Sodium Alginate-Based MgO Nanoparticles Coupled Antibiotics as Safe and Effective Antimicrobial Candidates against Staphylococcus aureus of Houbara Bustard Birds
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Nanoparticles and Composites
2.2. Characterization of Nanoparticles and Composites
2.3. Part I: Cytogenetic Assessment of Different Treatments
2.3.1. Allium cepa Ana-Telophase Test
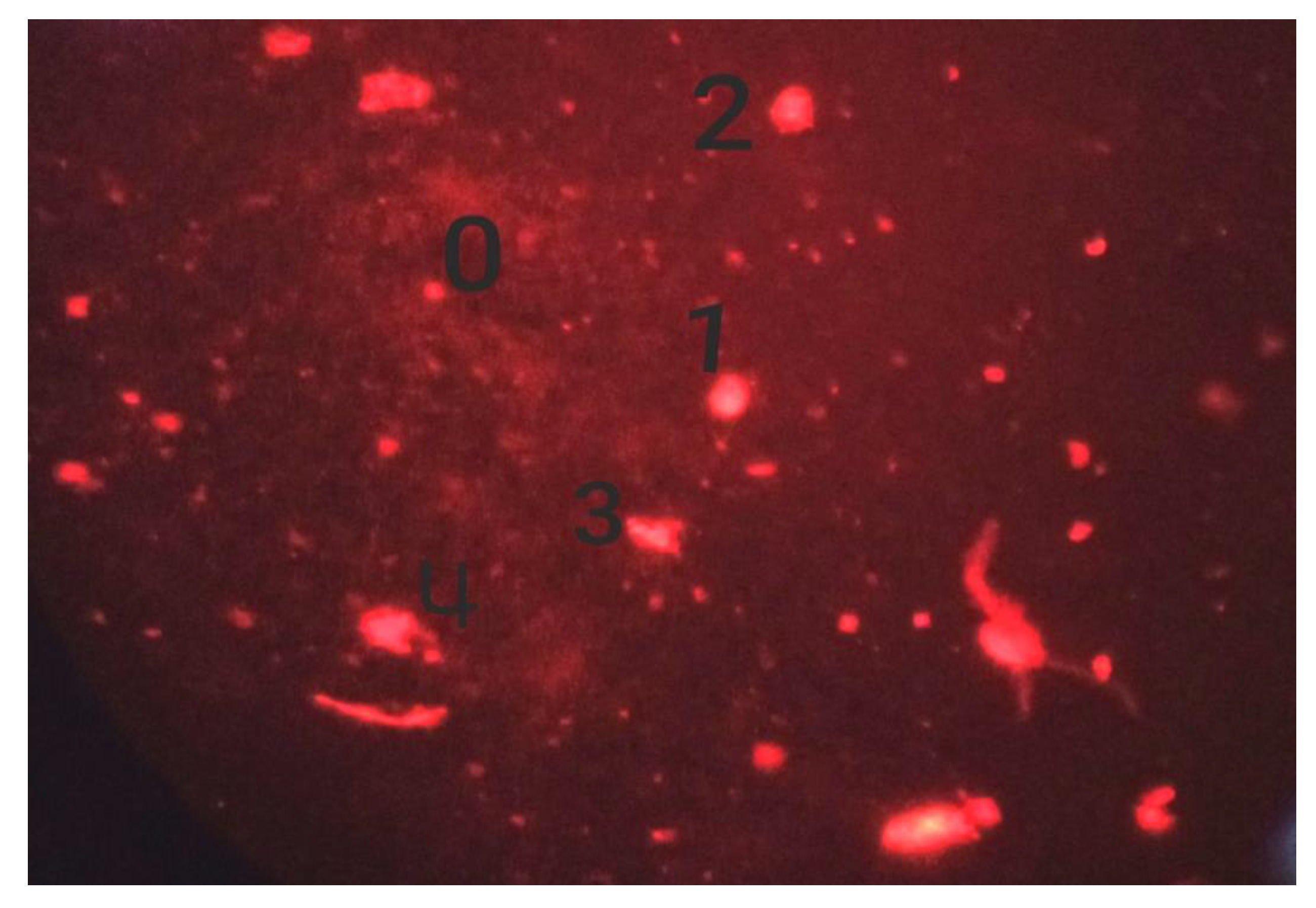
2.3.2. Comet Assay on A. cepa Root Tips
2.4. Part II: Antimicrobial Potential against Bacteria
2.4.1. Isolation of S. aureus
2.4.2. Molecular Analysis
2.4.3. Antibacterial Potential of Nanocomposites
Well Diffusion Assay
Broth Microdilution Assay
2.5. Statistical Analyses
3. Results
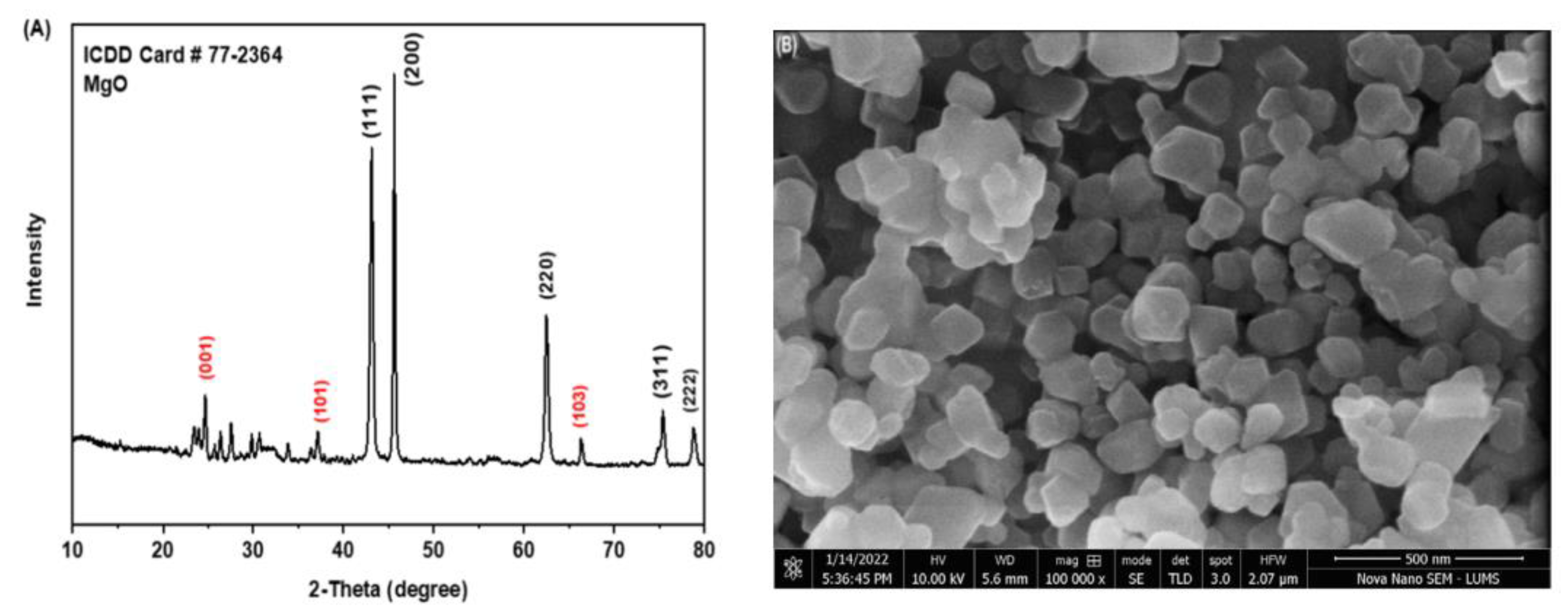
3.1. Characterization of Nanoparticles and Composites
3.2. Cytotoxicity and Genotoxicity of Different Preparations
3.2.1. Effect of Cefoxitin
3.2.2. Effect of Magnesium Oxide (M) and Gel (G)
3.2.3. Effect of Gel, Magnesium Oxide, and Cefoxitin (GMC)
3.2.4. Effect of Cefoxitin and Gel (GC)
3.2.5. Effect of Tylosin and Gel (GT)
3.2.6. Effect of Ampicillin and Gel (GA)
3.2.7. Effect of Gel, Magnesium Oxide, and Tylosin (GMT)
3.3. Antibacterial Potential of Composites against Bacteria
3.3.1. Comparison of the Zones of Inhibition
3.3.2. Comparison of Minimum Inhibitory Concentrations (MIC)
Antibacterial Efficacy of Composites with Respect to Time Intervals
Comparison of the Antibacterial Potential among Different Composites
4. Discussion
4.1. Characterization of Nanoparticles
4.2. Genotoxicity Assay
4.3. Antibacterial Potential Nanocomposites
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nabi, G.; Ullah, R.; Khan, S.; Amin, M.; Rauf, N. The Asian houbara bustard (Chlamydotis macqueenii): On an accelerating path to extinction? Biodivers. Conserv. 2019, 28, 1301–1302. [Google Scholar] [CrossRef]
- Arnold, K.E.; Williams, N.J.; Bennett, M. ‘Disperse abroad in the land’: The role of wildlife in the dissemination of antimicrobial resistance. Biol. Lett. 2016, 12, 20160137. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mwafy, A.; Youssef, D.Y.; Mohamed, M.M. Antibacterial activity of zinc oxide nanoparticles against some multidrug-resistant strains of Escherichia coli and Staphylococcus aureus. Int. J. Vet. Sci. 2023, 12, 284–289. [Google Scholar] [CrossRef]
- Raheel, I.; Orabi, A.; Erfan, A.; Raslan, M.A.; Wahab, S.H.A.E.; Mohamed, E.A.A. Intestinal tract of broiler chickens as a reservoir of potentially pathogenic curli producing ESβL Escherichia coli. Int. J. Vet. Sci. 2022, 11, 498–503. [Google Scholar] [CrossRef]
- Ahmed, A.; Ijaz, M.; Khan, J.A.; Anjum, A.A. Molecular characterization and therapeutic insights into biofilm positive Staphylococcus aureus isolated from bovine subclinical mastitis. Pak. Vet. J. 2022, 42, 584–590. [Google Scholar] [CrossRef]
- Liu, J.; Wang, X.; Bi, C.; Ali, F.; Saleem, M.U.; Qin, J.; Ashfaq, H.; Han, Z.; Alsayeqh, A.F. Epidemiological investigation of Staphylococcus aureus infection in dairy cattle in Anhui, China. Pak. Vet. J. 2022, 42, 580–583. [Google Scholar] [CrossRef]
- Sarwar, I.; Ashar, A.; Mahfooz, A.; Aqib, A.I.; Saleem, M.I.; Butt, A.A.; Bhutta, Z.A.; Shoaib, M.; Kulyar, M.F.E.A.; Ilyas, A. Evaluation of Antibacterial Potential of Raw Turmeric, Nano-Turmeric, and NSAIDs against Multiple Drug Resistant Staphylococcus aureus and E. coli Isolated from Animal Wounds. Pak. Vet. J. 2021, 41, 209–214. [Google Scholar]
- Telli, A.E.; Biçer, Y.; Telli, N.; Güngör, C.; Turkal, G.; Onmaz, N.E. Pathogenic Escherichia coli and Salmonella spp. in chicken rinse carcasses: Isolation and genotyping by ERIC-PCR. Pak. Vet. J. 2022, 42, 493–498. [Google Scholar] [CrossRef]
- Javed, M.U.; Ijaz, M.; Fatima, Z.; Anjum, A.A.; Aqib, A.I.; Ali, M.M.; Rehman, A.; Ahmed, A.; Ghaffar, A. Frequency and Antimicrobial Susceptibility of Methicillin and Vancomycin-Resistant Staphylococcus aureus from Bovine Milk. Pak. Vet. J. 2021, 41, 463–468. [Google Scholar] [CrossRef]
- Parveen, S.; Saqib, S.; Ahmed, A.; Shahzad, A.; Ahmed, N. Prevalence of MRSA colonization among healthcare-workers and effectiveness of decolonization regimen in ICU of a Tertiary care Hospital, Lahore, Pakistan. Adv. Life Sci. 2020, 8, 38–41. [Google Scholar]
- Gardam, M.A. Is methicillin-resistant Staphylococcus aureus an emerging community pathogen? A review of the literature. Can. J. Infect. Dis. 2000, 11, 202–211. [Google Scholar] [PubMed] [Green Version]
- Van Boeckel, T.P.; Pires, J.; Silvester, R.; Zhao, C.; Song, J.; Criscuolo, N.G.; Gilbert, M.; Bonhoeffer, S.; Laxminarayan, R. Global trends in antimicrobial resistance in animals in low-and middle-income countries. Science 2019, 365, eaaw1944. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lei, Z.Q.; Li, L.; Li, G.J.; Leung, C.W.; Shi, J.; Wong, C.M.; Lo, K.C.; Chan, W.K.; Mak, C.S.K.; Chan, S.B.; et al. Liver cancer immunoassay with magnetic nanoparticles and MgO-based magnetic tunnel junction sensors. J. Appl. Phys. 2012, 111, 07E505. [Google Scholar] [CrossRef] [Green Version]
- Di, D.R.; He, Z.Z.; Sun, Z.Q.; Liu, J. A new nano-cryosurgical modality for tumor treatment using biodegradable MgO nanoparticles. Nanomed. Nanotechnol. Biol. Med. 2012, 8, 1233–1241. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z.-X.; Lv, B.-F. MgO nanoparticles as antibacterial agent: Preparation and activity. Braz. J. Chem. Eng. 2014, 31, 591–601. [Google Scholar] [CrossRef]
- Sun, J.; Wang, S.; Zhao, D.; Hun, F.H.; Weng, L.; Liu, H. Cytotoxicity, permeability, and inflammation of metal oxide nanoparticles in human cardiac microvascular endothelial cells. Cell Biol. Toxicol. 2011, 27, 333–342. [Google Scholar] [CrossRef]
- Ge, S.; Wang, G.; Shen, Y.; Zhang, Q.; Jia, D.; Wang, H.; Dong, Q.; Yin, T. Cytotoxic effects of MgO nanoparticles on human umbilical vein endothelial cells in vitro. IET Nanobiotechnol. 2011, 5, 36–40. [Google Scholar] [CrossRef] [PubMed]
- Muneer, A.; Kumar, S.; Aqib, A.I.; Khan, S.R.; Shah, S.Q.A.; Zaheer, I.; ur Rehman, T.; Abbas, A.; Hussain, K.; Rehman, A.; et al. Evaluation of Sodium Alginate Stabilized Nanoparticles and Antibiotics against Drug Resistant Escherichia coli Isolated from Gut of Houbara Bustard Bird. Oxidative Med. Cell. Longev. 2022, 2022, 7627759. [Google Scholar] [CrossRef]
- Zaheer, T.; Kandeel, M.; Abbas, R.Z.; Khan, S.R.; Rehman, T.U.; Aqib, A.I. Acaricidal potential and ecotoxicity of metallic nano-pesticides used against the major life stages of hyalomma ticks. Life 2022, 12, 977. [Google Scholar] [CrossRef] [PubMed]
- Liman, R.; Ali, M.M.; Istifli, E.S.; Ciğerci, İ.H.; Bonciu, E. Genotoxic and cytotoxic effects of pethoxamid herbicide on Allium cepa cells and its molecular docking studies to unravel genotoxicity mechanism. Environ. Sci. Pollut. Res. 2022, 29, 63127–63140. [Google Scholar] [CrossRef]
- Ali, M.M.; Fatima, A.; Nawaz, S.; Rehman, A.; Javed, M.; Nadeem, A. Cytotoxic and genotoxic evaluation of bisphenol S on onion root tips by Allium cepa and comet tests. Environ. Sci. Pollut. Res. 2022, 29, 88803–88811. [Google Scholar] [CrossRef]
- El-Shahawy, I.; Abou Elenien, F. Enteric parasites of Egyptian captive birds: A general coprological survey with new records of the species. Trop Biomed 2015, 32, 650–658. [Google Scholar]
- Aditi, F.Y.; Rahman, S.S.; Hossain, M.M. A study on the microbiological status of mineral drinking water. Open Microbiol. J. 2017, 11, 31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anwar, M.A.; Aqib, A.I.; Ashfaq, K.; Deeba, F.; Khan, M.K.; Khan, S.R.; Muzammil, I.; Shoaib, M.; Naseer, M.A.; Riaz, T.; et al. Antimicrobial resistance modulation of MDR E. coli by antibiotic coated ZnO nanoparticles. Microb. Pathog. 2020, 148, 104450. [Google Scholar] [CrossRef] [PubMed]
- Bindhu, M.R.; Umadevi, M.; Micheal, M.K.; Arasu, M.V.; Al-Dhabi, N.A. Structural, morphological and optical properties of MgO nanoparticles for antibacterial applications. Mater. Lett. 2016, 166, 19–22. [Google Scholar] [CrossRef]
- Amaç, E.; Liman, R. Cytotoxic and genotoxic effects of clopyralid herbicide on Allium cepa roots. Environ. Sci. Pollut. Res. 2021, 28, 48450–48458. [Google Scholar] [CrossRef]
- Liman, R.; Başbuğ, B.; Ali, M.M.; Acikbas, Y.; Ciğerci, İ.H. Cytotoxic and genotoxic assessment of tungsten oxide nanoparticles in Allium cepa cells by Allium ana-telophase and comet assays. J. Appl. Genet. 2021, 62, 85–92. [Google Scholar] [CrossRef]
- Surwade, P.; Ghildyal, C.; Weikel, C.; Luxton, T.; Peloquin, D.; Fan, X.; Shah, V. Augmented antibacterial activity of ampicillin with silver nanoparticles against methicillin-resistant Staphylococcus aureus (MRSA). J. Antibiot. 2019, 72, 50–53. [Google Scholar] [CrossRef]
- El-Hamaky, A.M.A.; Hassan, A.A.; Wahba, A.K.A.; El Mosalamy, M.M.E.A. Influence of copper and zinc nanoparticles on genotyping characterizations of multi-drug resistance genes for some calf pathogens. Int. J. Vet. Sci. 2023, 12, 309–317. [Google Scholar] [CrossRef]
- Homem, N.C.; Miranda, C.A.F.D.S.; Antunes, J.I.D.C.; de Amorim, M.T.S.P.; Felgueiras, H.P. Modification of Ca2+-crosslinked sodium alginate/gelatin films with propolis for an improved antimicrobial action. Proceedings 2020, 69, 4. [Google Scholar]
- Aymen Naima Aqib, A.I.; Akram, K.; Majeed, H.; Murtaza, M.; Muneer, A.; Alsayeqh, A.F. Resistance modulation of dairy milk borne Streptococcus agalactiae and Klebsiella pneumoniae through metallic oxide nanoparticles. Pak. Vet. J. 2022, 42, 424–428. [Google Scholar] [CrossRef]
- Vimbela, G.V.; Ngo, S.M.; Fraze, C.; Yang, L.; Stout, D.A. Antibacterial properties, and toxicity from metallic nanomaterials. Int. J. Nanomed. 2017, 12, 3941. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mangalampalli, B.; Dumala, N.; Grover, P. Acute oral toxicity study of magnesium oxide nanoparticles and microparticles in female albino Wistar rats. Regul. Toxicol. Pharmacol. 2017, 90, 170–184. [Google Scholar] [CrossRef] [PubMed]
- Goetghebeur, M.; Landry, P.A.; Han, D.; Vicente, C. Methicillin-resistant Staphylococcus aureus: A public health issue with economic consequences. Can. J. Infect. Dis. Med. Microbiol. 2007, 18, 27–34. [Google Scholar] [CrossRef] [Green Version]
- Salsabil, S.S.; Ardana, V.P.; Larastiyasa, R.R.P.B.; Pratiwi, I.W.; Widianti, R.A.; Pratama, A.M. Nanoparticles of kirinyuh (Chromolaena odorata (L.) R.M. King & H. Rob.) leaves extract as a candidate for natural remedies lowering hypercholesterol: In silico and in vivo study. Pak. Vet. J. 2022, 42, 397–403. [Google Scholar] [CrossRef]
- Shnawa, B.H.; Jalil, P.J.; Aspoukeh, P.K.; Mohammed, D.A.; Biro, D.M. Protoscolicidal and biocompatibility properties of biologically fabricated zinc oxide nanoparticles using Ziziphus spina-christi leaves. Pak. Vet. J. 2022, 42, 517–525. [Google Scholar] [CrossRef]



| Concentration (mg/mL) | CCN | MI ± SD | Phase Index (%) ± SD | DNA Damage (Mean ± SD) | |||
|---|---|---|---|---|---|---|---|
| Prophase | Metaphase | Anaphase | Telophase | ||||
| 24 h | |||||||
| Control | 508 | 78.22 ± 0.12 a | 98.09 ± 0.72 a | 2.02 ± 0.12 a | 2.62 ± 0.28 a | 7.98 ± 0.19 a | 12 ± 0.99 a |
| MMS | 509 | 56.15 ± 0.67 b | 84.13 ± 0.06 b | 2.01 ± 0.16 b | 1.2 ± 0.01 b | 5.88 ± 0.87 b | 122 ± 0.75 b |
| 1.25 mg/mL Cefoxitin | 550 | 52.02 ± 0.11 b | 84.09 ± 0.11 b | 2.11 ± 0.02 b | 1.99 ± 1.16 b | 5.75 ± 0.12 b | 105 ± 0.99 b |
| 2.5 mg/mL Cefoxitin | 567 | 50.19 ± 0.28 b | 83.12 ± 0.02 b | 1.99 ± 0.02 b | 1.90 ± 0.01 b | 5.23 ± 0.05 b | 107 ± 0.19 b |
| 5 mg/mL Cefoxitin | 565 | 49.03 ± 0.92 b | 82.33 ± 0.41 b | 1.86 ± 0.13 b | 1.87 ± 0.28 b | 4.25 ± 0.14 b | 109 ± 2.11 b |
| 48 h | |||||||
| 1.25 mg/mL Cefoxitin | 567 | 50.32 ± 0.29 b | 83.09 ± 0.01 b | 1.85 ± 0.05 b | 1.89 ± 1.26 b | 5.55 ± 0.11 b | 116 ± 0.32 b |
| 2.5 mg/mL Cefoxitin | 555 | 49.11 ± 0.22 b | 82.12 ± 0.12 b | 1.59 ± 0.01 b | 1.70 ± 0.11 b | 5.33 ± 0.13 b | 126 ± 0.21 b |
| 5 mg/mL Cefoxitin | 545 | 48.26 ± 0.93 b | 82.03 ± 0.11 b | 1.56 ± 0.18 b | 1.77 ± 0.18 b | 5.13 ± 0.01 b | 120 ± 0.21 b |
| Concentration (mg/mL) | CCN | MI ± SD | Phase Index (%) ± SD | DNA Damage (Mean ± SD) | |||
|---|---|---|---|---|---|---|---|
| Prophase | Metaphase | Anaphase | Telophase | ||||
| 24 h | |||||||
| Control | 508 | 78.22 ± 0.12 a | 98.09 ± 0.72 a | 2.02 ± 0.12 a | 2.62 ± 0.28 a | 7.98 ± 0.19 a | 12 ± 0.99 a |
| MMS | 509 | 56.15 ± 0.67 b | 84.13 ± 0.02 b | 2.01 ± 0.16 b | 1.2 ± 0.01 b | 5.88 ± 0.87 b | 122 ± 0.75 b |
| 1.25 mg/mL MG | 550 | 51.21 ± 0.01 b | 83.09 ± 0.12 b | 2.25 ± 0.12 b | 1.89 ± 1.26 b | 5.71 ± 0.22 b | 105 ± 0.99 b |
| 2.5 mg/mL MG | 567 | 50.18 ± 0.32 b | 82.12 ± 0.12 b | 2.09 ± 0.01 b | 1.80 ± 0.22 b | 5.13 ± 0.15 b | 107 ± 0.19 b |
| 5 mg/mL MG | 565 | 49.13 ± 0.28 b | 81.33 ± 0.11 b | 1.76 ± 0.11 b | 1.17 ± 0.19 b | 4.25 ± 0.15 b | 109 ± 2.11 b |
| 48 h | |||||||
| 1.25 mg/mL MG | 567 | 49.12 ± 0.24 b | 82.09 ± 0.21 b | 1.75 ± 0.03 b | 1.99 ± 1.46 b | 5.15 ± 0.45 b | 116 ± 0.32 b |
| 2.5 mg/mL MG | 555 | 49.99 ± 0.02 b | 81.12 ± 0.32 b | 1.19 ± 0.04 b | 1.86 ± 0.31 b | 4.23 ± 0.56 b | 126 ± 0.21 b |
| 5 mg/mL MG | 545 | 48.16 ± 0.09 b | 80.03 ± 0.99 b | 1.66 ± 0.11 b | 1.77 ± 0.17 b | 4.83 ± 0.21 b | 120 ± 0.21 s |
| Concentration (mg/mL) | CCN | MI ± SD | Phase Index (%) ± SD | DNA Damage (Mean ± SD) | |||
|---|---|---|---|---|---|---|---|
| Prophase | Metaphase | Anaphase | Telophase | ||||
| 24 h | |||||||
| Control | 586 | 78.22 ± 0.12 a | 98.09 ± 0.72 a | 2.02 ± 0.12 a | 2.62 ± 0.28 a | 7.98 ± 0.19 a | 12 ± 0.99 a |
| MMS | 575 | 56.15 ± 0.67 b | 84.13 ± 0.06 b | 2.01 ± 0.16 b | 1.2 ± 0.01 b | 5.88 ± 0.87 b | 122 ± 0.75 b |
| 1.25 mg/mL GMC | 577 | 66.11 ± 0.11 a | 89.13 ± 0.23 a | 2.1 ± 0.12 a | 1.98 ± 0.16 a | 6.64 ± 0.90 a | 75 ± 0.19 a |
| 2.5 mg/mL | 550 | 63.19 ± 0.12 a | 88.19 ± 0.45 a | 2.02 ± 0.03 a | 1.78 ± 0.21 a | 6.33 ± 0.85 a | 85 ± 0.09 a |
| 5 mg/mL | 545 | 60.22 ± 0.12 a | 86.10 ± 0.11 a | 2.07 ± 0.18 a | 1.77 ± 0.22 a | 6.29 ± 0.21 a | 87 ± 1.02 a |
| 48 h | |||||||
| 1.25 mg/mL | 545 | 67.19 ± 0.01 a | 87.13 ± 0.13 a | 2.55 ± 0.12 a | 1.88 ± 0.18 a | 6.69 ± 0.09 a | 76 ± 0.06 a |
| 2.5 mg/mL GMC | 559 | 65.09 ± 0.02 a | 86.19 ± 0.05 a | 2.12 ± 0.13 a | 1.68 ± 0.11 a | 6.03 ± 0.05 a | 82 ± 0.07 a |
| 5 mg/mL | 550 | 62.12 ± 0.13 a | 86.98 ± 0.23 a | 2.08 ± 0.13 a | 1.57 ± 0.20 a | 6.09 ± 0.71 a | 85 ± 0.15 a |
| Concentration (mg/mL) | CCN | Phase Index (%) ± SD | DNA Damage (Mean ± SD) | ||||
|---|---|---|---|---|---|---|---|
| Prophase | Metaphase | Anaphase | Telophase | ||||
| 24 h | |||||||
| Control | 586 | 78.22 ± 0.12 a | 98.09 ± 0.72 a | 2.02 ± 0.12 a | 2.62 ± 0.28 a | 7.98 ± 0.19 a | 12 ± 0.99 a |
| MMS | 575 | 56.15 ± 0.67 b | 84.13 ± 0.06 b | 2.01 ± 0.16 b | 1.2 ± 0.01 b | 5.88 ± 0.87 b | 122 ± 0.75 b |
| 1.25 mg/mL GC | 577 | 65.11 ± 0.31 a | 88.19 ± 0.27 a | 2.99 ± 0.12 a | 1.78 ± 0.16 a | 6.94 ± 0.17 a | 64 ± 0.49 a |
| 2.5 mg/mL GC | 550 | 63.18 ± 0.02 a | 87.14 ± 0.49 a | 2.12 ± 0.23 a | 1.71 ± 0.21 a | 6.23 ± 0.15 a | 72 ± 0.68 a |
| 5 mg/mL GC | 545 | 62.28 ± 0.42 a | 86.02 ± 0.17 a | 2.99 ± 0.22 a | 1.08 ± 0.22 a | 5.99 ± 0.22 a | 72 ± 1.02 a |
| 48 h | |||||||
| 1.25 mg/mL GC | 545 | 66.89 ± 0.01 a | 87.99 ± 0.03 a | 2.95 ± 0.19 a | 1.68 ± 0.08 a | 6.69 ± 0.09 a | 79 ± 0.06 a |
| 2.5 mg/mL GC | 559 | 65.19 ± 0.22 a | 85.09 ± 0.04 a | 2.82 ± 0.53 a | 1.44 ± 0.16 a | 6.03 ± 0.02 a | 80 ± 0.97 a |
| 5 mg/mL GC | 555 | 64.99 ± 0.09 a | 84.11 ± 0.04 a | 2.92 ± 0.73 a | 1.32 ± 0.19 a | 5.923 ± 0.15 a | 80 ± 0.85 a |
| Concentration (ppm) | CCN | MI ± SD | Phase Index (%) ± SD | DNA Damage (Mean ± SD) | |||
|---|---|---|---|---|---|---|---|
| Prophase | Metaphase | Anaphase | Telophase | ||||
| Control | 5006 | 76.81 ± 0.92 a | 99.18 ± 0.61 a | 2.19 ± 0.21 a | 2.61 ± 0.11 a | 7.81 ± 0.88 a | 12 ± 0.99 a |
| MMS | 5704 | 59.15 ± 0.67 b | 86.13 ± 0.46 b | 2.01 ± 0.26 b | 1.3 ± 0.11 b | 6.15 ± 0.15 b | 122 ± 0.75 b |
| 24 h | |||||||
| 1.25 mg/mL GT | 504 | 75.25 ± 0.75 a | 95.43 ± 0.46 a | 2.13 ± 0.16 a | 2.09 ± 0.12 a | 7.85 ± 0.55 a | 22 ± 0.19 a |
| 2.5 mg/mL GT | 509 | 72.66 ± 0.99 a | 94.98 ± 0.63 a | 2.16 ± 0.13 a | 1.94 ± 0.28 a | 7.83 ± 0.63 a | 24 ± 0.05 a |
| 5 mg/mL | 566 | 71 ± 0.19 a | 94.92 ± 0.57 a | 2.18 ± 0.16 a | 1.96 ± 0.22 a | 7.01 ± 0.76 a | 25 ± 0.99 a |
| 48 h | |||||||
| 1.25 mg/mL GT | 516 | 74.25 ± 0.25 a | 95.03 ± 0.47 a | 2.01 ± 0.16 a | 2.19 ± 0.16 a | 7.01 ± 0.51 a | 22 ± 0.99 a |
| 2.5 mg/mL GT | 569 | 73.66 ± 0.91 a | 94.11 ± 0.06 a | 2.05 ± 0.11 a | 1.22 ± 0.18 a | 7.99 ± 0.12 a | 24 ± 0.75 a |
| 5 mg/mL | 568 | 74 ± 0.28 a | 94.92 ± 0.23 a | 2.22 ± 0.02 a | 1.98 ± 0.32 a | 7.91 ± 0.75 a | 25 ± 0.19 a |
| Concentration (ppm) | CCN | MI ± SD | Phase Index (%) ± SD | DNA Damage (Mean ± SD) | |||
|---|---|---|---|---|---|---|---|
| Prophase | Metaphase | Anaphase | Telophase | ||||
| 24 h | |||||||
| Control | 576 | 76.21 ± 0.92 a | 99.98 ± 0.61 a | 2.19 ± 0.11 a | 2.68 ± 0.29 a | 7.91 ± 0.89 a | 12 ± 0.19 a |
| MMS | 574 | 59.15 ± 0.67 b | 86.13 ± 0.46 b | 2.01 ± 0.26 b | 1.3 ± 0.11 b | 6.15 ± 0.15 b | 123 ± 0.75 b |
| 1.25 mg/mL GA | 577 | 72.11 ± 0.75 a | 95.43 ± 0.46 a | 2.13 ± 0.10 a | 2.99 ± 0.19 a | 7.85 ± 0.15 a | 19 ± 0.29 a |
| 2.5 mg/mL GA | 507 | 72.19 ± 0.99 a | 94.99 ± 0.13 a | 2.06 ± 0.03 a | 1.98 ± 0.08 a | 7.83 ± 0.60 a | 20 ± 0.22 a |
| 5 mg/mL GA | 545 | 71.72 ± 0.91 a | 93.15 ± 0.76 a | 2.07 ± 0.17 a | 1.97 ± 0.19 a | 7.98 ± 0.62 a | 19 ± 0.15 a |
| 48 h | |||||||
| 1.25 mg/mL GA | 518 | 73.86 ± 0.99 a | 93.92 ± 0.57 a | 2.18 ± 0.16 a | 1.96 ± 0.82 a | 7.21 ± 0.76 a | 20 ± 0.22 a |
| 2.5 mg/mL GA | 519 | 74.15 ± 0.25 a | 94.03 ± 0.47 a | 2.87 ± 0.19 a | 2.99 ± 0.86 a | 7.91 ± 0.71 a | 20 ± 1.99 a |
| 5 mg/mL GA | 569 | 73.16 ± 0.91 a | 94.11 ± 0.06 a | 2.85 ± 0.19 a | 1.12 ± 0.88 a | 7.97 ± 0.19 a | 20 ± 1.23 a |
| Concentration (ppm) | CCN | MI ± SD | Phase Index (%) ± SD | DNA Damage (Mean ± SD) | |||
|---|---|---|---|---|---|---|---|
| Prophase | Metaphase | Anaphase | Telophase | ||||
| 24 h | |||||||
| Control | 586 | 78.22 ± 0.12 a | 98.09 ± 0.72 a | 2.02 ± 0.12 a | 2.62 ± 0.28 a | 7.98 ± 0.19 a | 12 ± 0.99 a |
| MMS | 575 | 56.15 ± 0.67 b | 84.13 ± 0.06 b | 2.01 ± 0.16 b | 1.2 ± 0.01 b | 5.88 ± 0.87 b | 122 ± 0.75 b |
| 1.25 mg/mL GMT | 577 | 76.12 ± 0.81 a | 96.13 ± 0.36 a | 2.99 ± 0.12 a | 2.99 ± 0.17 a | 7.65 ± 0.92 a | |
| 2.5 mg/mL GMT | 550 | 75.09 ± 0.19 a | 94.19 ± 0.12 a | 2.09 ± 0.01 a | 1.68 ± 0.01 a | 7.13 ± 0.05 a | 25 ± 0.59 a |
| 5 mg/mL GMT | 545 | 75.02 ± 0.92 a | 94.10 ± 0.45 a | 2.06 ± 0.16 a | 1.87 ± 0.18 a | 7.25 ± 0.22 a | 27 ± 0.09 a |
| 48 h | |||||||
| 1.25 mg/mL GMT | 545 | 75.16 ± 0.19 a | 93.92 ± 0.06 a | 2.08 ± 0.26 a | 1.86 ± 0.82 a | 6.91 ± 0.06 a | 26 ± 0.02 a |
| 2.5 mg/mL GMT | 559 | 74.15 ± 0.25 a | 93.23 ± 0.17 a | 2.07 ± 0.79 a | 1.09 ± 0.86 a | 6.01 ± 0.22 a | 26 ± 0.01 a |
| 5 mg/mL GMT | 550 | 73.16 ± 0.91 a | 93.95 ± 0.06 a | 2.15 ± 0.02 a | 1.02 ± 0.88 a | 6.07 ± 0.35 a | 25 ± 0.12 a |
| Antibiotics/Nanoparticle | Combinations | Mean ± SD | Percentage (%) Variation |
|---|---|---|---|
| Tylosin | Alone | 14.000 ± 1.000 c | - |
| GT | 33.67 ± 5.13 a | 58.42% | |
| GMT | 26.00 ± 3.46 ab | 46.15% | |
| Cefoxitin | Alone | 18.00 ± 5.29 a | - |
| GC | 30.33 ± 1.528 a | 40.65% | |
| GMC | 25.00 ± 3.61 a | 40% | |
| Ampicillin | Alone | 24.00 ± 7.00 a | - |
| GA | 30.67 ± 8.08 a | 21.75% | |
| GMA | 33.67 ± 5.69 a | 28.72% | |
| MA | 23.33 ± 6.66 a | −2.87% | |
| Magnesium oxide | Alone | 18.67 ± 0.577 b | - |
| GMC | 25.00 ± 3.61 ab | 25.32% | |
| GMT | 26.00 ± 3.46 ab | 28.19% | |
| GMA | 33.67 ± 5.69 a | 44.55% | |
| MG | 22.67 ± 5.03 ab | 17.65% |
| Drug | 4 h | 8 h | 12 h | 16 h | 20 h | 24 h |
|---|---|---|---|---|---|---|
| GMA | 625.0 ± 0.0 a | 312.5 ± 0.0 b | 208.3 ± 90.2 a | 65.1 ± 22.6 b | 16.28 ± 5.64 c | 9.766 ± 0.000 b |
| GA | 833 ± 361 a | 625.0 ± 0.0 ab | 365 ± 239 a | 130.2 ± 45.1 ab | 52.1 ± 22.6 bc | 26.04 ± 11.28 ab |
| GT | 1042 ± 361 a | 833 ± 361 a | 521 ± 180 a | 104.2 ± 45.1 ab | 52.1 ± 22.6 bc | 13.02 ± 5.64 b |
| GMC | 625.0 ± 0.0 a | 312.5 ± 0.0 b | 156.3 ± 0.0 a | 130.2 ± 45.1 ab | 32.55 ± 11.28 bc | 19.53 ± 0.00 b |
| GMT | 625.0 ± 0.0 a | 312.5 ± 0.0 b | 156.3 ± 0.0 a | 78.13 ± 0.00 b | 39.06 ± 0.00 bc | 26.04 ± 11.28 ab |
| MG | 1042 ± 361 a | 521 ± 180 ab | 365 ± 239 a | 182.3 ± 119.3 ab | 65.1 ± 22.6 b | 39.06 ± 0.00 a |
| GC | 833 ± 361 a | 625.0 ± 0.0 ab | 312.5 ± 0.0 a | 260.4 ± 90.2 a | 156.3 ± 0.0 a | 39.06 ± 0.00 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Murtaza, M.; Aqib, A.I.; Khan, S.R.; Muneer, A.; Ali, M.M.; Waseem, A.; Zaheer, T.; Al-Keridis, L.A.; Alshammari, N.; Saeed, M. Sodium Alginate-Based MgO Nanoparticles Coupled Antibiotics as Safe and Effective Antimicrobial Candidates against Staphylococcus aureus of Houbara Bustard Birds. Biomedicines 2023, 11, 1959. https://doi.org/10.3390/biomedicines11071959
Murtaza M, Aqib AI, Khan SR, Muneer A, Ali MM, Waseem A, Zaheer T, Al-Keridis LA, Alshammari N, Saeed M. Sodium Alginate-Based MgO Nanoparticles Coupled Antibiotics as Safe and Effective Antimicrobial Candidates against Staphylococcus aureus of Houbara Bustard Birds. Biomedicines. 2023; 11(7):1959. https://doi.org/10.3390/biomedicines11071959
Chicago/Turabian StyleMurtaza, Maheen, Amjad Islam Aqib, Shanza Rauf Khan, Afshan Muneer, Muhammad Muddassir Ali, Ahmad Waseem, Tean Zaheer, Lamya Ahmed Al-Keridis, Nawaf Alshammari, and Mohd Saeed. 2023. "Sodium Alginate-Based MgO Nanoparticles Coupled Antibiotics as Safe and Effective Antimicrobial Candidates against Staphylococcus aureus of Houbara Bustard Birds" Biomedicines 11, no. 7: 1959. https://doi.org/10.3390/biomedicines11071959








