Noradrenergic Pathways Involved in Micturition in an Animal Model of Hydrocephalus—Implications for Urinary Dysfunction
Abstract
:1. Introduction
2. Materials and Methods
2.1. Surgical Induction of Hydrocephalus
2.2. Cystometric Analysis
2.3. Vascular Perfusion and Material Processing for Immunohistochemical Analysis
2.4. Immunohistochemical Analysis of DBH and Fos Expression
2.5. Statistical Analysis
3. Results
3.1. General Conditions of the Animals
3.2. Cystometric Analysis
3.3. Hydrocephalic Animals Presented Increases in the Expression of Noradrenaline Synthetizing Enzyme (Dopamine-β-Hydroxylase) in the Onuf’s Nucleus
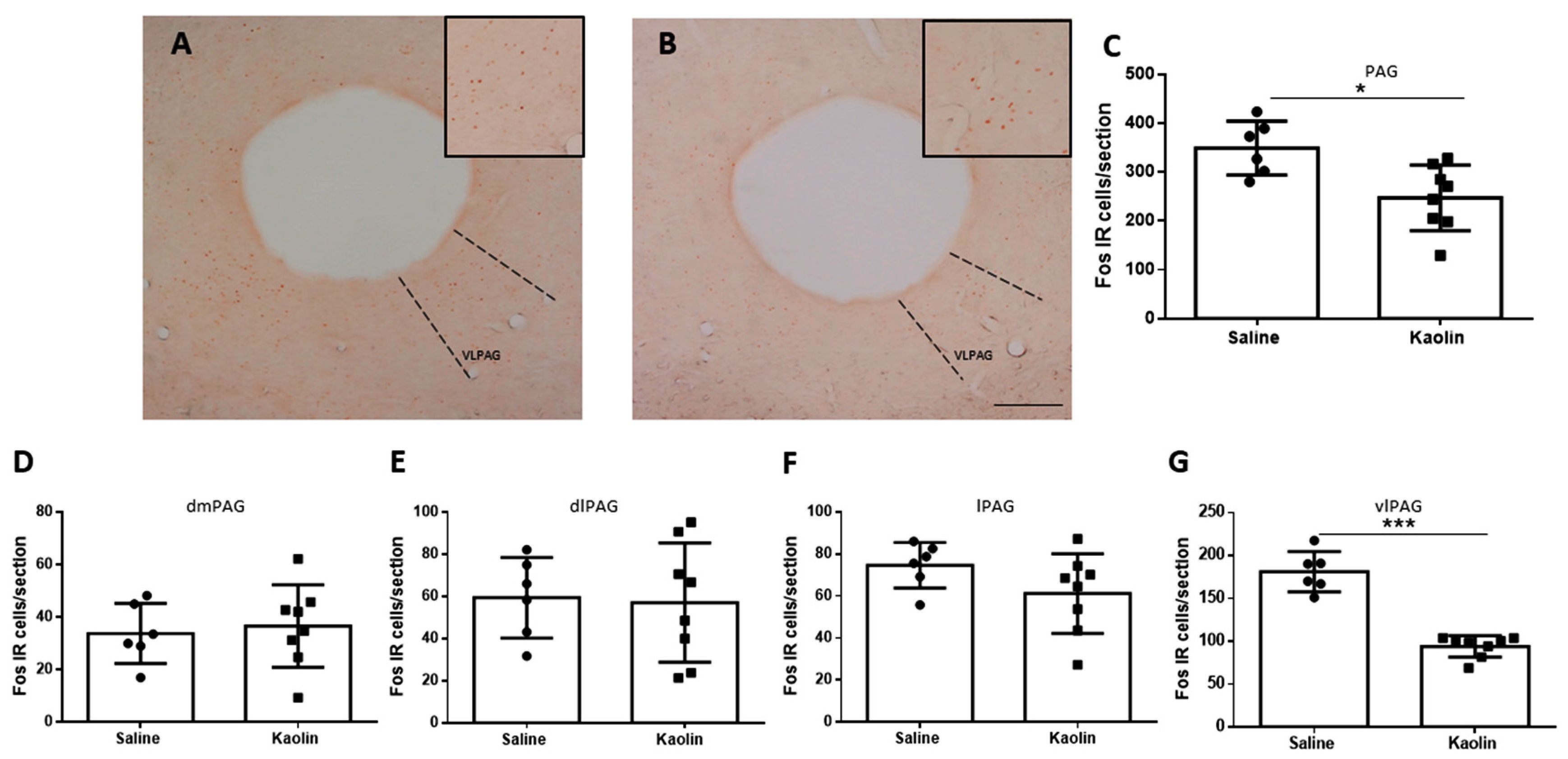
3.4. Hydrocephalic Animals Presented Decrease in Neuronal Activation of the vlPAG
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hochstetler, A.; Raskin, J.; Blazer-Yost, B.L. Hydrocephalus: Historical analysis and considerations for treatment. Eur. J. Med. Res. 2022, 27, 168. [Google Scholar] [CrossRef] [PubMed]
- Olopade, F.E.; Shokunbi, M.T.; Siren, A.L. The relationship between ventricular dilatation, neuropathological and neurobehavioural changes in hydrocephalic rats. Fluids Barriers CNS 2012, 9, 1–10. [Google Scholar] [CrossRef]
- Xu, H.; Zhang, S.L.; Tan, G.W.; Zhu, H.W.; Huang, C.Q.; Zhang, F.F.; Wang, Z.X. Reactive gliosis and neuroinflammation in rats with communicating hydrocephalus. Neuroscience 2012, 218, 317–325. [Google Scholar] [CrossRef] [PubMed]
- Del Bigio, M.R. Neuropathology and structural changes in hydrocephalus. Dev. Disabil. Res. Rev. 2010, 16, 16–22. [Google Scholar] [CrossRef]
- Sakakibara, R. Lower urinary tract dysfunction in patients with brain lesions. Handb. Clin. Neurol. 2015, 130, 269–287. [Google Scholar] [CrossRef] [PubMed]
- Sakakibara, R.; Uchida, Y.; Ishii, K.; Kazui, H.; Hashimoto, M.; Ishikawa, M.; Yuasa, T.; Kishi, M.; Ogawa, E.; Tateno, F.; et al. Correlation of right frontal hypoperfusion and urinary dysfunction in iNPH: A SPECT study. Neurourol. Urodyn. 2012, 31, 50–55. [Google Scholar] [CrossRef]
- Sakakibara, R.; Kanda, T.; Sekido, T.; Uchiyama, T.; Awa, Y.; Ito, T.; Liu, Z.; Yamamoto, T.; Yamanishi, T.; Yuasa, T. Mechanism of bladder dysfunction in idiopathic normal pressure hydrocephalus. Neurourol. Urodyn. 2008, 27, 507–510. [Google Scholar] [CrossRef]
- Griffiths, D. Functional imaging of structures involved in neural control of the lower urinary tract. Handb. Clin. Neurol. 2015, 130, 121–133. [Google Scholar] [CrossRef]
- Griffiths, D.J.; Fowler, C.J. The micturition switch and its forebrain influences. Acta Physiol. 2013, 207, 93–109. [Google Scholar] [CrossRef]
- Meriaux, C.; Hohnen, R.; Schipper, S.; Zare, A.; Jahanshahi, A.; Birder, L.A.; Temel, Y.; van Koeveringe, G.A. Neuronal activation in the periaqueductal gray matter upon electrical stimulation of the bladder. Front. Cell Neurosci. 2018, 12, 2018. [Google Scholar] [CrossRef]
- Rao, Y.; Gao, Z.; Li, X.; Li, X.; Li, J.; Liang, S.; Li, D.; Zhai, J.; Yan, J.; Yao, J.; et al. Ventrolateral Periaqueductal Gray Neurons Are Active During Urination. Front. Cell Neurosci. 2022, 16, 865186. [Google Scholar] [CrossRef] [PubMed]
- Zare, A.; Jahanshahi, A.; Rahnama’i, M.S.; Schipper, S.; van Koeveringe, G.A. The Role of the Periaqueductal Gray Matter in Lower Urinary Tract Function. Mol. Neurobiol. 2019, 56, 920–934. [Google Scholar] [CrossRef]
- Stone, E.; Coote, J.H.; Allard, J.; Lovick, T.A. GABAergic control of micturition within the periaqueductal grey matter of the male rat. J. Physiol. 2011, 589, 2065–2078. [Google Scholar] [CrossRef]
- Valentino, R.J.; Sheng, C.; Yan, Z.; Aston-Jones, G. Evidence for divergent projections to the brain noradrenergic system and the spinal parasympathetic system from Barrington’s nucleus. Brain Res. 1996, 732, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Bruinstroop, E.; Cano, G.; Vanderhorst, V.G.; Cavalcante, J.C.; Wirth, J.; Sena-Esteves, M.; Saper, C.B. Spinal projections of the A5, A6 (locus coeruleus), and A7 noradrenergic cell groups in rats. J. Comp. Neurol. 2012, 520, 1985–2001. [Google Scholar] [CrossRef] [PubMed]
- Bajic, D.; Proudfit, H.K.; Van Bockstaele, E.J. Periaqueductal gray neurons monosynaptically innervate extranuclear noradrenergic dendrites in the rat pericoerulear region. J. Comp. Neurol. 2000, 427, 649–662. [Google Scholar] [CrossRef]
- Manohar, A.; Curtis, A.L.; Zderic, S.A.; Valentino, R.J. Brainstem network dynamics underlying the encoding of bladder information. eLife 2017, 6, 2017. [Google Scholar] [CrossRef]
- Schellino, R.; Boido, M.; Vercelli, A. The Dual Nature of Onuf’s Nucleus: Neuroanatomical Features and Peculiarities, in Health and Disease. Front. Neuroanat. 2020, 14, 572013. [Google Scholar] [CrossRef]
- De Groat, W.C.; Griffiths, D.; Yoshimura, N. Neural control of the lower urinary tract. Compr. Physiol. 2015, 5, 327–396. [Google Scholar] [CrossRef]
- Rouzade-Dominguez, M.-L.; Curtis, A.L.; Valentino, R.J. ‘Role of Barrington’s Nucleus in the Activation of Rat Locus Coeruleus Neurons by Colonic Distension. 2001. Available online: www.elsevier.com/locate/bres (accessed on 10 August 2023).
- Louçano, M.; Oliveira, J.; Martins, I.; Vaz, R.; Tavares, I. Pain Modulation from the Locus Coeruleus in a Model of Hydrocephalus: Searching for Oxidative Stress-Induced Noradrenergic Neuroprotection. Int. J. Mol. Sci. 2022, 23, 3970. [Google Scholar] [CrossRef]
- Del Bigio, M.R. Neuropathological changes caused by hydrocephalus. Acta Neuropathol. 1993, 85, 573–585. [Google Scholar] [CrossRef] [PubMed]
- Di Curzio, D.L. ‘Animal Models of Hydrocephalus. Open J. Mod. Neurosurg. 2018, 8, 57–71. [Google Scholar] [CrossRef]
- Cabuk, B.; Etus, V.; Bozkurt, S.U.; Sav, A.; Ceylan, S. Neuroprotective effect of memantine on hippocampal neurons in infantile rat hydrocephalus. Turk. Neurosurg. 2011, 21, 352–358. [Google Scholar] [CrossRef] [PubMed]
- Yuan, W.; McAllister, J.P.; Lindquist, D.M.; Gill, N.; Holland, S.K.; Henkel, D.; Rajagopal, A.; Mangano, F.T. Diffusion tensor imaging of white matter injury in a rat model of infantile hydrocephalus. Child’s Nerv. Syst. 2012, 28, 47–54. [Google Scholar] [CrossRef]
- Okii, N.; Amano, T.; Seki, T.; Matsubayashi, H.; Mukai, H.; Ono, Y.; Kurisu, K.; Sakai, N. Fragmentation of protein kinase N (PKN) in the hydrocephalic rat brain. Acta Histochem. Cytochem. 2007, 40, 113–121. [Google Scholar] [CrossRef]
- Olopade, F.E.; Shokunbi, M.T. Neurobehavioral Deficits in Progressive Experimental Hydrocephalus in Neonatal Rats. Niger. J. Physiol. Sci. 2017, 31, 105–113. [Google Scholar] [PubMed]
- Potes, C.S.; Neto, F.L.; Castro-Lopes, J.M. Inhibition of pain behavior by GABAB receptors in the thalamic ventrobasal complex: Effect on normal rats subjected to the formalin test of nociception. Brain Res. 2006, 1115, 37–47. [Google Scholar] [CrossRef]
- Von Euler, M.; Akesson, E.; Samuelsson, E.B.; Seiger, A.; Sundstrom, E. Motor performance score: A new algorithm for accurate behavioral testing of spinal cord injury in rats. Exp. Neurol. 1996, 137, 242–254. [Google Scholar] [CrossRef]
- Coelho, A.; Oliveira, R.; Cruz, F.; Cruz, C.D. Impairment of sensory afferents by intrathecal administration of botulinum toxin A improves neurogenic detrusor overactivity in chronic spinal cord injured rats. Exp. Neurol. 2016, 285, 159–166. [Google Scholar] [CrossRef]
- Allard, S.; Gosein, V.; Cuello, A.C.; Ribeiro-da-Silva, A. Changes with aging in the dopaminergic and noradrenergic innervation of rat neocortex. Neurobiol. Aging 2011, 32, 2244–2253. [Google Scholar] [CrossRef]
- Cerpa, J.-C.; Marchand, A.R.; Coutureau, E. Distinct regional patterns in noradrenergic innervation of the rat prefrontal cortex. J. Chem. Neuroanat. 2019, 96, 102–109. [Google Scholar] [CrossRef] [PubMed]
- Hughes, S.W.; Hickey, L.; Hulse, R.P.; Lumb, B.M.; Pickering, A.E. Endogenous analgesic action of the pontospinal noradrenergic system spatially restricts and temporally delays the progression of neuropathic pain following tibial nerve injury. Pain. 2013, 154, 1680–1690. [Google Scholar] [CrossRef]
- Kovács, K.J. Measurement of Immediate-Early Gene Activation-c-fos and Beyond. J. Neuroendocrinol. 2008, 20, 665–672. [Google Scholar] [CrossRef] [PubMed]
- Perrin-Terrin, A.-S.; Jeton, F.; Pichon, A.; Frugière, A.; Richalet, J.-P.; Bodineau, L.; Voituron, N. The c-FOS Protein Immunohistological Detection: A Useful Tool as a Marker of Central Pathways Involved in Specific Physiological Responses In Vivo and Ex Vivo. J. Vis. Exp. 2016, 110, e53613. [Google Scholar] [CrossRef]
- Santos, P.L.; Brito, R.G.; Matos, J.P.S.C.F.; Quintans, J.S.S.; Quintans-Júnior, L.J. Fos Protein as a Marker of Neuronal Activity: A Useful Tool in the Study of the Mechanism of Action of Natural Products with Analgesic Activity. Mol. Neurobiol. 2018, 55, 4560–4579. [Google Scholar] [CrossRef] [PubMed]
- Paxinos, G.; Watson, C. The Rat Brain in Stereotaxic Coordinates, 5th ed.; Elsevier Science & Technology Books: San Diego, CA, USA, 2004. [Google Scholar]
- Ma, L.; Tang, J.-Y.; Zhou, J.-Y.; Zhou, P.; Zhu, C.; Zhang, X.; Wang, Y.; Ding, J.-Q.; Jia, H.-Q.; Gu, X.-J. The role of lumbosacral innervating noradrenergic neurons in micturition control. Brain Res. 2022, 1777, 147754. [Google Scholar] [CrossRef]
- Pinto, M.; Lima, D.; Tavares, I. Neuronal activation at the spinal cord and medullary pain control centers after joint stimulation: A c-fos study in acute and chronic articular inflammation. Neuroscience 2007, 147, 1076–1089. [Google Scholar] [CrossRef]
- Kitta, T.; Matsumoto, M.; Tanaka, H.; Mitsui, T.; Yoshioka, M.; Nonomura, K. GABAergic mechanism mediated via D receptors in the rat periaqueductal gray participates in the micturition reflex: An in vivo microdialysis study. Eur. J. Neurosci. 2008, 27, 3216–3225. [Google Scholar] [CrossRef]
- Sakakibara, R.; Yamamoto, T.; Sekido, N.; Sawai, S. How brain diseases affect the lower urinary tract function? Bladder 2023, 10, e21200001. [Google Scholar] [CrossRef]
- Rendtorff, R.; Novak, A.; Tunn, R. Normal pressure hydrocephalus as cause of urinary incontinence—A shunt for incontinence. Geburtshilfe Frauenheilkd. 2012, 72, 1130–1131. [Google Scholar] [CrossRef]
- Romeiro, T.H.; Da Silva, S.C.; Beggiora, P.d.S.; Sampaio, G.B.; Brandão, R.A.; Santos, M.V.; Machado, H.R.; Lopes, L.d.S. The association of Edaravone with shunt surgery improves behavioral performance, reduces astrocyte reaction and apoptosis, and promotes neuroprotection in young hydrocephalic rats. J. Chem. Neuroanat. 2022, 119, 102059. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Louçano, M.; Coelho, A.; Chambel, S.S.; Prudêncio, C.; Cruz, C.D.; Tavares, I. Noradrenergic Pathways Involved in Micturition in an Animal Model of Hydrocephalus—Implications for Urinary Dysfunction. Biomedicines 2024, 12, 215. https://doi.org/10.3390/biomedicines12010215
Louçano M, Coelho A, Chambel SS, Prudêncio C, Cruz CD, Tavares I. Noradrenergic Pathways Involved in Micturition in an Animal Model of Hydrocephalus—Implications for Urinary Dysfunction. Biomedicines. 2024; 12(1):215. https://doi.org/10.3390/biomedicines12010215
Chicago/Turabian StyleLouçano, Marta, Ana Coelho, Sílvia Sousa Chambel, Cristina Prudêncio, Célia Duarte Cruz, and Isaura Tavares. 2024. "Noradrenergic Pathways Involved in Micturition in an Animal Model of Hydrocephalus—Implications for Urinary Dysfunction" Biomedicines 12, no. 1: 215. https://doi.org/10.3390/biomedicines12010215




