Radioprotective Effects of Carvacrol and/or Thymol against Gamma Irradiation-Induced Acute Nephropathy: In Silico and In Vivo Evidence of the Involvement of Insulin-like Growth Factor-1 (IGF-1) and Calcitonin Gene-Related Peptide
Abstract
:1. Introduction
2. Material and Methods
2.1. Drugs and Reagents
2.2. Radiation Facility
2.3. Animals and Treatments
2.4. Assessment of Serum Urea and Renal Oxidative Stress Biomarkers
2.5. Histopathological Examination
2.6. Assessment of Renal Inflammatory and Apoptotic Biomarkers
2.7. Assessment of IGF-1 and CGRP
2.8. Molecular Docking
2.9. Statistical Analysis
3. Results
3.1. Carvacrol and/or Thymol Ameliorates the γ-Irradiation-Induced Deterioration of Kidney Indices and Oxidative Stress
3.2. Carvacrol and/or Thymol Improves the γ-Irradiation-Induced Deterioration of Renal Histopathology
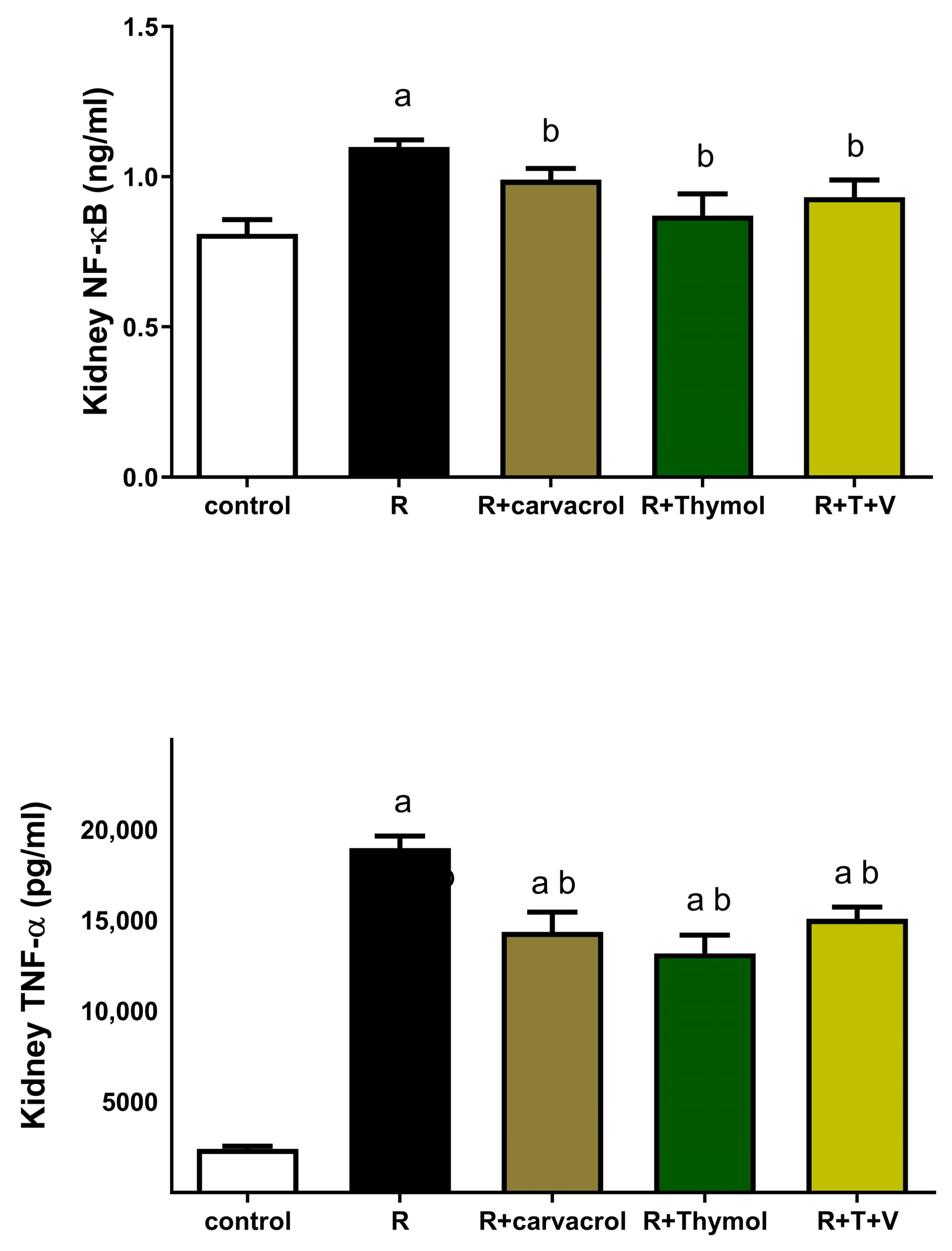
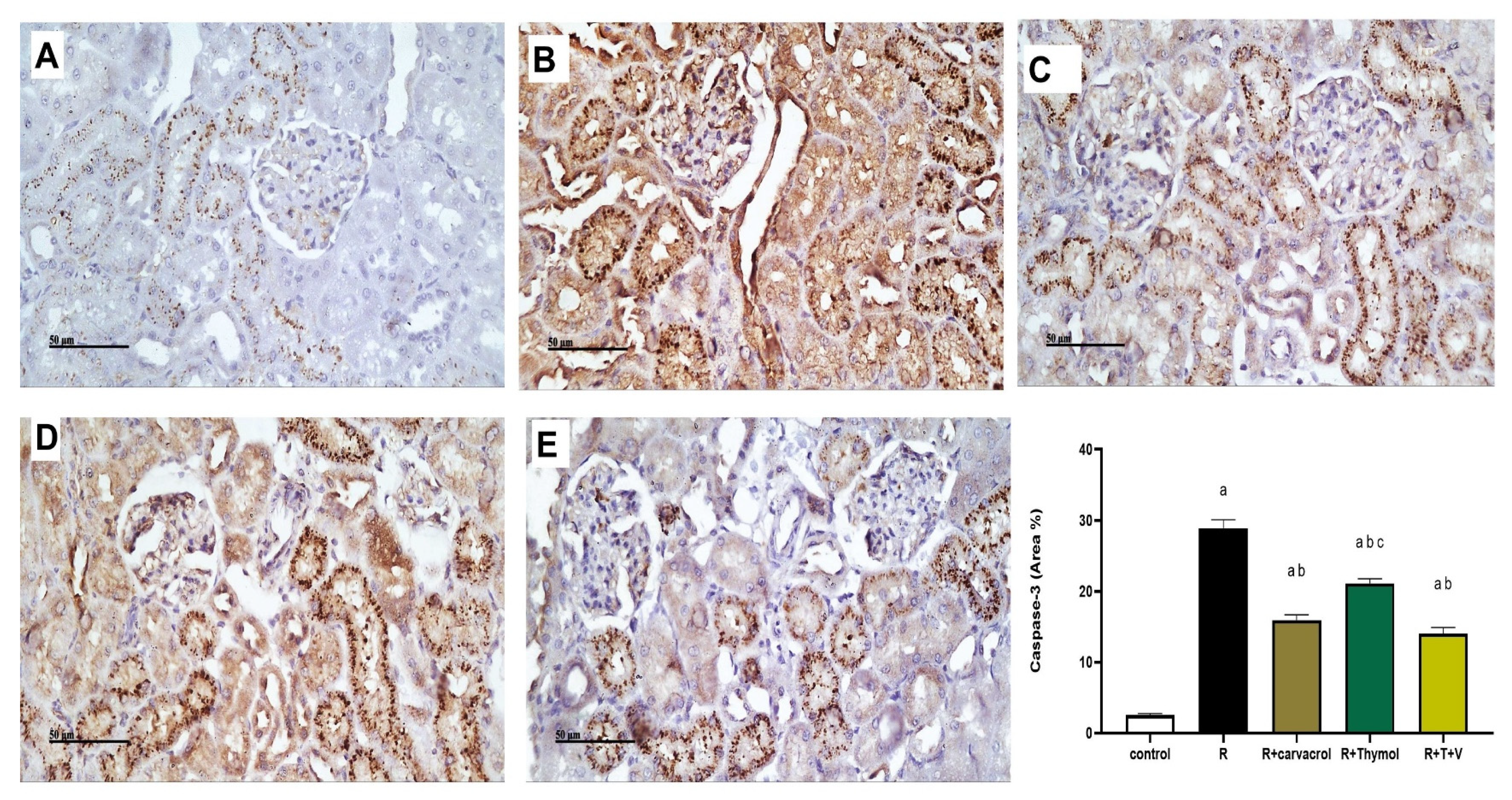
3.3. Carvacrol and/or Thymol Mitigates the γ-Irradiation-Induced Renal Inflammatory and Apoptotic Responses
3.4. Carvacrol and/or Thymol Suppresses the γ-Irradiation-Induced Upregulation of IGF-1 and CGRP Expressions
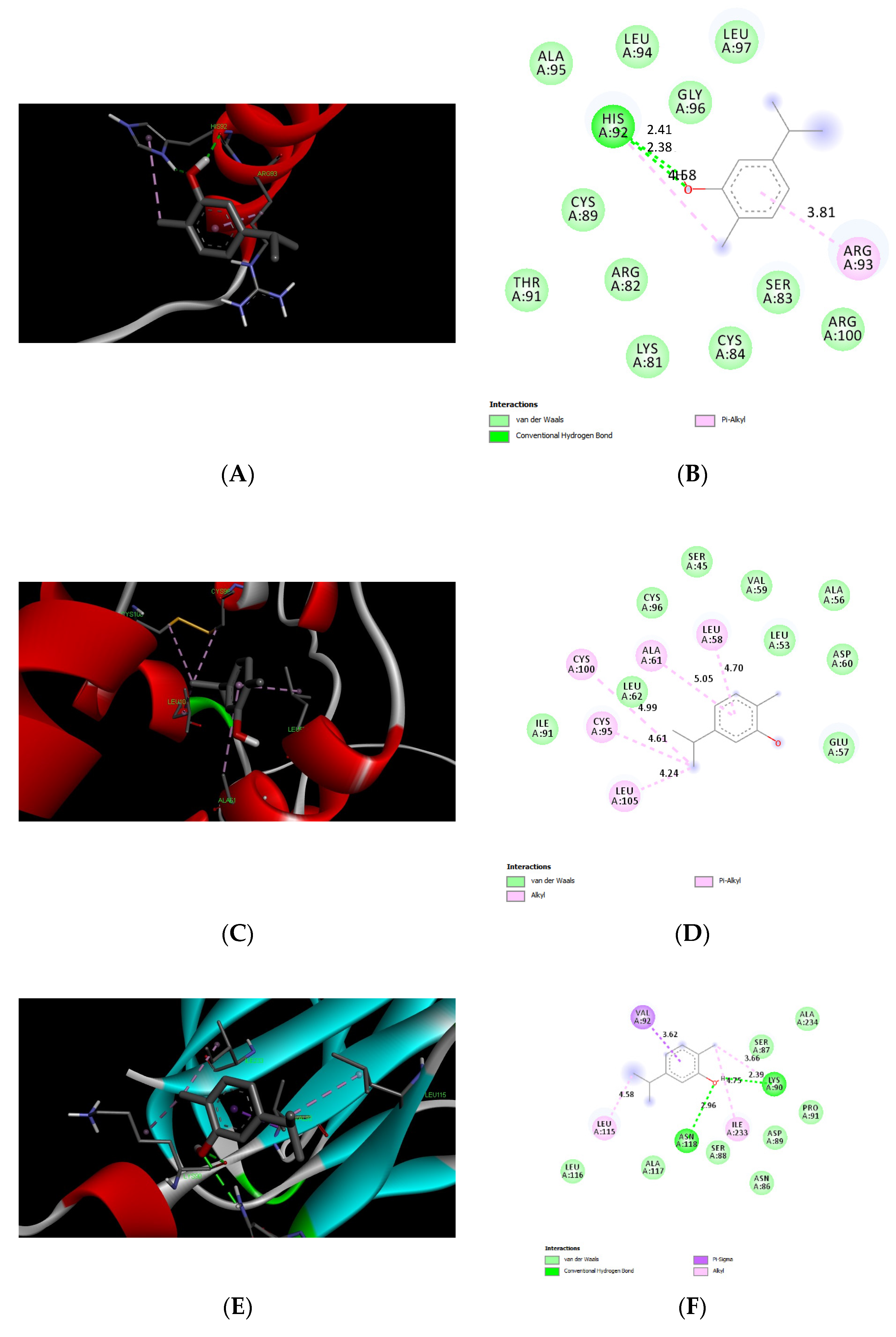
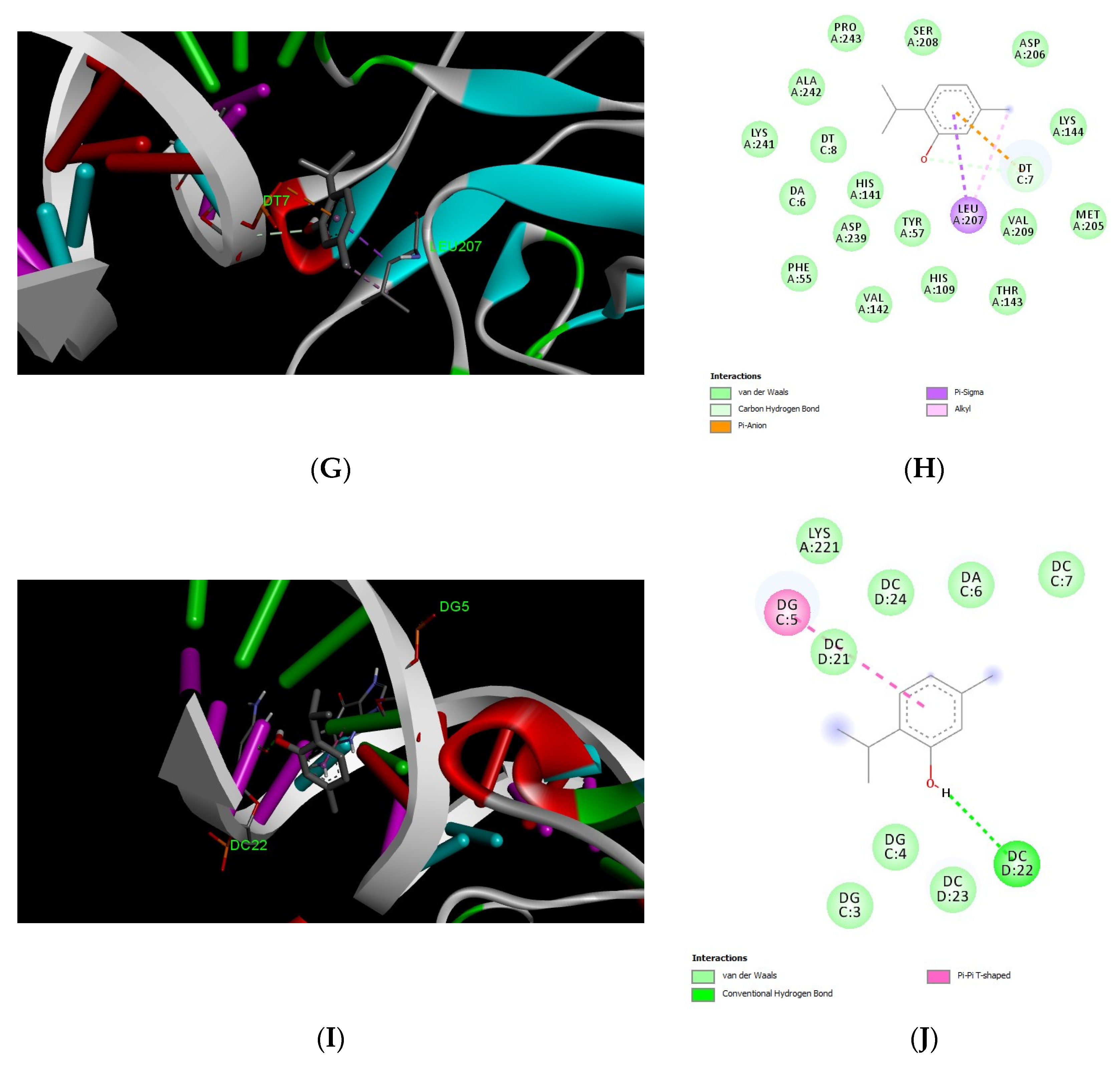
3.5. Docking Results of Carvacrol and Thymol
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA A Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Gersten, O.; Wilmoth, J.R. The Cancer Transition in Japan since 1951. Demogr. Res. 2002, 7, 271–306. [Google Scholar] [CrossRef]
- Glynne-Jones, R.; Wyrwicz, L.; Tiret, E.; Brown, G.; Rödel, C.; Cervantes, A.; Arnold, D. Rectal cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2017, 28 (Suppl. S4), iv22–iv40. [Google Scholar] [CrossRef]
- Early and Locally Advanced Non-Small-Cell Lung Cancer (NSCLC): ESMO Clinical Practice Guidelines for Diagnosis, Treatment and Follow-up†—Annals of Oncology. Available online: https://www.annalsofoncology.org/article/S0923-7534(19)42150-9/fulltext (accessed on 28 August 2023).
- Parker, C.; Gillessen, S.; Heidenreich, A.; Horwich, A. Cancer of the prostate: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up†. Ann. Oncol. 2015, 26, v69–v77. [Google Scholar] [CrossRef] [PubMed]
- Delaney, G.; Jacob, S.; Featherstone, C.; Barton, M. The role of radiotherapy in cancer treatment: Estimating optimal utilization from a review of evidence-based clinical guidelines. Cancer 2005, 104, 1129–1137. [Google Scholar] [CrossRef] [PubMed]
- Bristow, R.G.; Alexander, B.; Baumann, M.; Bratman, S.V.; Brown, J.M.; Camphausen, K.; Choyke, P.; Citrin, D.; Contessa, J.N.; Dicker, A.; et al. Combining precision radiotherapy with molecular targeting and immunomodulatory agents: A guideline by the American Society for Radiation Oncology. Lancet Oncol. 2018, 19, e240–e251. [Google Scholar] [CrossRef]
- Ward, J.F. Molecular mechanisms of radiation induced damage to nucleic acids California Univ., Los Angeles (USA). Lab. of Nuclear Medicine and Radiation Biology; 1973 January. Report No.: UCLA-12-924. Available online: https://www.osti.gov/biblio/6700951 (accessed on 28 August 2023).
- Piraux, E.; Caty, G.; Aboubakar Nana, F.; Reychler, G. Effects of exercise therapy in cancer patients undergoing radiotherapy treatment: A narrative review. SAGE Open Med. 2020, 8, 2050312120922657. [Google Scholar] [CrossRef]
- Klaus, R.; Niyazi, M.; Lange-Sperandio, B. Radiation-induced kidney toxicity: Molecular and cellular pathogenesis. Radiat. Oncol. 2021, 16, 43. [Google Scholar] [CrossRef]
- Schaue, D.; McBride, W.H. Opportunities and challenges of radiotherapy for treating cancer. Nat. Rev. Clin. Oncol. 2015, 12, 527–540. [Google Scholar] [CrossRef]
- Nevinny-Stickel, M.; Poljanc, K.; Forthuber, B.C.; Heute, D.; Posch, A.; Lechner, J.; Beer, B.; Lukas, P.; Seppi, T. Optimized conformal paraaortic lymph node irradiation is not associated with enhanced renal toxicity. Strahlenther. Onkol. 2007, 183, 385–391. [Google Scholar] [CrossRef]
- Bölling, T.; Schuck, A.; Rübe, C.; Hesselmann, S.; Pape, H.; Dieckmann, K.; Pöllinger, B.; Kortmann, R.D.; Speiser-Held, I.; Meyer, F.M.; et al. Therapy-associated late effects after irradiation of malignant diseases in childhood and adolescence. Feasibility analyses of a prospective multicenter register study. Strahlenther. Onkol. 2006, 182, 443–449. [Google Scholar] [CrossRef] [PubMed]
- Kaldir, M.; Cosar-Alas, R.; Cermik, T.F.; Yurut-Caloglu, V.; Saynak, M.; Altaner, S.; Caloglu, M.; Kocak, Z.; Tokatli, F.; Türe, M.; et al. Amifostine use in radiation-induced kidney damage. Preclinical evaluation with scintigraphic and histopathologic parameters. Strahlenther. Onkol. 2008, 184, 370–375. [Google Scholar] [CrossRef] [PubMed]
- Kala, J. Radiation-induced kidney injury. J. Onco-Nephrol. 2019, 3, 160–167. [Google Scholar] [CrossRef]
- Wang, J.Y.J. Cell Death Response to DNA Damage. Yale J. Biol. Med. 2019, 92, 771–779. [Google Scholar] [PubMed]
- Goligorsky, M.S. Chronic Kidney Disease: A Vicarious Relation to Premature Cell Senescence. Am. J. Pathol. 2020, 190, 1164–1171. [Google Scholar] [CrossRef]
- Morgan, M.A.; Lawrence, T.S. Molecular Pathways: Overcoming Radiation Resistance by Targeting DNA Damage Response Pathways. Clin. Cancer Res. 2015, 21, 2898–2904. [Google Scholar] [CrossRef]
- De Ruysscher, D.; Niedermann, G.; Burnet, N.G.; Siva, S.; Lee, A.W.M.; Hegi-Johnson, F. Radiotherapy toxicity. Nat. Rev. Dis. Primers 2019, 5, 13. [Google Scholar] [CrossRef]
- Yurut-Caloglu, V.; Caloglu, M.; Deniz-Yalta, T.; Aktoz, T.; Nurlu1, D.; Kilic-Durankus, N.; Arda, E.; Turkkan1, G.; İnci, O. Radiation-induced acute kidney toxicity: Protective effect of L-carnitine versus amifostine. Int. J. Radiat. Res. 2015, 13, 317–324. Available online: http://ijrr.com/article-1-1586-en.html (accessed on 28 August 2023).
- Brain, S.D.; Williams, T.J.; Tippins, J.R.; Morris, H.R.; MacIntyre, I. Calcitonin gene-related peptide is a potent vasodilator. Nature 1985, 313, 54–56. [Google Scholar] [CrossRef]
- Edwards, R.M.; Trizna, W. Calcitonin gene-related peptide: Effects on renal arteriolar tone and tubular cAMP levels. Am. J. Physiol. 1990, 258, F121–F125. [Google Scholar] [CrossRef]
- Zaidi, M.; Datta, H.; Bevis, P.J. Kidney: A target organ for calcitonin gene-related peptide. Exp. Physiol. 1990, 75, 27–32. [Google Scholar] [CrossRef]
- Zhong, B.; Ma, S.; Wang, D.H. Knockout of TRPV1 Exacerbates Ischemia-reperfusion-induced Renal Inflammation and Injury in Obese Mice. Vivo 2020, 34, 2259–2268. [Google Scholar] [CrossRef]
- Schnaiter, A.; Cai, L.; Andratschke, N.; Schill, S.; Weber, W.; Molls, M.; Nieder, C. A Model of Experimental Kidney Irradiation for Screening of Response Modifiers: Evaluation of Insulin-like Growth Factor-1 (IGF-1) and Erythropoietin (EPO). Int. J. Radiat. Oncol. Biol. Phys. 2005, 63, S471–S472. [Google Scholar] [CrossRef]
- Mahran, Y.F. New insights into the protection of growth hormone in cisplatin-induced nephrotoxicity: The impact of IGF-1 on the Keap1-Nrf2/HO-1 signaling. Life Sci. 2020, 253, 117581. [Google Scholar] [CrossRef]
- Borkum, J.M. CGRP and Brain Functioning: Cautions for Migraine Treatment. Headache 2019, 59, 1339–1357. [Google Scholar] [CrossRef]
- Won, L.; Kraig, R.P. Insulin-like growth factor-1 inhibits spreading depression-induced trigeminal calcitonin gene related peptide, oxidative stress & neuronal activation in rat. Brain Res. 2020, 1732, 146673. Available online: https://www.sciencedirect.com/science/article/pii/S0006899320300299 (accessed on 28 August 2023). [PubMed]
- Al-Khrashi, L.A.; Badr, A.M.; AL-Amin, M.A.; Mahran, Y.F. Thymol Ameliorates 5-Fluorouracil-Induced Intestinal Mucositis: Evidence of Down-Regulatory Effect on TGF-β/MAPK Pathways through NF-κB. J. Biochem. Mol. Toxicol. 2022, 36, e22932. [Google Scholar] [CrossRef] [PubMed]
- Antimicrobial Agents from Plants: Antibacterial Activity of Plant Volatile oils-Dorman-2000-Journal of Applied Microbiology—Wiley Online Library. Available online: https://sfamjournals.onlinelibrary.wiley.com/doi/full/10.1046/j.1365-2672.2000.00969.x (accessed on 28 August 2023).
- Abu-Elfotuh, K.; Abdel-Sattar, S.A.; Abbas, A.N.; Mahran, Y.F.; Alshanwani, A.R.; Hamdan, A.M.E.; Atwa, A.M.; Reda, E.; Ahmed, Y.M.; Zaghlool, S.S.; et al. The protective effect of thymoquinone or/and thymol against monosodium glutamate-induced attention-deficit/hyperactivity disorder (ADHD)-like behavior in rats: Modulation of Nrf2/HO-1, TLR4/NF-κB/NLRP3/caspase-1 and Wnt/β-Catenin signaling pathways in rat model. Biomed. Pharmacother. 2022, 155, 113799. Available online: https://www.sciencedirect.com/science/article/pii/S075333222201188X (accessed on 28 August 2023). [PubMed]
- Abedi, S.M.; Yarmand, F.; Motallebnejad, M.; Seyedmajidi, M.; Moslemi, D.; Bijani, A.; Hosseinimehr, S.J. Radioprotective Effect of Thymol Against Salivary Glands Dysfunction Induced by Ionizing Radiation in Rats. Iran. J. Pharm. Res. 2016, 15, 861–866. [Google Scholar]
- Mahran, Y.F.; Badr, A.M.; Aldosari, A.; Bin-Zaid, R.; Alotaibi, H.N. Carvacrol and Thymol Modulate the Cross-Talk between TNF-α and IGF-1 Signaling in Radiotherapy-Induced Ovarian Failure. Oxid. Med. Cell Longev. 2019, 2019, 3173745. [Google Scholar] [CrossRef]
- Archana, P.R.; Nageshwar Rao, B.; Satish Rao, B.S. Modulation of Gamma Ray–Induced Genotoxic Effect by Thymol, a Monoterpene Phenol Derivative of Cymene. Integr. Cancer Ther. 2011, 10, 374–383. [Google Scholar] [CrossRef]
- Arivalagan, S.; Thomas, N.S.; Kuppusamy, T.; Namashivayam, N. Radioprotective Effect of Carvacrol Against X-Radiation-Induced Cellular Damage in Cultured Human Peripheral Blood Lymphocytes. J. Environ. Pathol. Toxicol. Oncol. 2015, 34, 263–275. [Google Scholar] [CrossRef]
- Özmen, H.K.; Tanyelİ, F.N.E.A.A.; Yildirim, S.; Bayir, Y.; Eser, Y.Ş.G.; Kahramanlar, A. Thymol May be an Effective Agent in the Treatment ofLiver and Kidney Damages Caused by Ionizing Radiation. Turk. J. Oncol. 2021, 36, 71–78. [Google Scholar]
- El-Sayed, E.M.; Abd-Allah, A.R.; Mansour, A.M.; El-Arabey, A.A. Thymol and carvacrol prevent cisplatin-induced nephrotoxicity by abrogation of oxidative stress, inflammation, and apoptosis in rats. J. Biochem. Mol. Toxicol. 2015, 29, 165–172. [Google Scholar]
- Panahi kokhdan, E.; Sadeghi, H.; Kazemi, S.; Doustimotlagh, A.H. Nephroprotective Effects of Zataria multiflora Boiss. Hydroalcoholic Extract, Carvacrol, and Thymol on Kidney Toxicity Induced by Cisplatin in Rats. Evid.-Based Complement. Altern. Med. 2021, 2021, e8847212. [Google Scholar] [CrossRef]
- Gunes, S.; Ayhanci, A.; Sahinturk, V.; Altay, D.U.; Uyar, R. Carvacrol attenuates cyclophosphamide-induced oxidative stress in rat kidney. Can. J. Physiol. Pharmacol. 2017, 95, 844–849. [Google Scholar] [CrossRef]
- Badr, A.M.; Alkharashi, L.A.; Sherif, I.O.; Alanteet, A.A.; Alotaibi, H.N.; Mahran, Y.F. IL-17/Notch1/STAT3 Pathway Contributes to 5-Fluorouracil-Induced Intestinal Mucositis in Rats: Amelioration by Thymol Treatment. Pharmaceuticals 2022, 15, 1412. [Google Scholar] [CrossRef] [PubMed]
- Masuda, H.; Mori, N.; Matsui, Y.; Tsukiyama, K.; Nishimura, O.; Takeuchi, S.; Terada, Y.; Watanabe, T.; Nadamoto, T. Effects of Carvacrol and Volatile Fraction of Winter Savory (Satureja montana L.) on Body Temperature in Humans Who Experience Cold Sensitivity. Food Sci. Technol. Res. 2013, 19, 1085–1092. [Google Scholar]
- Yang, Y.; Chen, Q.; Jia, S.; He, L.; Wang, A.; Li, D.; Li, Y.; Li, X. Involvement of TRPV1 in the expression and release of calcitonin gene-related peptide induced by rutaecarpine. Mol. Med. Rep. 2018, 17, 5168–5174. [Google Scholar] [CrossRef] [PubMed]
- Koc, K.; Cerig, S.; Ucar, S.; Colak, S.; Bakir, M.; Erol, H.S.; Yildirim, S.; Hosseinigouzdagani, M.; Ozek, N.S.; Aysin, F.; et al. Gastroprotective effects of oleuropein and thymol on indomethacin-induced gastric ulcer in Sprague-Dawley rats. Drug Chem. Toxicol. 2020, 43, 441–453. [Google Scholar] [CrossRef] [PubMed]
- Türkcü, G.; Alabalık, U.; Keleş, A.N.; Bozkurt, M.; Ibiloğlu, I.; Fırat, U.; Büyükbayram, H. Protective effects of carvacrol and pomegranate against methotrexate-induced intestinal damage in rats. Int. J. Clin. Exp. Med. 2015, 8, 15474–15481. [Google Scholar]
- Andre, W.P.; Ribeiro, W.L.; Cavalcante, G.S.; Santos, J.M.; Macedo, I.T.; Paula, H.C.; de Freitas, R.M.; de Morais, S.M.; Melo, J.V.; Bevilaqua, C.M. Comparative efficacy and toxic effects of carvacryl acetate and carvacrol on sheep gastrointestinal nematodes and mice. Vet. Parasitol. 2016, 218, 52–58. [Google Scholar] [CrossRef]
- Jenner, P.M.; Hagan, E.C.; Taylor, J.M.; Cook, E.L.; Fitzhugh, O.G. Food flavourings and compounds of related structure I. Acute oral toxicity. Food Cosmet. Toxicol. 1964, 2, 327–343. Available online: https://www.sciencedirect.com/science/article/pii/S0015626464801929 (accessed on 28 August 2023). [CrossRef]
- Zotti, M.; Colaianna, M.; Morgese, M.G.; Tucci, P.; Schiavone, S.; Avato, P.; Trabace, L. Carvacrol: From ancient flavoring to neuromodulatory agent. Molecules 2013, 18, 6161–6172. [Google Scholar] [CrossRef]
- Frontiers|Pharmacological Properties and Molecular Mechanisms of Thymol: Prospects for Its Therapeutic Potential and Pharmaceutical Development. Available online: https://www.frontiersin.org/articles/10.3389/fphar.2017.00380/full (accessed on 28 August 2023).
- Beutler, E.; Duron, O.; Kelly, B.M. Improved method for the determination of blood glutathione. J. Lab. Clin. Med. 1963, 61, 882–888. [Google Scholar]
- Ohkawa, H.; Ohishi, N.; Yagi, K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 1979, 95, 351–358. [Google Scholar] [CrossRef]
- Banchroft, J.; Stevens, A.; Turner, D. Theory and Practice of Histological Techniques, 4th ed.; Churchill Livingstone: London, UK, 1996. [Google Scholar]
- Parker, G.A.; Cohen, E.P.; Li, N.; Takayama, K.; Farese, A.M.; MacVittie, T.J. Radiation Nephropathy in a Nonhuman Primate Model of Partial-Body Irradiation With Minimal Bone Marrow Sparing-Part 2: Histopathology, Mediators, and Mechanisms. Health Phys. 2019, 116, 409–425. [Google Scholar] [CrossRef] [PubMed]
- Wei, Q.; Wang, M.H.; Dong, Z. Differential Gender Differences in Ischemic and Nephrotoxic Acute Renal Failure. Am. J. Nephrol. 2005, 25, 491–499. [Google Scholar] [CrossRef]
- Bioengineering|Free Full-Text|Radiotherapy Advances in Renal Disease—Focus on Renal Ischemic Preconditioning. Available online: https://www.mdpi.com/2306-5354/10/1/68 (accessed on 28 August 2023).
- Cohen, E.P.; Lawton, C.A.; Moulder, J.E.; Becker, C.G.; Ash, R.C. Clinical course of late-onset bone marrow transplant nephropathy. Nephron 1993, 64, 626–635. [Google Scholar] [CrossRef] [PubMed]
- Sokolović, D.T.; Stojanović, N.M.; Mitić, K.; Randjelović, P.; Popović, D.; Karutanović, T.; Miljković, N.; Lalić, J.; Stevanović, M.; Radulović, N. Effects of a combined thymol and carvacrol application on rat kidney damage parameters after L-arginine application. Facta Univ. Ser. Phys. Chem. Technol. 2018, 16, 91. Available online: http://casopisi.junis.ni.ac.rs/index.php/FUPhysChemTech/article/view/4005 (accessed on 28 August 2023). [CrossRef]
- Wei, J.; Wang, B.; Wang, H.; Meng, L.; Zhao, Q.; Li, X.; Xin, Y.; Jiang, X. Radiation-Induced Normal Tissue Damage: Oxidative Stress and Epigenetic Mechanisms. Oxid. Med. Cell Longev. 2019, 2019, 3010342. [Google Scholar] [CrossRef] [PubMed]
- Lord, C.J.; Ashworth, A. The DNA damage response and cancer therapy. Nature 2012, 481, 287–294. [Google Scholar] [CrossRef]
- Mercantepe, T.; Topcu, A.; Rakici, S.; Tumkaya, L.; Yilmaz, A.; Mercantepe, F. The radioprotective effect of N-acetylcysteine against x-radiation-induced renal injury in rats. Envrion. Sci. Pollut. Res. Int. 2019, 26, 29085–29094. [Google Scholar] [CrossRef]
- Hormati, A.; Ahmadpour, S.; Afkhami Ardekani, M.; Khodadust, F.; Refahi, S. Radioprotective effects of montelukast, a selective leukotriene CysLT1 receptor antagonist, against nephrotoxicity induced by gamma radiation in mice. J. Biochem. Mol. Toxicol. 2020, 34, e22479. [Google Scholar] [CrossRef]
- Badr, A.; Fouad, D. Anti-Apoptotic and Anti-Inflammatory Effects of Olive Leaf Extract against Cisplatin-Induced Nephrotoxicity in Male Rats. Int. J. Pharmacol. 2016, 12, 675–688. [Google Scholar] [CrossRef]
- Chen, Y.-C.; Chen, C.-H.; Hsu, Y.-H.; Chen, T.-H.; Sue, Y.-M.; Cheng, C.-Y.; Chen, T.-W. Leptin reduces gentamicin-induced apoptosis in rat renal tubular cells via the PI3K-Akt signaling pathway. Eur. J. Pharmacol. 2011, 658, 213–218. [Google Scholar] [CrossRef]
- Li, W.; Yang, Y.; Li, Y.; Zhao, Y.; Jiang, H. Sirt5 Attenuates Cisplatin-Induced Acute Kidney Injury through Regulation of Nrf2/HO-1 and Bcl-2. Biomed. Res. Int. 2019, 2019, 4745132. [Google Scholar] [CrossRef]
- Chien, L.H.; Wu, C.T.; Deng, J.S.; Jiang, W.P.; Huang, W.C.; Huang, G.J. Salvianolic Acid C Protects against Cisplatin-Induced Acute Kidney Injury through Attenuation of Inflammation, Oxidative Stress and Apoptotic Effects and Activation of the CaMKK-AMPK-Sirt1-Associated Signaling Pathway in Mouse Models. Antioxidants 2021, 10, 1620. [Google Scholar] [CrossRef]
- Richter, C.; Gogvadze, V.; Laffranchi, R.; Schlapbach, R.; Schweizer, M.; Suter, M.; Walter, P.; Yaffee, M. Oxidants in mitochondria: From physiology to diseases. Biochim. Biophys. Acta. 1995, 1271, 67–74. [Google Scholar] [CrossRef]
- Khbouz, B.; Gu, S.; Pinto Coelho, T.; Lallemand, F.; Jouret, F. Radiotherapy Advances in Renal Disease-Focus on Renal Ischemic Preconditioning. Bioengineering 2023, 10, 68. [Google Scholar] [CrossRef] [PubMed]
- Borgmann, K.; Köcher, S.; Kriegs, M.; Mansour, W.Y.; Parplys, A.C.; Rieckmann, T.; Rothcamm, K. DNA Repair. Recent. Results Cancer Res. 2016, 198, 1–24. [Google Scholar] [PubMed]
- Mahamud, O.; So, J.; Chua, M.L.K.; Bristow, R.G. Targeting DNA repair for precision radiotherapy: Balancing the therapeutic ratio. Curr. Probl. Cancer 2017, 41, 265–272. [Google Scholar] [CrossRef] [PubMed]
- Maeba, S.; Ichiyama, T.; Ueno, Y.; Makata, H.; Matsubara, T.; Furukawa, S. Effect of montelukast on nuclear factor kappaB activation and proinflammatory molecules. Ann. Allergy Asthma Immunol. 2005, 94, 670–674. [Google Scholar] [CrossRef] [PubMed]
- Zheng, R.; Tan, Y.; Gu, M.; Kang, T.; Zhang, H.; Guo, L. N-acetyl cysteine inhibits lipopolysaccharide-mediated synthesis of interleukin-1β and tumor necrosis factor-α in human periodontal ligament fibroblast cells through nuclear factor-kappa B signaling. Medicine 2019, 98, e17126. [Google Scholar] [CrossRef] [PubMed]
- Bisht, A.; Dickens, M.; Rutherfurd-Markwick, K.; Thota, R.; Mutukumira, A.N.; Singh, H. Chlorogenic Acid Potentiates the Anti-Inflammatory Activity of Curcumin in LPS-Stimulated THP-1 Cells. Nutrients 2020, 12, E2706. [Google Scholar] [CrossRef] [PubMed]
- Morrison, J.F.B.; Dhanasekaran, S.; Howarth, F.C. Neuropeptides in the rat corpus cavernosum and seminal vesicle: Effects of age and two types of diabetes. Auton. Neurosci. Basic. Clin. 2009, 146, 76–80. [Google Scholar] [CrossRef]
- De Winter, B.Y.; Bredenoord, A.J.; Van Nassauw, L.; De Man, J.G.; De Schepper, H.U.; Timmermans, J.-P.; Pelckmans, P.A. Involvement of afferent neurons in the pathogenesis of endotoxin-induced ileus in mice: Role of CGRP and TRPV1 receptors. Eur. J. Pharmacol. 2009, 615, 177–184. [Google Scholar] [CrossRef]
- Smith, A.S.; Smid, S.D. Impaired capsaicin and neurokinin-evoked colonic motility in inflammatory bowel disease. J. Gastroenterol. Hepatol. 2005, 20, 697–704. [Google Scholar] [CrossRef]
- Engel, M.A.; Khalil, M.; Siklosi, N.; Mueller-Tribbensee, S.M.; Neuhuber, W.L.; Neurath, M.F.; Becker, C.; Reeh, P.W. Opposite effects of substance P and calcitonin gene-related peptide in oxazolone colitis. Dig. Liver Dis. 2012, 44, 24–29. [Google Scholar] [CrossRef]
- Engel, M.A.; Becker, C.; Reeh, P.W.; Neurath, M.F. Role of sensory neurons in colitis: Increasing evidence for a neuroimmune link in the gut. Inflamm. Bowel Dis. 2011, 17, 1030–1033. [Google Scholar] [CrossRef]
- Khan, A.A.; Diogenes, A.; Jeske, N.A.; Henry, M.A.; Akopian, A.; Hargreaves, K.M. Tumor necrosis factor α enhances the sensitivity of rat trigeminal neurons to capsaicin. Neuroscience 2008, 155, 503–509. [Google Scholar] [CrossRef] [PubMed]
- Tsuji, F.; Murai, M.; Oki, K.; Seki, I.; Ueda, K.; Inoue, H.; Nagelkerken, L.; Sasano, M.; Aono, H. Transient receptor potential vanilloid 1 agonists as candidates for anti-inflammatory and immunomodulatory agents. Eur. J. Pharmacol. 2010, 627, 332–339. [Google Scholar] [CrossRef]
- Demirbilek, S.; Ersoy, M.O.; Demirbilek, S.; Karaman, A.; Gürbüz, N.; Bayraktar, N.; Bayraktar, M. Small-Dose Capsaicin Reduces Systemic Inflammatory Responses in Septic Rats. Anesth. Analg. 2004, 99, 1501. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.-J.; Li, N.-S.; Zhang, Y.-S.; Liu, B.; Yang, Z.-C.; Li, Y.-J.; Dong, X.-R.; Peng, J. Vanillyl nonanoate protects rat gastric mucosa from ethanol-induced injury through a mechanism involving calcitonin gene-related peptide. Eur. J. Pharmacol. 2011, 666, 211–217. [Google Scholar] [CrossRef] [PubMed]
- El-Naga, R.N.; Mahran, Y.F. Indole-3-carbinol protects against cisplatin-induced acute nephrotoxicity: Role of calcitonin gene-related peptide and insulin-like growth factor-1. Sci. Rep. 2016, 6, 29857. [Google Scholar] [CrossRef]
- Li, J.; Carnevale, K.A.; Dipette, D.J.; Supowit, S.C. Renal protective effects of α-calcitonin gene-related peptide in deoxycorticosterone-salt hypertension. Am. J. Physiol. Ren. Physiol. 2013, 304, F1000–F1008. [Google Scholar] [CrossRef]
- Okajima, K.; Harada, N.; Uchiba, M.; Isobe, H. Activation of capsaicin-sensitive sensory neurons by carvedilol, a nonselective beta-blocker, in spontaneous hypertensive rats. J. Pharmacol. Exp. Ther. 2004, 309, 684–691. [Google Scholar] [CrossRef]
- Ledeganck, K.J.; Boulet, G.A.; Bogers, J.J.; Verpooten, G.A.; De Winter, B.Y. The TRPM6/EGF pathway is downregulated in a rat model of cisplatin nephrotoxicity. PLoS ONE 2013, 8, e57016. [Google Scholar] [CrossRef]
- Yasuda, H.; Kato, A.; Miyaji, T.; Zhou, H.; Togawa, A.; Hishida, A. Insulin-like growth factor-I increases p21 expression and attenuates cisplatin-induced acute renal injury in rats. Clin. Exp. Nephrol. 2004, 8, 27–35. [Google Scholar] [CrossRef]
- Badr, A.M.; El-Orabi, N.F.; Mahran, Y.F.; Badr, A.M.; Bayoumy, N.M.; Hagar, H.; Elmongy, E.I.; Atawia, R.T. In vivo and In silico evidence of the protective properties of carvacrol against experimentally-induced gastric ulcer: Implication of antioxidant, anti-inflammatory, and antiapoptotic mechanisms. Chem.-Biol. Interact. 2023, 382, 110649. [Google Scholar] [CrossRef]




| Treated Groups | BUN (mg/dL) | Glutathione (mM/mg Protein) | TBARS (nmol/mg Protein) |
|---|---|---|---|
| Control | 0.033 ± 0.004 | 0.109 ± 0.013 | 8.050 ± 0.645 |
| Irradiated | 0.095 a ± 0.020 | 0.003 a ± 0.005 | 12.330 a ± 0.551 |
| R+V | 0.048 a,b ± 0.004 | 0.195 a,b,c ± 0.019 | 8.020 b ± 0.560 |
| R+T | 0.042 a,b ± 0.004 | 0.209 a,b,c ± 0.024 | 7.484 b ± 0.317 |
| R+T+V | 0.067 a,b ± 0.005 | 0.304 a,b ± 0.016 | 6.660 b ± 0.528 |
| Protein Name | ID | ∆G (Binding Affinity Kcal/mol) | Amino Acids in Interaction | Type of Interaction |
|---|---|---|---|---|
| CGRP | AF_P01256_F1 | −4.4 | His 92 | Pi-alkyl H-bond |
| ARG 93 | Pi-alkyl | |||
| IGF1 | AF-P08025-F1 | −4.5 | Leu 58 | Pi-alkyl |
| Ala 61 | Pi-alkyl | |||
| Cys 100 | alkyl | |||
| Cys 95 | Alkyl | |||
| Leu 105 | alkyl | |||
| TNF-α | AF-P16599-F1 | −4.8 | Val 92 | Pi-sigma |
| Leu 115 | alkyl | |||
| Asn 118 | H-Bond | |||
| Lys 90 | H-Bond | |||
| Ile 233 | Alkyl | |||
| NF-κB | 1nfk | −6.2 | Pro 68 | alkyl |
| Pi-alkyl | ||||
| Phe 53 | Pi-alkyl | |||
| Gly 66 | h-bond | |||
| P65 | 1vkx | −5.5 | C 22 | h-bond |
| Protein Name | ID | ∆G (Binding Affinity Kcal/mol) | Amino Acids in Interaction | Type of Interaction |
|---|---|---|---|---|
| CGRP | AF_P01256_F1 | −4.4 | Leu 97 | Alkyl |
| Arg 93 | Pi-alkyl | |||
| His 92 | h-bond | |||
| IGF1 | AF-P08025-F1 | −4.5 | LUE 53 | Alkyl |
| Leu 58 | Pi-alkyl | |||
| Ala 61 | ||||
| TNF-α | AF-P16599-F1 | −4.8 | Lys 90 | Pi-alkyl |
| Pro 91 | Alkyl | |||
| Pi-alkyl | ||||
| Ala 234 | Pi-alkyl | |||
| NF-κB | 1nfk | −5.9 | T 7 | Pi-anion |
| Carbon hydrogen | ||||
| Leu 207 | Pi-sigma | |||
| alkyl | ||||
| P65 | 1vkx | −5.4 | G 5 | Pi-pi t shaped |
| C 22 | h-bond |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mahran, Y.F.; Al-Kharashi, L.A.; Atawia, R.T.; Alanazi, R.T.; Dhahi, A.M.B.; Alsubaie, R.; Badr, A.M. Radioprotective Effects of Carvacrol and/or Thymol against Gamma Irradiation-Induced Acute Nephropathy: In Silico and In Vivo Evidence of the Involvement of Insulin-like Growth Factor-1 (IGF-1) and Calcitonin Gene-Related Peptide. Biomedicines 2023, 11, 2521. https://doi.org/10.3390/biomedicines11092521
Mahran YF, Al-Kharashi LA, Atawia RT, Alanazi RT, Dhahi AMB, Alsubaie R, Badr AM. Radioprotective Effects of Carvacrol and/or Thymol against Gamma Irradiation-Induced Acute Nephropathy: In Silico and In Vivo Evidence of the Involvement of Insulin-like Growth Factor-1 (IGF-1) and Calcitonin Gene-Related Peptide. Biomedicines. 2023; 11(9):2521. https://doi.org/10.3390/biomedicines11092521
Chicago/Turabian StyleMahran, Yasmen F., Layla A. Al-Kharashi, Reem T. Atawia, Rawan Turki Alanazi, Amal M. Bin Dhahi, Rawd Alsubaie, and Amira M. Badr. 2023. "Radioprotective Effects of Carvacrol and/or Thymol against Gamma Irradiation-Induced Acute Nephropathy: In Silico and In Vivo Evidence of the Involvement of Insulin-like Growth Factor-1 (IGF-1) and Calcitonin Gene-Related Peptide" Biomedicines 11, no. 9: 2521. https://doi.org/10.3390/biomedicines11092521





