Neuroprotective Effects of Krypton Inhalation on Photothrombotic Ischemic Stroke
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Animals
2.2. Modeling of Photothrombotic Ischemic Stroke
2.3. Exposure to Krypton
2.4. Assessment of Neurological Status
2.5. Brain MRI
2.6. Immunohistochemical Study
2.7. Western Blot Analysis
2.8. Statistical Analysis
3. Results
3.1. Krypton Protects Rat Brains Exposed to PIS
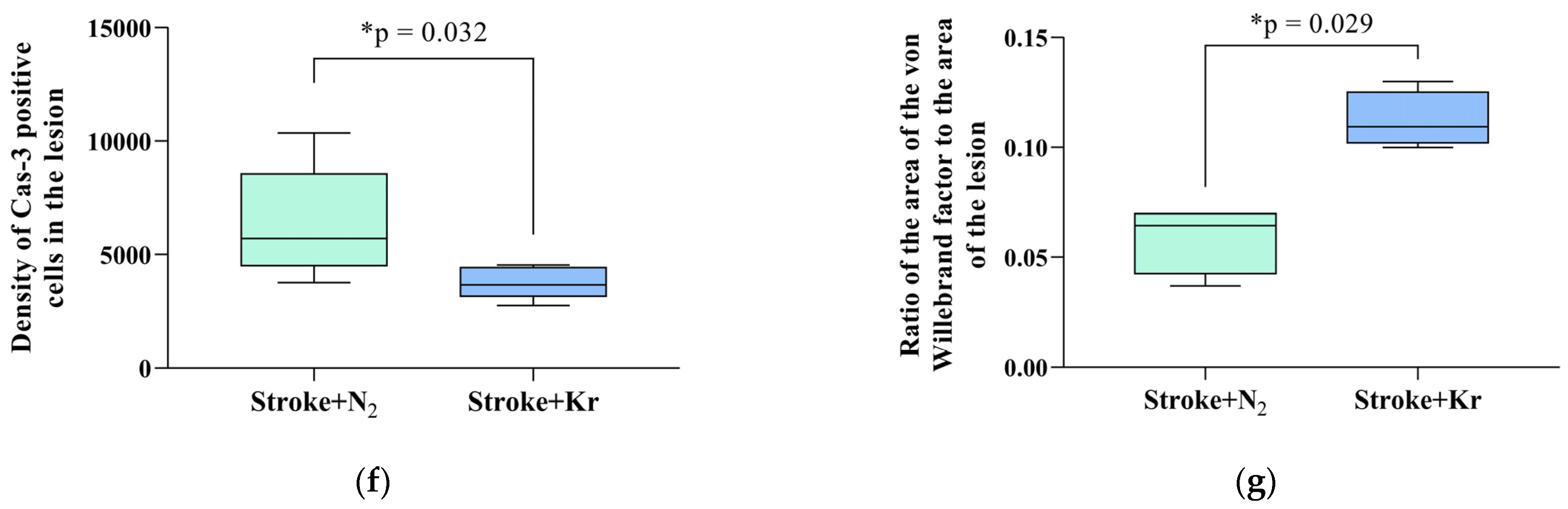
3.2. Krypton Inhibits Neuroinflammation and Cell Death after Ischemic Stroke
3.3. Molecular Mechanisms of Neuroprotection
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yin, H.; Chen, Z.; Zhao, H.; Huang, H.; Liu, W. Noble Gas and Neuroprotection: From Bench to Bedside. Front. Pharmacol. 2022, 13, 1028688. [Google Scholar] [CrossRef]
- Zhao, H.; Mitchell, S.; Ciechanowicz, S.; Savage, S.; Wang, T.; Ji, X.; Ma, D. Argon Protects against Hypoxic-Ischemic Brain Injury in Neonatal Rats through Activation of Nuclear Factor (Erythroid-Derived 2)-like 2. Oncotarget 2016, 7, 25640–25651. [Google Scholar] [CrossRef]
- Rojo, A.I.; Sagarra, M.R.D.; Cuadrado, A. GSK-3β down-Regulates the Transcription Factor Nrf2 after Oxidant Damage: Relevance to Exposure of Neuronal Cells to Oxidative Stress. J. Neurochem. 2008, 105, 192–202. [Google Scholar] [CrossRef]
- Ma, S.; Chu, D.; Li, L.; Creed, J.A.; Ryang, Y.M.; Sheng, H.; Yang, W.; Warner, D.S.; Turner, D.A.; Hoffmann, U. Argon Inhalation for 24 Hours After Onset of Permanent Focal Cerebral Ischemia in Rats Provides Neuroprotection and Improves Neurologic Outcome. Crit. Care Med. 2019, 47, E693–E699. [Google Scholar] [CrossRef]
- Bellezza, I.; Giambanco, I.; Minelli, A.; Donato, R. Nrf2-Keap1 Signaling in Oxidative and Reductive Stress. Biochim. Biophys. Acta Mol. Cell Res. 2018, 1865, 721–733. [Google Scholar] [CrossRef]
- Ma, Q. Role of Nrf2 in Oxidative Stress and Toxicity. Annu. Rev. Pharmacol. Toxicol. 2013, 53, 401–426. [Google Scholar] [CrossRef]
- Moro, F.; Fossi, F.; Magliocca, A.; Pascente, R.; Sammali, E.; Baldini, F.; Tolomeo, D.; Micotti, E.; Citerio, G.; Stocchetti, N.; et al. Efficacy of Acute Administration of Inhaled Argon on Traumatic Brain Injury in Mice. Br. J. Anaesth. 2021, 126, 256–264. [Google Scholar] [CrossRef]
- Antonova, V.V.; Silachev, D.N.; Ryzhkov, I.A.; Lapin, K.N.; Kalabushev, S.N.; Ostrova, I.V.; Varnakova, L.A.; Grebenchikov, O.A. Three-Hour Argon Inhalation Has No Neuroprotective Effect after Open Traumatic Brain Injury in Rats. Brain Sci. 2022, 12, 920. [Google Scholar] [CrossRef]
- Creed, J.; Cantillana-Riquelme, V.; Yan, B.H.; Ma, S.; Chu, D.; Wang, H.; Turner, D.A.; Laskowitz, D.T.; Hoffmann, U. Argon Inhalation for 24 h After Closed-Head Injury Does Not Improve Recovery, Neuroinflammation, or Neurologic Outcome in Mice. Neurocrit. Care 2021, 34, 833–843. [Google Scholar] [CrossRef]
- Soldatov, P.E.; Shulaguin, Y.A.; Tyurin-Kuzmin, A.Y.; Dyachenko, A.I. Endurance of Hypoxic Hypoxia After Preliminary Breathing of Normoxic Mixtures Containing Argon or Krypton. Aviakosm. I Ekol. Meditsina 2021, 55, 74–80. [Google Scholar] [CrossRef]
- Ananev, V.N. Effect of Inert Gases on Argon and Krypton Absorption of Oxygen in the Closed Space in Rats. Fundam. Res. 2012, 1, 11–13. [Google Scholar]
- Soldatov, P.E.; Shulaguin, Y.A.; Tyurin-Kuzmin, A.Y.; Krychenkov, D.A.; Nosovsky, A.M.; Suvorov, A.V.; Smolenskaya, T.S.; Smirnov, I.A.S.T. Complex Monitoring-Based Evaluation of the Cardiorespiratory System and Gas Exchange in Laboratory Animals Breathing Hypoxic Breathing Mixtures Containing Inertial Gasses. Aerosp. Environ. Med. 2019, 53, 65–76. [Google Scholar] [CrossRef]
- Kussmaul, A.R.; Bogacheva, M.A.; Shkurat, T.P.; Pavlov, B.N. Effects of Xenon and Krypton-Containing Breathing Mixtures on Clinical and Biochemical Blood Indices in Animals. Aerosp. Environ. Med. 2007, 2, 30–32. [Google Scholar]
- Silachev, D.N.; Usatikova, E.A.; Pevzner, I.B.; Zorova, L.D.; Babenko, V.A.; Gulyaev, M.V.; Pirogov, Y.A.; Plotnikov, E.Y.; Zorov, D.B. Effect of Anesthetics on Efficiency of Remote Ischemic Preconditioning. Biochem. Biokhimiia 2017, 82, 1006–1016. [Google Scholar] [CrossRef]
- Watson, B.D.; Dietrich, W.D.; Busto, R.; Wachtel, M.S.; Ginsberg, M.D. Induction of Reproducible Brain Infarction by Photochemically Initiated Thrombosis. Ann. Neurol. 1985, 17, 497–504. [Google Scholar] [CrossRef]
- De Ryck, M.; Van Reempts, J.; Borgers, M.; Wauquier, A.; Janssen, P.A.J. Photochemical Stroke Model: Flunarizine Prevents Sensorimotor Deficits after Neocortical Infarcts in Rats. Stroke 1989, 20, 1383–1390. [Google Scholar] [CrossRef]
- Jolkkonen, J.; Puurunen, K.; Rantakömi, S.; Härkönen, A.; Haapalinna, A.; Sivenius, J. Behavioral Effects of the A2-Adrenoceptor Antagonist, Atipamezole, after Focal Cerebral Ischemia in Rats. Eur. J. Pharmacol. 2000, 400, 211–219. [Google Scholar] [CrossRef]
- Silachev, D.N.; Uchevatkin, A.A.; Pirogov, Y.A.; Zorov, D.B.; Isaev, N.K. Comparative Evaluation of Two Methods for Studies of Experimental Focal Ischemia: Magnetic Resonance Tomography and Triphenyltetrazoleum Detection of Brain Injuries. Bull. Exp. Biol. Med. 2009, 147, 269–272. [Google Scholar] [CrossRef]
- Sergio, C.M.; Rolando, C.A. Erythropoietin Regulates Signaling Pathways Associated with Neuroprotective Events. Exp. Brain Res. 2022, 240, 1303–1315. [Google Scholar] [CrossRef]
- Sopjani, M.; Millaku, L.; Nebija, D.; Emini, M.; Rifati-Nixha, A.; Dërmaku-Sopjani, M. The Glycogen Synthase Kinase-3 in the Regulation of Ion Channels and Cellular Carriers. Curr. Med. Chem. 2019, 26, 6817–6829. [Google Scholar] [CrossRef]
- Harris, K.; Armstrong, S.P.; Campos-Pires, R.; Kiru, L.; Franks, N.P.; Dickinson, R. Neuroprotection against Traumatic Brain Injury by Xenon, but Not Argon, Is Mediated by Inhibition at the n-Methyl-d-Aspartate Receptor Glycine Site. Anesthesiology 2013, 119, 1137–1148. [Google Scholar] [CrossRef]
- Liang, M.; Ahmad, F.; Dickinson, R. Neuroprotection by the Noble Gases Argon and Xenon as Treatments for Acquired Brain Injury: A Preclinical Systematic Review and Meta-Analysis. Br. J. Anaesth. 2022, 129, 200–218. [Google Scholar] [CrossRef]
- Filev, A.D.; Silachev, D.N.; Ryzhkov, I.A.; Lapin, K.N.; Babkina, A.S.; Grebenchikov, O.A.; Pisarev, V.M. Effect of Xenon Treatment on Gene Expression in Brain Tissue after Traumatic Brain Injury in Rats. Brain Sci. 2021, 11, 889. [Google Scholar] [CrossRef]
- Koziakova, M.; Harris, K.; Edge, C.J.; Franks, N.P.; White, I.L.; Dickinson, R. Noble Gas Neuroprotection: Xenon and Argon Protect against Hypoxic–Ischaemic Injury in Rat Hippocampus in Vitro via Distinct Mechanisms. Br. J. Anaesth. 2019, 123, 601–609. [Google Scholar] [CrossRef]
- Edge, C.J.; Dickinson, R. Argon: A Noble, but Not Inert, Treatment for Brain Trauma? Br. J. Anaesth. 2021, 126, 41–43. [Google Scholar] [CrossRef]
- Silachev, D.N.; Boeva, E.A.; Yakupova, E.I.; Milovanova, M.A.; Varnakova, L.A.; Kalabushev, S.N.; Antonova, V.V.; Cherpakov, R.A.; Ryzhkov, I.A.; Lapin, K.N.; et al. Positive Neuroprotective Effect of Argon Inhalation after Photochemically Induced Ischemic Stroke Model in Rats. Bull. Exp. Biol. Med. 2024, 176, 167–174. [Google Scholar] [CrossRef]
- Boeva, E.A.; Silachev, D.N.; Yakupova, E.I.; Milovanova, M.A.; Varnakova, L.A.; Kalabushev, S.N.; Denisov, S.O.; Antonova, V.V.; Ryzhkov, I.A.; Lapin, K.N.; et al. Experimental Study of Neuroprotective Properties of Inhaled Argon-Oxygen Mixture in a Photoinduced Ischemic Stroke Model. Gen. Reanimatol. 2023, 19, 46–53. [Google Scholar] [CrossRef]
- Jawad, N.; Rizvi, M.; Gu, J.; Adeyi, O.; Tao, G.; Maze, M.; Ma, D. Neuroprotection (and Lack of Neuroprotection) Afforded by a Series of Noble Gases in an in Vitro Model of Neuronal Injury. Neurosci. Lett. 2009, 460, 232–236. [Google Scholar] [CrossRef]
- Le Nogue, D.; Lavaur, J.; Milet, A.; Ramirez-Gil, J.F.; Katz, I.; Lemaire, M.; Farjot, G.; Hirsch, E.C.; Michel, P.P. Neuroprotection of Dopamine Neurons by Xenon against Low-Level Excitotoxic Insults Is Not Reproduced by Other Noble Gases. J. Neural Transm. 2020, 127, 27–34. [Google Scholar] [CrossRef]
- Collins, R.C.; Kennedy, M.C. Serving Families Who Have Served: Providing Family Therapy and Support in Interdisciplinary Polytrauma Rehabilitation. J. Clin. Psychol. 2008, 64, 993–1003. [Google Scholar] [CrossRef]
- Randi, A.M.; Smith, K.E.; Castaman, G. Von Willebrand Factor Regulation of Blood Vessel Formation. Blood 2018, 132, 132. [Google Scholar] [CrossRef] [PubMed]
- Rauch, A.; Wohner, N.; Christophe, O.D.; Denis, C.V.; Susen, S.; Lenting, P.J. On the Versatility of von Willebrand Factor. Mediterr. J. Hematol. Infect. Dis. 2013, 5, e2013046. [Google Scholar] [CrossRef] [PubMed]
- Anatskaya, L.N.; Goncharova, N. Endogenous Neovasculogenesis in Poststroke Cerebral Reparation. Int. Neurol. J. 2013, 6, 11–19. [Google Scholar]
- Fan, W.; Dai, Y.; Xu, H.; Zhu, X.; Cai, P.; Wang, L.; Sun, C.; Hu, C.; Zheng, P.; Zhao, B.Q. Caspase-3 Modulates Regenerative Response After Stroke. Stem Cells 2014, 32, 473–486. [Google Scholar] [CrossRef] [PubMed]
- Uzdensky, A.B. Apoptosis Regulation in the Penumbra after Ischemic Stroke: Expression of pro- and Antiapoptotic Proteins. Apoptosis Int. J. Program. Cell Death 2019, 24, 687–702. [Google Scholar] [CrossRef] [PubMed]
- Brücken, A.; Bleilevens, C.; Föhr, P.; Nolte, K.; Rossaint, R.; Marx, G.; Fries, M.; Derwall, M. Influence of Argon on Temperature Modulation and Neurological Outcome in Hypothermia Treated Rats Following Cardiac Arrest. Resuscitation 2017, 117, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Marmolejo-martínez-artesero, S.; Casas, C.; Romeo-guitart, D. Endogenous Mechanisms of Neuroprotection: To Boost or Not to Boost. Cells 2021, 10, 370. [Google Scholar] [CrossRef]
- Ng, S.Y.; Lee, A.Y.W. Traumatic Brain Injuries: Pathophysiology and Potential Therapeutic Targets. Front. Cell. Neurosci. 2019, 13, 528. [Google Scholar] [CrossRef]
- Fadoul, G.; Ikonomovic, M.; Zhang, F.; Yang, T. The Cell-Specific Roles of Nrf2 in Acute and Chronic Phases of Ischemic Stroke. CNS Neurosci. Ther. 2023, 30, e14462. [Google Scholar] [CrossRef]
- Kobayashi, A.; Kang, M.-I.; Okawa, H.; Ohtsuji, M.; Zenke, Y.; Chiba, T.; Igarashi, K.; Yamamoto, M. Oxidative Stress Sensor Keap1 Functions as an Adaptor for Cul3-Based E3 Ligase to Regulate Proteasomal Degradation of Nrf2. Mol. Cell. Biol. 2004, 24, 7130–7139. [Google Scholar] [CrossRef]
- Yang, T.; Zhang, F. Targeting Transcription Factor Nrf2 (Nuclear Factor Erythroid 2-Related Factor 2) for the Intervention of Vascular Cognitive Impairment and Dementia. Arterioscler. Thromb. Vasc. Biol. 2021, 41, 97–116. [Google Scholar] [CrossRef] [PubMed]
- An, Y.; Li, H.; Wang, M.; Xia, Z.; Ding, L.; Xia, X. Nuclear Factor Erythroid 2-Related Factor 2 Agonist Protects Retinal Ganglion Cells in Glutamate Excitotoxicity Retinas. Biomed. Pharmacother. Biomed. Pharmacother. 2022, 153, 113378. [Google Scholar] [CrossRef] [PubMed]
- Demyanenko, S.; Uzdensky, A. Profiling of Signaling Proteins in Penumbra After Focal Photothrombotic Infarct in the Rat Brain Cortex. Mol. Neurobiol. 2017, 54, 6839–6856. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.Z.; Li, T.T.; Cao, H.L.; Yang, W.C. Recent Advances in the Neuroprotective Effects of Medical Gases. Med. Gas Res. 2019, 9, 80–87. [Google Scholar] [CrossRef]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Antonova, V.V.; Silachev, D.N.; Plotnikov, E.Y.; Pevzner, I.B.; Yakupova, E.I.; Pisarev, M.V.; Boeva, E.A.; Tsokolaeva, Z.I.; Lyubomudrov, M.A.; Shumov, I.V.; et al. Neuroprotective Effects of Krypton Inhalation on Photothrombotic Ischemic Stroke. Biomedicines 2024, 12, 635. https://doi.org/10.3390/biomedicines12030635
Antonova VV, Silachev DN, Plotnikov EY, Pevzner IB, Yakupova EI, Pisarev MV, Boeva EA, Tsokolaeva ZI, Lyubomudrov MA, Shumov IV, et al. Neuroprotective Effects of Krypton Inhalation on Photothrombotic Ischemic Stroke. Biomedicines. 2024; 12(3):635. https://doi.org/10.3390/biomedicines12030635
Chicago/Turabian StyleAntonova, Viktoriya V., Denis N. Silachev, Egor Y. Plotnikov, Irina B. Pevzner, Elmira I. Yakupova, Mikhail V. Pisarev, Ekaterina A. Boeva, Zoya I. Tsokolaeva, Maxim A. Lyubomudrov, Igor V. Shumov, and et al. 2024. "Neuroprotective Effects of Krypton Inhalation on Photothrombotic Ischemic Stroke" Biomedicines 12, no. 3: 635. https://doi.org/10.3390/biomedicines12030635




