger, N.; Milekovic, T.; Shkorbatova, P.; Pavlova, N.; Musienko, P.; Bezard, E.; et al. Configuration of Electrical Spinal Cord Stimulation through Real-Time Processing of Gait Kinematics. Nat. Protoc. 2018, 13, 2031–2061. [Google Scholar] [CrossRef] [Green Version]
- Nissbrandt, H.; Sundström, E.; Jonsson, G.; Hjorth, S.; Carlsson, A. Synthesis and Release of Dopamine in Rat Brain: Comparison between Substantia Nigra Pars Compacts, Pars Reticulata, and Striatum. J. Neurochem. 1989, 52, 1170–1182. [Google Scholar] [CrossRef]
- Dauer, W.; Przedborski, S. Parkinson’s Disease: Mechanisms and Models. Neuron 2003, 39, 889–909. [Google Scholar] [CrossRef] [Green Version]
- Sedelis, M.; Schwarting, R.K.; Huston, J.P. Behavioral Phenotyping of the MPTP Mouse Model of Parkinson’s Disease. Behav. Brain Res. 2001, 125, 109–125. [Google Scholar] [CrossRef]
- Fox, S.H.; Brotchie, J.M. The MPTP-Lesioned Non-Human Primate Models of Parkinson’s Disease. Past, Present, and Future. Prog. Brain Res. 2010, 184, 133–157. [Google Scholar] [CrossRef]
- Ogawa, M.; Zhou, Y.; Tsuji, R.; Goto, S.; Kasahara, J. Video-Based Assessments of the Hind Limb Stepping in a Mouse Model of Hemi-Parkinsonism. Neurosci. Res. 2020, 154, 56–59. [Google Scholar] [CrossRef] [PubMed]
- Jackson-Lewis, V.; Blesa, J.; Przedborski, S. Animal Models of Parkinson’s Disease. Park. Relat. Disord. 2012, 18, S183–S185. [Google Scholar] [CrossRef]
- Shih, L.C.; Tarsy, D. Dopamine Depletors and Movement Disorders. Encycl. Mov. Disord. 2010, 1, 321–323. [Google Scholar] [CrossRef]
- Widerlöv, E. Dose-Dependent Pharmacokinetics of Alpha-Methyl-p-Tyrosine (Alpha-MT) and Comparison of Catecholamine Turnover Rates after Two Doses of Alpha-MT. J. Neural Transm. 1979, 44, 145–158. [Google Scholar] [CrossRef] [PubMed]
- Horak, F.B.; Frank, J.; Nutt, J. Effects of Dopamine on Postural Control in Parkinsonian Subjects: Scaling, Set, and Tone. J. Neurophysiol. 1996, 75, 2380–2396. [Google Scholar] [CrossRef]
- Sherman, D.; Fuller, P.M.; Marcus, J.; Yu, J.; Zhang, P.; Chamberlin, N.L.; Saper, C.B.; Lu, J. Anatomical Location of the Mesencephalic Locomotor Region and Its Possible Role in Locomotion, Posture, Cataplexy, and Parkinsonism. Front. Neurol. 2015, 6, 140. [Google Scholar] [CrossRef] [Green Version]
- Vitrac, C.; Benoit-Marand, M. Monoaminergic Modulation of Motor Cortex Function. Front. Neural Circuits 2017, 11, 72. [Google Scholar] [CrossRef] [Green Version]
- Lapointe, N.P.; Rouleau, P.; Ung, R.-V.; Guertin, P.A. Specific Role of Dopamine D1 Receptors in Spinal Network Activation and Rhythmic Movement Induction in Vertebrates. J. Physiol. 2009, 587, 1499–1511. [Google Scholar] [CrossRef]
- Musienko, P.; van den Brand, R.; Märzendorfer, O.; Roy, R.R.; Gerasimenko, Y.; Edgerton, V.R.; Courtine, G. Controlling Specific Locomotor Behaviors through Multidimensional Monoaminergic Modulation of Spinal Circuitries. J. Neurosci. 2011, 31, 9264–9278. [Google Scholar] [CrossRef]
- Barbeau, H.; Rossignol, S. Initiation and Modulation of the Locomotor Pattern in the Adult Chronic Spinal Cat by Noradrenergic, Serotonergic and Dopaminergic Drugs. Brain Res. 1991, 546, 250–260. [Google Scholar] [CrossRef]
- Metz, G.A.; Tse, A.; Ballermann, M.; Smith, L.K.; Fouad, K. The Unilateral 6-OHDA Rat Model of Parkinson’s Disease Revisited: An Electromyographic and Behavioural Analysis. Eur. J. Neurosci. 2005, 22, 735–744. [Google Scholar] [CrossRef] [PubMed]
- Xia, R.; Markopoulou, K.; Puumala, S.E.; Rymer, W.Z. A Comparison of the Effects of Imposed Extension and Flexion Movements on Parkinsonian Rigidity. Clin. Neurophysiol. 2006, 117, 2302–2307. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, T.-H.; Chen, J.-J.J.; Chen, L.-H.; Chiang, P.-T.; Lee, H.-Y. Time-Course Gait Analysis of Hemiparkinsonian Rats Following 6-Hydroxydopamine Lesion. Behav. Brain Res. 2011, 222, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Bovonsunthonchai, S.; Vachalathiti, R.; Pisarnpong, A.; Khobhun, F.; Hiengkaew, V. Spatiotemporal Gait Parameters for Patients with Parkinson’s Disease Compared with Normal Individuals. Physiother. Res. Int. 2014, 19, 158–165. [Google Scholar] [CrossRef]
- Rinalduzzi, S.; Trompetto, C.; Marinelli, L.; Alibardi, A.; Missori, P.; Fattapposta, F.; Pierelli, F.; Currà, A. Balance Dysfunction in Parkinson’s Disease. BioMed Res. Int. 2015, 2015, 434683. [Google Scholar] [CrossRef]
- Benninger, F.; Khlebtovsky, A.; Roditi, Y.; Keret, O.; Steiner, I.; Melamed, E.; Djaldetti, R. Beneficial Effect of Levodopa Therapy on Stooped Posture in Parkinson’s Disease. Gait Posture 2015, 42, 263–268. [Google Scholar] [CrossRef] [PubMed]
- Macpherson, J.M. Strategies That Simplify the Control of Quadrupedal Stance. I. Forces at the Ground. J. Neurophysiol. 1988, 60, 204–217. [Google Scholar] [CrossRef]
- Kuhman, D.J.; Walker, H.C.; Hurt, C.P. Dopamine-Mediated Improvements in Dynamic Balance Control in Parkinson’s Disease. Gait Posture 2020, 82, 68–74. [Google Scholar] [CrossRef]
- Kato, M.; Murakami, S.; Hirayama, H.; Hikino, K. Recovery of Postural Control Following Chronic Bilateral Hemisections at Different Spinal Cord Levels in Adult Cats. Exp. Neurol. 1985, 90, 350–364. [Google Scholar] [CrossRef]
- Musienko, P.E.; Zelenin, P.V.; Lyalka, V.F.; Orlovsky, G.N.; Deliagina, T.G. Postural Performance in Decerebrated Rabbit. Behav. Brain Res. 2008, 190, 124–134. [Google Scholar] [CrossRef] [Green Version]
- Karayannidou, A.; Zelenin, P.V.; Orlovsky, G.N.; Sirota, M.G.; Beloozerova, I.N.; Deliagina, T.G. Maintenance of Lateral Stability During Standing and Walking in the Cat. J. Neurophysiol. 2009, 101, 8–19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murray, A.J.; Croce, K.; Belton, T.; Akay, T.; Jessell, T.M. Balance Control Mediated by Vestibular Circuits Directing Limb Extension or Antagonist Muscle Co-Activation. Cell Rep. 2018, 22, 1325–1338. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deliagina, T.; Beloozerova, I.N.; Popova, L.B.; Sirota, M.G.; Swadlow, H.A.; Grant, G.; Orlovsky, G.N. Role of Different Sensory Inputs for Maintenance of Body Posture in Sitting Rat and Rabbit. Mot. Control 2000, 4, 439–452. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dimitrova, D.; Horak, F.B.; Nutt, J.G. Postural Muscle Responses to Multidirectional Translations in Patients with Parkinson’s Disease. J. Neurophysiol. 2004, 91, 489–501. [Google Scholar] [CrossRef] [Green Version]
- Henry, S.M.; Fung, J.; Horak, F.B. EMG Responses to Maintain Stance during Multidirectional Surface Translations. J. Neurophysiol. 1998, 80, 1939–1950. [Google Scholar] [CrossRef] [Green Version]
- Miklyaeva, E.I.; Woodward, N.C.; Nikiforov, E.G.; Tompkins, G.J.; Klassen, F.; Ioffe, M.E.; Whishaw, I.Q. The Ground Reaction Forces of Postural Adjustments during Skilled Reaching in Unilateral Dopamine-Depleted Hemiparkinson Rats. Behav. Brain Res. 1997, 88, 143–152. [Google Scholar] [CrossRef]
- Mori, S.; Kawahara, K.; Sakamoto, T.; Aoki, M.; Tomiyama, T. Setting and Resetting of Level of Postural Muscle Tone in Decerebrate Cat by Stimulation of Brain Stem. J. Neurophysiol. 1982, 48, 737–748. [Google Scholar] [CrossRef]

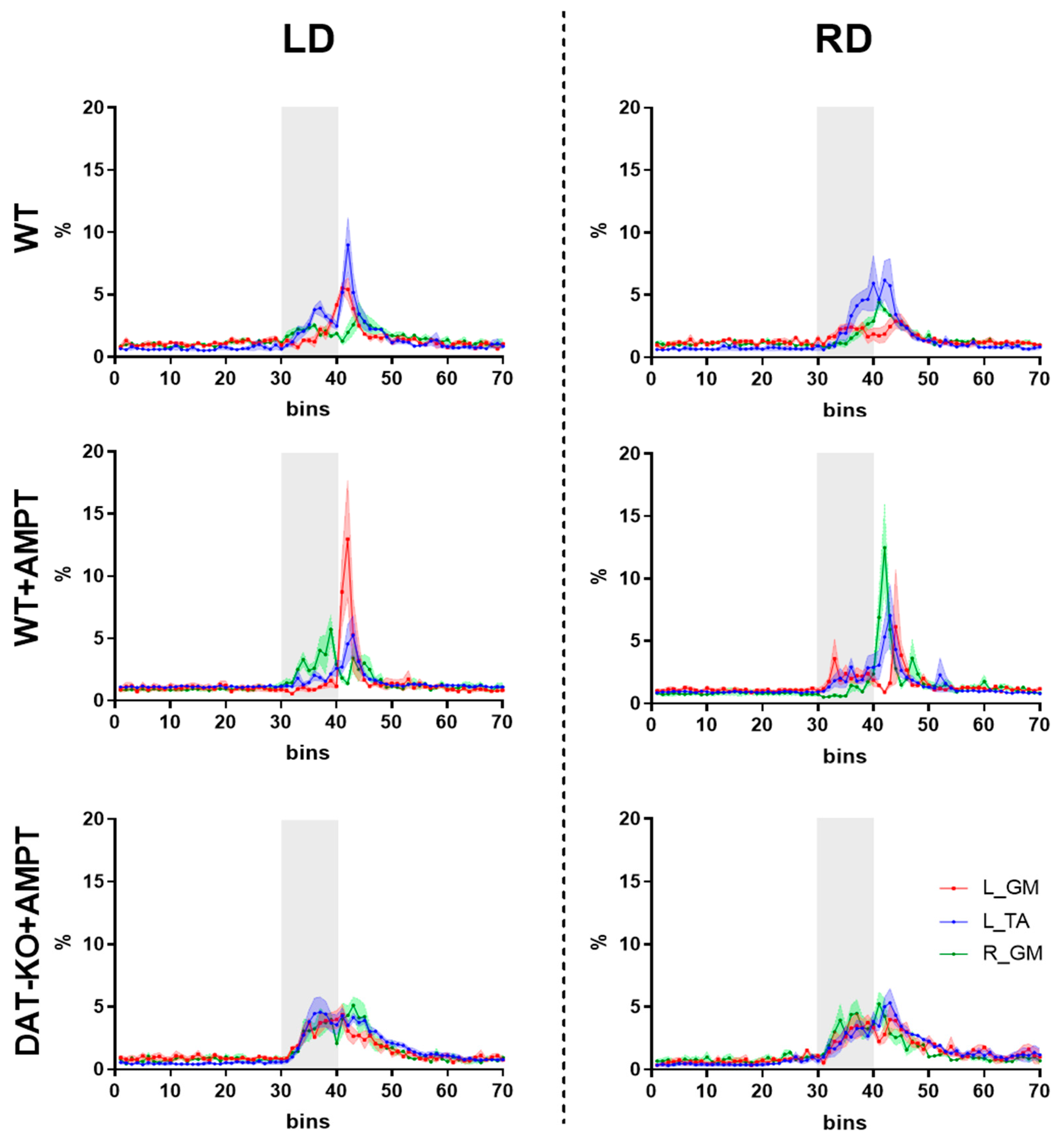
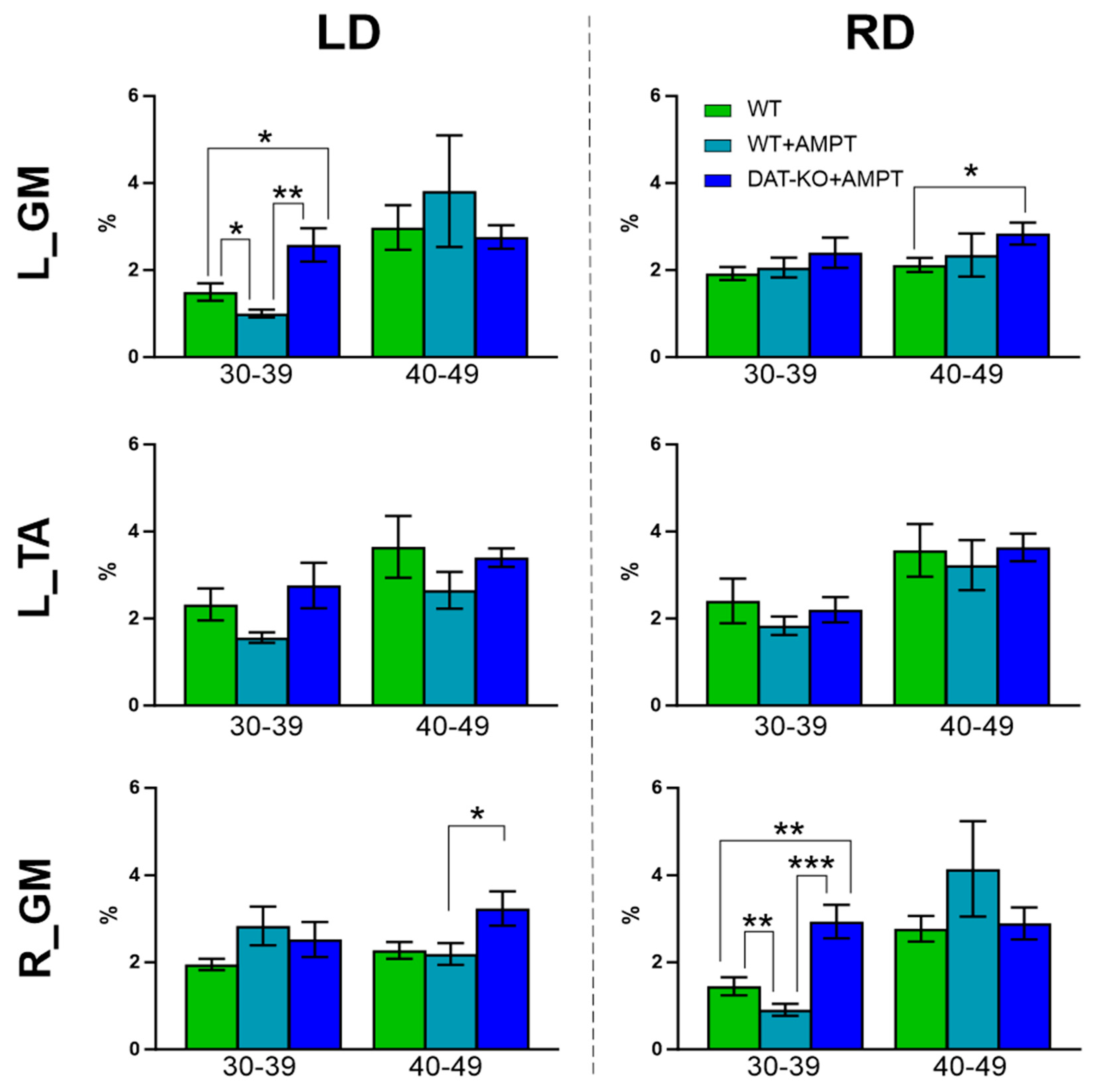
| Bins | L_TA vs. L_GM | L_TA vs. R_GM | L_GM vs. R_GM | F (2, 9) | ||
|---|---|---|---|---|---|---|
| WT | LD | 30–39 | 0.0675 | 0.8207 | 0.3198 | 4.021 |
| 40–49 | 0.4449 | 0.3208 | 0.8752 | 2.434 | ||
| RD | 30–39 | 0.8988 | 0.0489 * | 0.1499 | 4.057 | |
| 40–49 | 0.1239 | 0.2971 | 0.1378 | 4.989 | ||
| WT + AMPT | LD | 30–39 | 0.0008 * | 0.0198 * | 0.0021 * | 19.39 |
| 40–49 | 0.8662 | 0.834 | 0.7886 | 1.368 | ||
| RD | 30–39 | >0.9999 | 0.0003 * | 0.0032 * | 17.19 | |
| 40–49 | 0.8078 | 0.94 | 0.6801 | 1.508 | ||
| DAT-KO + AMPT | LD | 30–39 | >0.9999 | 0.7 | >0.9999 | 1.454 |
| 40–49 | 0.0357 * | >0.9999 | 0.8089 | 2.487 | ||
| RD | 30–39 | 0.711 | 0.0372 * | 0.0897 | 7.026 | |
| 40–49 | 0.0116 * | 0.1939 | >0.9999 | 3.355 |
| Bins | WT vs. WT + AMT | WT vs. DAT + AMT | WT + AMPT vs. DAT-KO + AMPT | F (2, 9) | ||
|---|---|---|---|---|---|---|
| LD | L_GM | 30–39 | 0.0155 * | 0.0108 * | 0.0031 * | 19.05 |
| 40–49 | >0.9999 | >0.9999 | >0.9999 | 0.8075 | ||
| L_TA | 30–39 | 0.0751 | 0.1264 | 0.0741 | 7.057 | |
| 40–49 | 0.1555 | >0.9999 | 0.1391 | 2.33 | ||
| R_GM | 30–39 | 0.2391 | 0.4616 | 0.6162 | 3.112 | |
| 40–49 | >0.9999 | 0.098 | 0.0452 * | 6.363 | ||
| RD | L_GM | 30–39 | >0.9999 | 0.3009 | 0.8376 | 1.759 |
| 40–49 | >0.9999 | 0.0033 * | 0.822 | 2.335 | ||
| L_TA | 30–39 | 0.5353 | >0.9999 | 0.4232 | 1.667 | |
| 40–49 | >0.9999 | >0.9999 | 0.5307 | 0.772 | ||
| R_GM | 30–39 | 0.0046 * | 0.0041 * | 0.0005 * | 29 | |
| 40–49 | 0.4926 | >0.9999 | 0.5678 | 2.125 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kalinina, D.S.; Lyakhovetskii, V.A.; Gorskii, O.V.; Shkorbatova, P.Y.; Pavlova, N.V.; Bazhenova, E.Y.; Sysoev, Y.I.; Gainetdinov, R.R.; Musienko, P.E. Alteration of Postural Reactions in Rats with Different Levels of Dopamine Depletion. Biomedicines 2023, 11, 1958. https://doi.org/10.3390/biomedicines11071958
Kalinina DS, Lyakhovetskii VA, Gorskii OV, Shkorbatova PY, Pavlova NV, Bazhenova EY, Sysoev YI, Gainetdinov RR, Musienko PE. Alteration of Postural Reactions in Rats with Different Levels of Dopamine Depletion. Biomedicines. 2023; 11(7):1958. https://doi.org/10.3390/biomedicines11071958
Chicago/Turabian StyleKalinina, Daria S., Vsevolod A. Lyakhovetskii, Oleg V. Gorskii, Polina Yu. Shkorbatova, Natalia V. Pavlova, Elena Yu. Bazhenova, Yurii I. Sysoev, Raul R. Gainetdinov, and Pavel E. Musienko. 2023. "Alteration of Postural Reactions in Rats with Different Levels of Dopamine Depletion" Biomedicines 11, no. 7: 1958. https://doi.org/10.3390/biomedicines11071958







