Oxidative Stress and the Pathogenesis of Aortic Aneurysms
Abstract
:1. Introduction
2. Aortic Aneurysms
3. Inflammatory Mediators of Aortic Aneurysm Formation
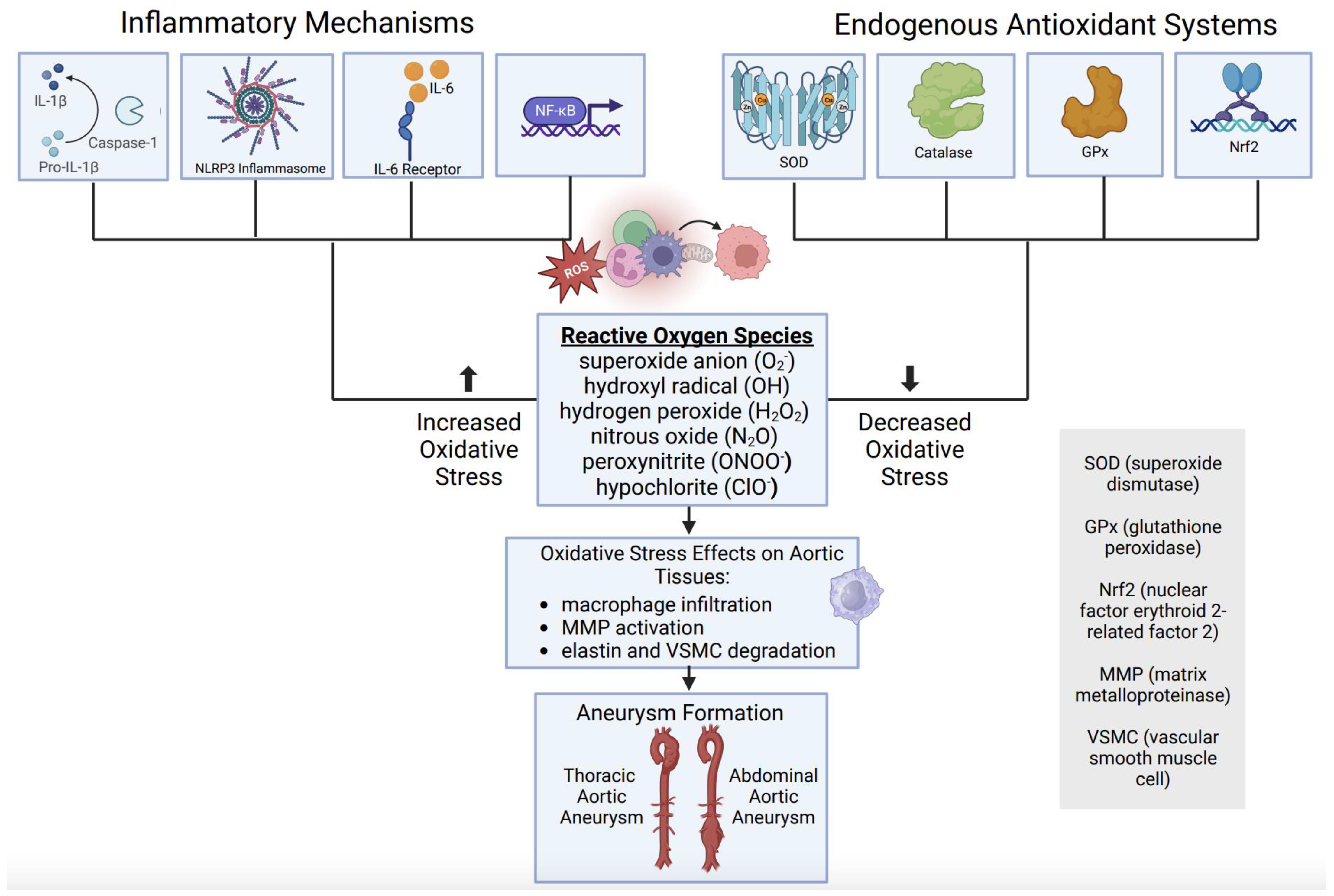
4. Reactive Oxygen Species Link to Aortic Aneurysms
5. Role of Endogenous Antioxidant Systems in Aortic Aneurysms
6. Oxidative Stress as a Therapeutic Target in Aortic Aneurysms
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| AA | aortic aneurysms |
| AAA | abdominal aortic aneurysm |
| aAA | ascending aortic aneurysm |
| ApoE | apolipoprotein E |
| dTAA | descending thoracic aortic aneurysm |
| BAPN | B-aminopropionitrile |
| CD4 | cluster of differentiation 4 |
| CuZnSOD | copper zinc superoxide dismutase |
| dTAA | descending thoracic aortic aneurysm |
| ECM | extracellular matrix |
| eNOS | endothelial nitric oxide synthase |
| GPx | glutathione peroxidase |
| IL-6 | interleukin 6 |
| IL-17 | interleukin 17 |
| IL-23 | interleukin 23 |
| IL-1α | interleukin-1 alpha |
| IL-1β | interleukin-1 beta |
| IL-1R1 | interleukin-1 receptor 1 |
| IL-R1 | interleukin receptor 1 |
| iNOS | inducible nitric oxide synthase |
| IFN𝛾 | interferon gamma |
| MCP1 | monocyte chemoattractant protein 1 |
| MMP-2 | matrix metalloproteinase 2 |
| MMP-9 | matrix metalloproteinase 9 |
| MnSOD | manganese superoxide dismutase |
| NLRP3 | NLR family pyrin domain containing 3 |
| NF-Kβ | nuclear factor kappa beta |
| NRF2 | Nuclear factor erythroid 2-related factor 2 |
| PPAR𝛾 | peroxisome proliferator-activated receptor gamma |
| ROS | reactive oxygen species |
| SOD | superoxide dismutase |
| TNF-α | tumor necrosis factor alpha |
| TGF-β | transforming growth factor beta |
| VEGF | vascular endothelial growth factor |
| VSMC | vascular smooth muscle cell |
References
- Sethi, S.; Parekh, U. Aortic Arch Aneurysm; StatPearls: Treasure Island, FL, USA, 2023. [Google Scholar]
- Panth, N.; Paudel, K.R.; Parajuli, K. Reactive Oxygen Species: A Key Hallmark of Cardiovascular Disease. Adv. Med. 2016, 2016, 9152732. [Google Scholar] [CrossRef] [PubMed]
- Taverne, Y.J.H.J.; Bogers, A.J.J.C.; Duncker, D.J.; Merkus, D. Reactive oxygen species and the cardiovascular system. Oxidative Med. Cell. Longev. 2013, 2013, 862423. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; You, Y.; Yin, Z.; Bao, Q.; Lei, S.; Yu, J.; Xie, C.; Ye, F.; Xie, X. Burden of Aortic Aneurysm and Its Attributable Risk Factors from 1990 to 2019: An Analysis of the Global Burden of Disease Study 2019. Front. Cardiovasc. Med. 2022, 9, 901225. [Google Scholar] [CrossRef] [PubMed]
- Kattoor, A.J.; Pothineni, N.V.K.; Palagiri, D.; Mehta, J.L. Oxidative Stress in Atherosclerosis. Curr. Atheroscler. Rep. 2017, 19, 42. [Google Scholar] [CrossRef] [PubMed]
- Nowak, W.N.; Deng, J.; Ruan, X.Z.; Xu, Q. Reactive Oxygen Species Generation and Atherosclerosis. Arter. Thromb. Vasc. Biol. 2017, 37, e41–e52. [Google Scholar] [CrossRef] [PubMed]
- Writing Group Members; Hiratzka, L.F.; Bakris, G.L.; Beckman, J.A.; Bersin, R.M.; Carr, V.F.; Casey, D.E., Jr.; Eagle, K.A.; Hermann, L.K.; Isselbacher, E.M.; et al. 2010 ACCF/AHA/AATS/ACR/ASA/SCA/SCAI/SIR/STS/SVM guidelines for the diagnosis and management of patients with Thoracic Aortic Disease: A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, American Association for Thoracic Surgery, American College of Radiology, American Stroke Association, Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, Society of Interventional Radiology, Society of Thoracic Surgeons, and Society for Vascular Medicine. Circulation 2010, 121, e266–e369. [Google Scholar] [CrossRef]
- Salmon, M. NADPH Oxidases in Aortic Aneurysms. Antioxidants 2022, 11, 1830. [Google Scholar] [CrossRef]
- Kent, K.C. Abdominal Aortic Aneurysms. N. Engl. J. Med. 2014, 371, 2101–2108. [Google Scholar] [CrossRef]
- Song, P.; He, Y.; Adeloye, D.; Zhu, Y.; Ye, X.; Yi, Q.; Rahimi, K.; Rudan, I.; Global Health Epidemiology Research Group (GHERG). The Global and Regional Prevalence of Abdominal Aortic Aneurysms: A Systematic Review and Modeling Analysis. Ann. Surg. 2023, 277, 912–919. [Google Scholar] [CrossRef]
- Ghorpade, A.; Baxter, B.T. Biochemistry and molecular regulation of matrix macromolecules in abdominal aortic aneurysms. Ann. N. Y. Acad. Sci. 1996, 800, 138–150. [Google Scholar] [CrossRef]
- Gittenberger-de Groot, A.C.; DeRuiter, M.C.; Bergwerff, M.; Poelmann, R.E. Smooth muscle cell origin and its relation to heterogeneity in development and disease. Arter. Thromb. Vasc. Biol. 1999, 19, 1589–1594. [Google Scholar] [CrossRef] [PubMed]
- Bergwerff, M.; Verberne, M.E.; DeRuiter, M.C.; Poelmann, R.E.; Gittenberger-De-Groot, A.C. Neural crest cell contribution to the developing circulatory system: Implications for vascular morphology? Circ. Res. 1998, 82, 221–231. [Google Scholar] [CrossRef]
- Rombouts, K.B.; van Merrienboer, T.A.R.; Ket, J.C.F.; Bogunovic, N.; van der Velden, J.; Yeung, K.K. The role of vascular smooth muscle cells in the development of aortic aneurysms and dissections. Eur. J. Clin. Investig. 2022, 52, e13697. [Google Scholar] [CrossRef] [PubMed]
- Keisler, B.; Carter, C. Abdominal aortic aneurysm. Am. Fam. Physician 2015, 91, 538–543. [Google Scholar] [PubMed]
- Millar, J.; Nasser, E.; Ailawadi, G.; Salmon, M. IL-1 in Abdominal Aortic Aneurysms. J. Cell. Immunol. 2023, 5, 22–31. [Google Scholar] [CrossRef] [PubMed]
- Johnston, W.F.; Salmon, M.; Pope, N.H.; Meher, A.; Su, G.; Stone, M.L.; Lu, G.; Owens, G.K.; Upchurch, G.R., Jr.; Ailawadi, G. Inhibition of interleukin-1beta decreases aneurysm formation and progression in a novel model of thoracic aortic aneurysms. Circulation 2014, 130 (Suppl. S1), S51–S59. [Google Scholar] [CrossRef]
- Schlösser, F.J.; Vaartjes, I.; van der Heijden, G.J.; Moll, F.L.; Verhagen, H.J.; Muhs, B.E.; de Borst, G.J.; Groenestege, A.T.T.; Kardaun, J.W.; de Bruin, A.; et al. Mortality after elective abdominal aortic aneurysm repair. Ann. Surg. 2010, 251, 158–164. [Google Scholar] [CrossRef]
- Lu, H.-Y.; Shih, C.-M.; Sung, S.-H.; Wu, A.T.H.; Cheng, T.-M.; Lin, Y.-C. Galectin-3 as a Biomarker for Stratifying Abdominal Aortic Aneurysm Size in a Taiwanese Population. Front. Cardiovasc. Med. 2021, 8, 663152. [Google Scholar] [CrossRef]
- Wenjing, F.; Tingting, T.; Qian, Z.; Hengquan, W.; Simin, Z.; Agyare, O.K.; Zhisheng, J.; Shunlin, Q. The role of IL-1β in aortic aneurysm. Clin. Chim. Acta 2020, 504, 7–14. [Google Scholar] [CrossRef]
- Andreata, F.; Syvannarath, V.; Clement, M.; Delbosc, S.; Guedj, K.; Fornasa, G.; Khallou-Laschet, J.; Morvan, M.; Even, G.; Procopio, E.; et al. Macrophage CD31 Signaling in Dissecting Aortic Aneurysm. J. Am. Coll. Cardiol. 2018, 72, 45–57. [Google Scholar] [CrossRef]
- Rondeau, J.-M.; Ramage, P.; Zurini, M.; Gram, H. The molecular mode of action and species specificity of canakinumab, a human monoclonal antibody neutralizing IL-1β. mAbs 2015, 7, 1151–1160. [Google Scholar] [CrossRef] [PubMed]
- Ridker, P.M.; Thuren, T.; Zalewski, A.; Libby, P. Interleukin-1β inhibition and the prevention of recurrent cardiovascular events: Rationale and Design of the Canakinumab Anti-inflammatory Thrombosis Outcomes Study (CANTOS). Am. Heart J. 2011, 162, 597–605. [Google Scholar] [CrossRef] [PubMed]
- Wei, Z.; Wang, Y.; Zhang, K.; Liao, Y.; Ye, P.; Wu, J.; Wang, Y.; Li, F.; Yao, Y.; Zhou, Y.; et al. Inhibiting the Th17/IL-17A-related inflammatory responses with digoxin confers protection against experimental abdominal aortic aneurysm. Arter. Thromb. Vasc. Biol. 2014, 34, 2429–2438. [Google Scholar] [CrossRef] [PubMed]
- Kelley, N.; Jeltema, D.; Duan, Y.; He, Y. The NLRP3 Inflammasome: An Overview of Mechanisms of Activation and Regulation. Int. J. Mol. Sci. 2019, 20, 3328. [Google Scholar] [CrossRef] [PubMed]
- Swanson, K.V.; Deng, M.; Ting, J.P.-Y. The NLRP3 inflammasome: Molecular activation and regulation to therapeutics. Nat. Rev. Immunol. 2019, 19, 477–489. [Google Scholar] [CrossRef] [PubMed]
- Ren, P.; Wu, D.; Appel, R.; Zhang, L.; Zhang, C.; Luo, W.; Robertson, A.A.B.; Cooper, M.A.; Coselli, J.S.; Milewicz, D.M.; et al. Targeting the NLRP3 Inflammasome with Inhibitor MCC950 Prevents Aortic Aneurysms and Dissections in Mice. J. Am. Heart Assoc. 2020, 9, e014044. [Google Scholar] [CrossRef]
- Usui, F.; Shirasuna, K.; Kimura, H.; Tatsumi, K.; Kawashima, A.; Karasawa, T.; Yoshimura, K.; Aoki, H.; Tsutsui, H.; Noda, T.; et al. Inflammasome activation by mitochondrial oxidative stress in macrophages leads to the development of angiotensin II-induced aortic aneurysm. Arter. Thromb. Vasc. Biol. 2015, 35, 127–136. [Google Scholar] [CrossRef]
- Abbate, A.; Toldo, S.; Marchetti, C.; Kron, J.; Van Tassell, B.W.; Dinarello, C.A. Interleukin-1 and the Inflammasome as Therapeutic Targets in Cardiovascular Disease. Circ. Res. 2020, 126, 1260–1280. [Google Scholar] [CrossRef]
- Johnston, W.F.; Salmon, M.; Su, G.; Lu, G.; Stone, M.L.; Zhao, Y.; Owens, G.K.; Upchurch, G.R., Jr.; Ailawadi, G. Genetic and Pharmacologic Disruption of Interleukin-1β Signaling Inhibits Experimental Aortic Aneurysm Formation. Arter. Thromb. Vasc. Biol. 2013, 33, 294–304. [Google Scholar] [CrossRef]
- Flondell-Sité, D.; Lindblad, B.; Kölbel, T.; Gottsäter, A. Cytokines and systemic biomarkers are related to the size of abdominal aortic aneurysms. Cytokine 2009, 46, 211–215. [Google Scholar] [CrossRef]
- Mi, T.; Nie, B.; Zhang, C.; Zhou, H. The elevated expression of osteopontin and NF-κB in human aortic aneurysms and its implication. Curr. Med. Sci. 2011, 31, 602–607. [Google Scholar] [CrossRef] [PubMed]
- Saito, T.; Hasegawa, Y.; Ishigaki, Y.; Yamada, T.; Gao, J.; Imai, J.; Uno, K.; Kaneko, K.; Ogihara, T.; Shimosawa, T.; et al. Importance of endothelial NF-κB signalling in vascular remodelling and aortic aneurysm formation. Cardiovasc. Res. 2013, 97, 106–114. [Google Scholar] [CrossRef] [PubMed]
- Touyz, R.M. Molecular and cellular mechanisms in vascular injury in hypertension: Role of angiotensin II. Curr. Opin. Nephrol. Hypertens. 2005, 14, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Pope, N.H.; Salmon, M.; Johnston, W.F.; Lu, G.; Lau, C.L.; Upchurch, G.R., Jr.; Ailawadi, G. Interleukin-6 Receptor Inhibition Prevents Descending Thoracic Aortic Aneurysm Formation. Ann. Thorac. Surg. 2015, 100, 1620–1626. [Google Scholar] [CrossRef] [PubMed]
- Cahill, C.M.; Rogers, J.T. Interleukin (IL) 1beta induction of IL-6 is mediated by a novel phosphatidylinositol 3-kinase-dependent AKT/IkappaB kinase alpha pathway targeting activator protein-1. J. Biol. Chem. 2008, 283, 25900–25912. [Google Scholar] [CrossRef] [PubMed]
- Paige, E.; Clément, M.; Lareyre, F.; Sweeting, M.; Raffort, J.; Grenier, C.; Finigan, A.; Harrison, J.; Peters, J.E.; Sun, B.B.; et al. Interleukin-6 Receptor Signaling and Abdominal Aortic Aneurysm Growth Rates. Circ. Genom. Precis. Med. 2019, 12, e002413. [Google Scholar] [CrossRef] [PubMed]
- Nishihara, M.; Aoki, H.; Ohno, S.; Furusho, A.; Hirakata, S.; Nishida, N.; Ito, S.; Hayashi, M.; Imaizumi, T.; Fukumoto, Y. The role of IL-6 in pathogenesis of abdominal aortic aneurysm in mice. PLoS ONE 2017, 12, e0185923. [Google Scholar] [CrossRef]
- Nakashima, H.; Aoki, M.; Miyake, T.; Kawasaki, T.; Iwai, M.; Jo, N.; Oishi, M.; Kataoka, K.; Ohgi, S.; Ogihara, T.; et al. Inhibition of experimental abdominal aortic aneurysm in the rat by use of decoy oligodeoxynucleotides suppressing activity of nuclear factor kappaB and ets transcription factors. Circulation 2004, 109, 132–138. [Google Scholar] [CrossRef]
- Dröge, W. Free radicals in the physiological control of cell function. Physiol. Rev. 2002, 82, 47–95. [Google Scholar] [CrossRef]
- Fei, J.; Demillard, L.J.; Ren, J. Reactive oxygen species in cardiovascular diseases: An update. Explor. Med. 2022, 3, 188–204. [Google Scholar] [CrossRef]
- Schramm, A.; Matusik, P.; Osmenda, G.; Guzik, T.J. Targeting NADPH oxidases in vascular pharmacology. Vasc. Pharmacol. 2012, 56, 216–231. [Google Scholar] [CrossRef] [PubMed]
- Guzik, B.; Sagan, A.; Ludew, D.; Mrowiecki, W.; Chwała, M.; Bujak-Gizycka, B.; Filip, G.; Grudzien, G.; Kapelak, B.; Żmudka, K.; et al. Mechanisms of oxidative stress in human aortic aneurysms—Association with clinical risk factors for atherosclerosis and disease severity. Int. J. Cardiol. 2013, 168, 2389–2396. [Google Scholar] [CrossRef] [PubMed]
- Miller, F.J., Jr.; Sharp, W.J.; Fang, X.; Oberley, L.W.; Oberley, T.D.; Weintraub, N.L. Oxidative stress in human abdominal aortic aneurysms: A potential mediator of aneurysmal remodeling. Arterioscler. Thromb. Vasc. Biol. 2002, 22, 560–565. [Google Scholar] [CrossRef] [PubMed]
- Xiong, W.; Mactaggart, J.; Knispel, R.; Worth, J.; Zhu, Z.; Li, Y.; Sun, Y.; Baxter, B.T.; Johanning, J. Inhibition of reactive oxygen species attenuates aneurysm formation in a murine model. Atherosclerosis 2009, 202, 128–134. [Google Scholar] [CrossRef] [PubMed]
- Hong, F.-F.; Liang, X.-Y.; Liu, W.; Lv, S.; He, S.-J.; Kuang, H.-B.; Yang, S.-L. Roles of eNOS in atherosclerosis treatment. Inflamm. Res. 2019, 68, 429–441. [Google Scholar] [CrossRef] [PubMed]
- Siu, K.L.; Miao, X.N.; Cai, H. Recoupling of eNOS with folic acid prevents abdominal aortic aneurysm formation in angiotensin II-infused apolipoprotein E null mice. PLoS ONE 2014, 9, e88899. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Chalupsky, K.; Stefani, E.; Cai, H. Mechanistic insights into folic acid-dependent vascular protection: Dihydrofolate reductase (DHFR)-mediated reduction in oxidant stress in endothelial cells and angiotensin II-infused mice: A novel HPLC-based fluorescent assay for DHFR activity. J. Mol. Cell. Cardiol. 2009, 47, 752–760. [Google Scholar] [CrossRef]
- Ahmad, M.; Wolberg, A.; Kahwaji, C.I. Biochemistry, Electron Transport Chain; StatPearls: Treasure Island, FL, USA, 2018. [Google Scholar]
- Yu, E.; Foote, K.; Bennett, M. Mitochondrial function in thoracic aortic aneurysms. Cardiovasc. Res. 2018, 114, 1696–1698. [Google Scholar] [CrossRef]
- Yuan, K.; Liang, W.; Zhang, J. A comprehensive analysis of differentially expressed genes and pathways in abdominal aortic aneurysm. Mol. Med. Rep. 2015, 12, 2707–2714. [Google Scholar] [CrossRef]
- Gabrielson, M.; Vorkapic, E.; Folkesson, M.; Welander, M.; Matussek, A.; Dimberg, J.; Länne, T.; Skogberg, J.; Wågsäter, D. Altered PPARγ Coactivator-1 Alpha Expression in Abdominal Aortic Aneurysm: Possible Effects on Mitochondrial Biogenesis. J. Vasc. Res. 2016, 53, 17–26. [Google Scholar] [CrossRef]
- Menteşe, U.; Turan, I.; Usta, S.; Demir, S.; Koral, Ö.; Menteşe, S.Ö.; Çavuşoğlu, I.G.; Karahan, S.C.; Alver, A.; Doğan, O.V.; et al. Systemic oxidant/antioxidant balance in human abdominal aortic aneurysm. Perfusion 2016, 31, 288–294. [Google Scholar] [CrossRef] [PubMed]
- van de Veerdonk, F.L.; Smeekens, S.P.; Joosten, L.A.B.; Kullberg, B.J.; Dinarello, C.A.; van der Meer, J.W.M.; Netea, M.G. Reactive oxygen species–independent activation of the IL-1β inflammasome in cells from patients with chronic granulomatous disease. Proc. Natl. Acad. Sci. USA 2010, 107, 3030–3033. [Google Scholar] [CrossRef] [PubMed]
- Sakalihasan, N.; Pincemail, J.; Defraigne, J.O.; Nusgens, B.; Lapiere, C.; Limet, R. Decrease of plasma vitamin E (alpha-tocopherol) levels in patients with abdominal aortic aneurysm. Ann. N. Y. Acad. Sci. 1996, 800, 278–282. [Google Scholar] [CrossRef] [PubMed]
- Dubick, M.A.; Hunter, G.C.; Casey, S.M.; Keen, C.L. Aortic ascorbic acid, trace elements, and superoxide dismutase activity in human aneurysmal and occlusive disease. Proc. Soc. Exp. Biol. Med. 1987, 184, 138–143. [Google Scholar] [CrossRef] [PubMed]
- Sinha, I.; Pearce, C.G.; Cho, B.S.; Hannawa, K.K.; Roelofs, K.J.; Stanley, J.C.; Henke, P.K.; Upchurch, G.R., Jr. Differential regulation of the superoxide dismutase family in experimental aortic aneurysms and rat aortic explants. J. Surg. Res. 2007, 138, 156–162. [Google Scholar] [CrossRef] [PubMed]
- Maiellaro-Rafferty, K.; Weiss, D.; Joseph, G.; Wan, W.; Gleason, R.L.; Taylor, W.R. Catalase overexpression in aortic smooth muscle prevents pathological mechanical changes underlying abdominal aortic aneurysm formation. Am. J. Physiol. Circ. Physiol. 2011, 301, H355–H362. [Google Scholar] [CrossRef]
- Yu, Z.; Morimoto, K.; Yu, J.; Bao, W.; Okita, Y.; Okada, K. Endogenous superoxide dismutase activation by oral administration of riboflavin reduces abdominal aortic aneurysm formation in rats. J. Vasc. Surg. 2016, 64, 737–745. [Google Scholar] [CrossRef]
- Soto, M.E.; Manzano-Pech, L.G.; Guarner-Lans, V.; Díaz-Galindo, J.A.; Vásquez, X.; Castrejón-Tellez, V.; Gamboa, R.; Huesca, C.; Fuentevilla-Alvárez, G.; Pérez-Torres, I. Oxidant/Antioxidant Profile in the Thoracic Aneurysm of Patients with the Loeys-Dietz Syndrome. Oxidative Med. Cell. Longev. 2020, 2020, 5392454. [Google Scholar] [CrossRef]
- Lubos, E.; Loscalzo, J.; Handy, D.E. Glutathione peroxidase-1 in health and disease: From molecular mechanisms to therapeutic opportunities. Antioxid. Redox Signal. 2011, 15, 1957–1997. [Google Scholar] [CrossRef]
- Hubackova, S.; Krejcikova, K.; Bartek, J.; Hodny, Z. IL1- and TGFβ-Nox4 signaling, oxidative stress and DNA damage response are shared features of replicative, oncogene-induced, and drug-induced paracrine ‘bystander senescence’. Aging 2012, 4, 932–951. [Google Scholar] [CrossRef]
- Takeda, N.; Hara, H.; Fujiwara, T.; Kanaya, T.; Maemura, S.; Komuro, I. TGF-β Signaling-Related Genes and Thoracic Aortic Aneurysms and Dissections. Int. J. Mol. Sci. 2018, 19, 2125. [Google Scholar] [CrossRef] [PubMed]
- Parastatidis, I.; Weiss, D.; Joseph, G.; Taylor, W.R. Overexpression of catalase in vascular smooth muscle cells prevents the formation of abdominal aortic aneurysms. Arter. Thromb. Vasc. Biol. 2013, 33, 2389–2396. [Google Scholar] [CrossRef] [PubMed]
- Tang, T.; Fan, W.; Zeng, Q.; Wan, H.; Zhao, S.; Jiang, Z.; Qu, S. The TGF-β pathway plays a key role in aortic aneurysms. Clin. Chim. Acta 2020, 501, 222–228. [Google Scholar] [CrossRef]
- Grigoryants, V.; Hannawa, K.K.; Pearce, C.G.; Sinha, I.; Roelofs, K.J.; Ailawadi, G.; Deatrick, K.B.; Woodrum, D.T.; Cho, B.S.; Henke, P.K.; et al. Tamoxifen up-regulates catalase production, inhibits vessel wall neutrophil infiltration, and attenuates development of experimental abdominal aortic aneurysms. J. Vasc. Surg. 2005, 41, 108–114. [Google Scholar] [CrossRef] [PubMed]
- Budbazar, E.; Rodriguez, F.; Sanchez, J.M.; Seta, F. The Role of Sirtuin-1 in the Vasculature: Focus on Aortic Aneurysm. Front. Physiol. 2020, 11, 1047. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.-Z.; Wang, F.; Gao, P.; Pei, J.-F.; Liu, Y.; Xu, T.-T.; Tang, X.; Fu, W.-Y.; Lu, J.; Yan, Y.-F.; et al. Age-Associated Sirtuin 1 Reduction in Vascular Smooth Muscle Links Vascular Senescence and Inflammation to Abdominal Aortic Aneurysm. Circ. Res. 2016, 119, 1076–1088. [Google Scholar] [CrossRef] [PubMed]
- Kopacz, A.; Werner, E.; Grochot-Przęczek, A.; Klóska, D.; Hajduk, K.; Neumayer, C.; Józkowicz, A.; Piechota-Polanczyk, A. Simvastatin Attenuates Abdominal Aortic Aneurysm Formation Favoured by Lack of Nrf2 Transcriptional Activity. Oxidative Med. Cell. Longev. 2020, 2020, 6340190. [Google Scholar] [CrossRef]
- Li, Z.; Zheng, W.; Kong, W.; Zeng, T. Itaconate: A Potent Macrophage Immunomodulator. Inflammation 2023, 46, 1177–1191. [Google Scholar] [CrossRef]
- Song, H.; Xu, T.; Feng, X.; Lai, Y.; Yang, Y.; Zheng, H.; He, X.; Wei, G.; Liao, W.; Liao, Y.; et al. Itaconate prevents abdominal aortic aneurysm formation through inhibiting inflammation via activation of Nrf2. EBioMedicine 2020, 57, 102832. [Google Scholar] [CrossRef]
- Shang, T.; Liu, Z.; Liu, C.-J. Antioxidant Vitamin C attenuates experimental abdominal aortic aneurysm development in an elastase-induced rat model. J. Surg. Res. 2014, 188, 316–325. [Google Scholar] [CrossRef]
- d’Uscio, L.V.; Milstien, S.; Richardson, D.; Smith, L.; Katusic, Z.S. Long-term vitamin C treatment increases vascular tetrahydrobiopterin levels and nitric oxide synthase activity. Circ. Res. 2003, 92, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Baker, T.A.; Milstien, S.; Katusic, Z.S. Effect of vitamin C on the availability of tetrahydrobiopterin in human endothelial cells. J. Cardiovasc. Pharmacol. 2001, 37, 333–338. [Google Scholar] [CrossRef] [PubMed]
- Heller, R.; Unbehaun, A.; Schellenberg, B.; Mayer, B.; Werner-Felmayer, G.; Werner, E.R. L-ascorbic acid potentiates endothelial nitric oxide synthesis via a chemical stabilization of tetrahydrobiopterin. J. Biol. Chem. 2001, 276, 40–47. [Google Scholar] [CrossRef]
- Duffy, M.J.; O’kane, C.M.; Stevenson, M.; Young, I.S.; Harkin, D.W.; Mullan, B.A.; McAuley, D.F. A randomized clinical trial of ascorbic acid in open abdominal aortic aneurysm repair. Intensiv. Care Med. Exp. 2015, 3, 50. [Google Scholar] [CrossRef] [PubMed]
- Gavrila, D.; Li, W.G.; McCormick, M.L.; Thomas, M.; Daugherty, A.; Cassis, L.A.; Miller, F.J., Jr.; Oberley, L.W.; Dellsperger, K.C.; Weintraub, N.L.; et al. Vitamin E inhibits abdominal aortic aneurysm formation in angiotensin II-infused apolipoprotein E-deficient mice. Arter. Thromb. Vasc. Biol. 2005, 25, 1671–1677. [Google Scholar] [CrossRef] [PubMed]
- Tornwall, M.E.; Virtamo, J.; Haukka, J.K.; Albanes, D.; Huttunen, J.K. α-Tocopherol (vitamin E) and β-carotene supplementation does not affect the risk for large abdominal aortic aneurysm in a controlled trial. Atherosclerosis 2001, 157, 167–173. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.-H.; Chang, H.-H.; Guo, Y.-R.; Chang, W.-C.; Chen, Y.-F. Vitamin B Mitigates Thoracic Aortic Dilation in Marfan Syndrome Mice by Restoring the Canonical TGF-β Pathway. Int. J. Mol. Sci. 2021, 22, 11737. [Google Scholar] [CrossRef]
- Xia, L.; Sun, C.; Zhu, H.; Zhai, M.; Zhang, L.; Jiang, L.; Hou, P.; Li, J.; Li, K.; Liu, Z.; et al. Melatonin protects against thoracic aortic aneurysm and dissection through SIRT1-dependent regulation of oxidative stress and vascular smooth muscle cell loss. J. Pineal Res. 2020, 69, e12661. [Google Scholar] [CrossRef]
- Liu, W.; Wang, B.; Wang, T.; Liu, X.; He, X.; Liu, Y.; Li, Z.; Zeng, H. Ursodeoxycholic Acid Attenuates Acute Aortic Dissection Formation in Angiotensin II-Infused Apolipoprotein E-Deficient Mice Associated with Reduced ROS and Increased Nrf2 Levels. Cell. Physiol. Biochem. 2016, 38, 1391–1405. [Google Scholar] [CrossRef]
- Sandhar, H.K.; Kumar, B.; Prasher, S.; Tiwari, P.; Salhan, M.; Sharma, P. A review of phytochemistry and pharmacology of flavonoids. Int. Pharm. Sci. 2011, 1, 25–41. [Google Scholar]
- Pisano, C.; Benedetto, U.; Ruvolo, G.; Balistreri, C.R. Oxidative Stress in the Pathogenesis of Aorta Diseases as a Source of Potential Biomarkers and Therapeutic Targets, with a Particular Focus on Ascending Aorta Aneurysms. Antioxidants 2022, 11, 182. [Google Scholar] [CrossRef] [PubMed]
- Saghaei, E.; Boroujeni, S.N.; Safavi, P.; Boroujeni, Z.B.; Bijad, E. Diosmetin Mitigates Cognitive and Memory Impairment Provoked by Chronic Unpredictable Mild Stress in Mice. Evid.-Based Complement. Altern. Med. 2020, 2020, 5725361. [Google Scholar] [CrossRef] [PubMed]
- Meephat, S.; Prasatthong, P.; Rattanakanokchai, S.; Bunbupha, S.; Maneesai, P.; Pakdeechote, P. Diosmetin attenuates metabolic syndrome and left ventricular alterations via the suppression of angiotensin II/AT1 receptor/gp91phox/p-NF-κB protein expression in high-fat diet fed rats. Food Funct. 2021, 12, 1469–1481. [Google Scholar] [CrossRef] [PubMed]
- David, A.V.A.; Arulmoli, R.; Parasuraman, S. Overviews of biological importance of quercetin: A bioactive flavonoid. Pharmacogn. Rev. 2016, 10, 84–89. [Google Scholar] [CrossRef]
- Kondo, M.; Izawa-Ishizawa, Y.; Goda, M.; Hosooka, M.; Kagimoto, Y.; Saito, N.; Matsuoka, R.; Zamami, Y.; Chuma, M.; Yagi, K.; et al. Preventive Effects of Quercetin against the Onset of Atherosclerosis-Related Acute Aortic Syndromes in Mice. Int. J. Mol. Sci. 2020, 21, 7226. [Google Scholar] [CrossRef]
- Wang, L.; Wang, B.; Li, H.; Lu, H.; Qiu, F.; Xiong, L.; Xu, Y.; Wang, G.; Liu, X.; Wu, H.; et al. Quercetin, a flavonoid with anti-inflammatory activity, suppresses the development of abdominal aortic aneurysms in mice. Eur. J. Pharmacol. 2012, 690, 133–141. [Google Scholar] [CrossRef]

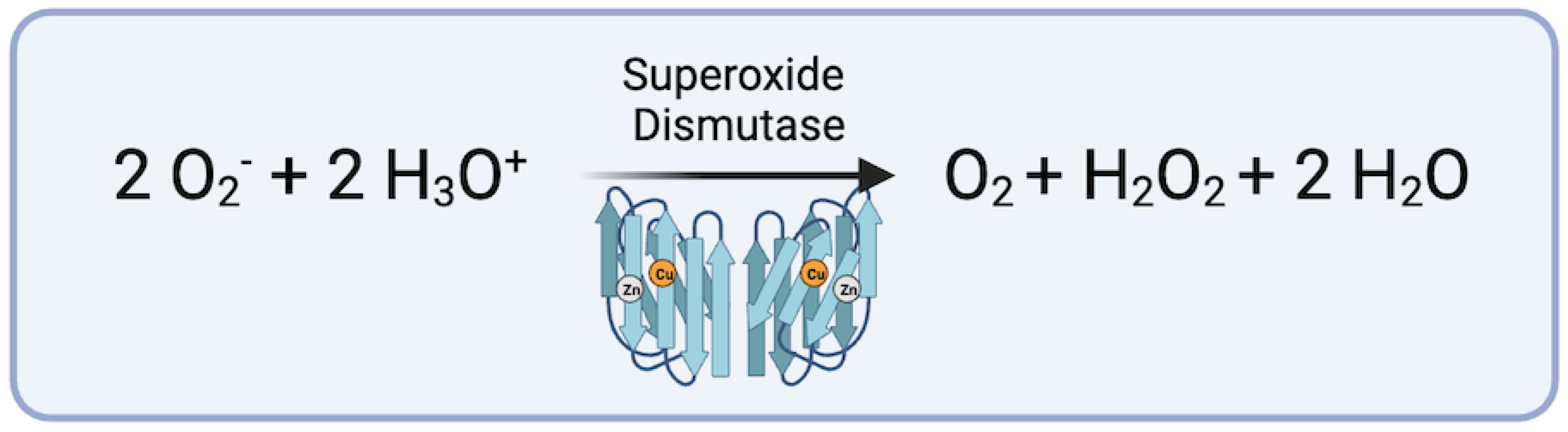


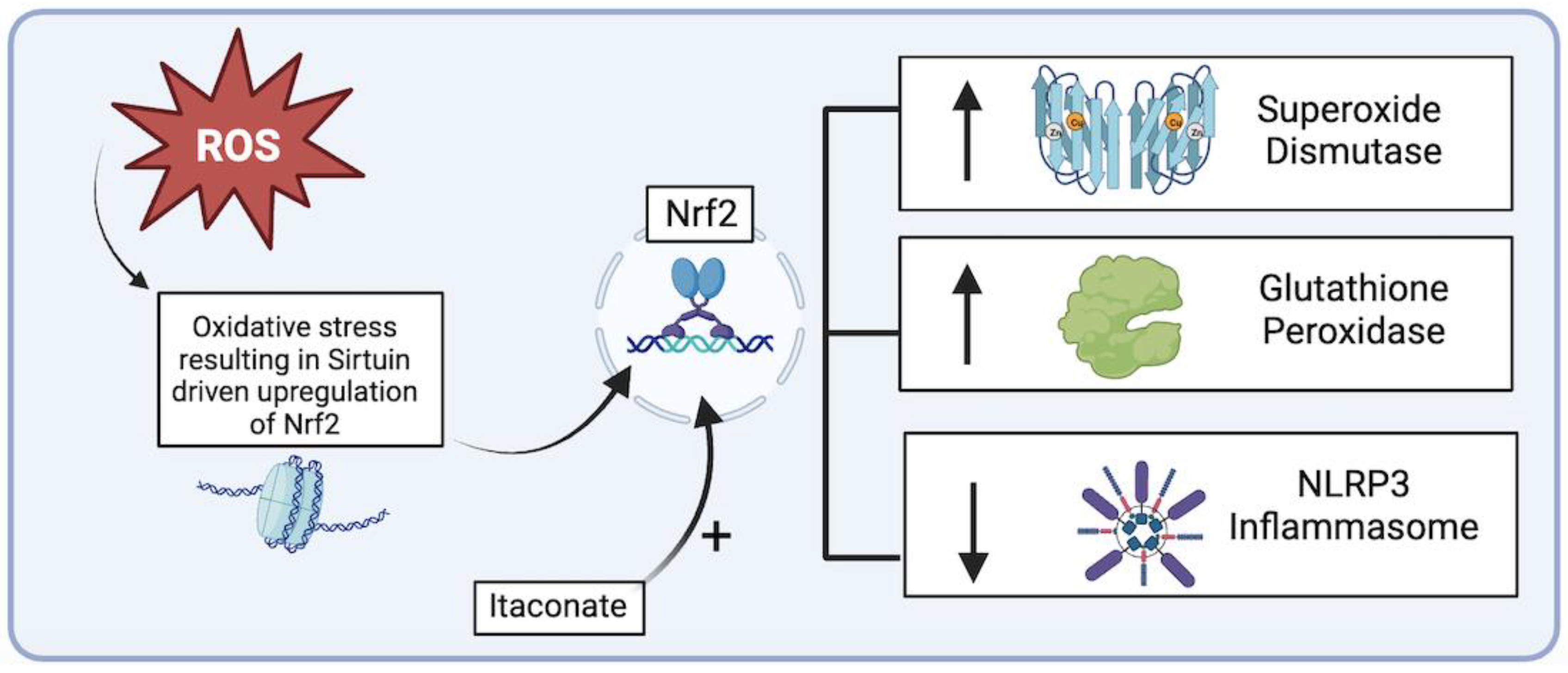
| Exogenous Antioxidants | In Vivo/In Vitro Effects on Aortic Aneurysms (AA) [Murine Models] | References |
|---|---|---|
| riboflavin (vit. B2) | Reduction of Abdominal AA maximal diameter Preservation of aortic elastin concentration Upregulation of endogenous Superoxide Dismutase Increased collagen deposition in Thoracic AA | [59,79] |
| Itaconic acid (itaconate) | Attenuation of Abdominal AA formation through Nuclear factor erythroid 2-related factor 2 (Nrf2) upregulation | [71] |
| Ascorbic acid (vit. C) | Preservation of aortic elastin concentration Downregulation of matrix metalloproteinase (MMP-2, MMP-9), and Interleukin-6 | [72,73,74,75] |
| α-tocopherol (vit. E) | Reduction of AA maximal diameter Decrease aortic rupture rate Lowers markers of aortic oxidative stress | [77,78] |
| Melatonin | Lowers incidence of Thoracic AA formation and rupture Increased endogenous Superoxide Dismutase Lowers markers of oxidative stress in aortic tissues | [80] |
| Ursodeoxycholic acid | Reduction of acute aortic dissection Decreased expression of NADPH oxidase Increased endogenous Superoxide Dismutase and catalase | [81] |
| Diosmetin | Endogenous Nrf2 upregulation Relief of cardiometabolic disorders Enhanced ventricular function Coronary artery vasodilation | [84,85] |
| Quercetin | Downregulation cyclooxygenase-2 and VEGF Upregulation of Superoxide Dismutase and caspase Endothelial cell protection * | [86,87,88] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kazaleh, M.; Gioscia-Ryan, R.; Ailawadi, G.; Salmon, M. Oxidative Stress and the Pathogenesis of Aortic Aneurysms. Biomedicines 2024, 12, 3. https://doi.org/10.3390/biomedicines12010003
Kazaleh M, Gioscia-Ryan R, Ailawadi G, Salmon M. Oxidative Stress and the Pathogenesis of Aortic Aneurysms. Biomedicines. 2024; 12(1):3. https://doi.org/10.3390/biomedicines12010003
Chicago/Turabian StyleKazaleh, Matthew, Rachel Gioscia-Ryan, Gorav Ailawadi, and Morgan Salmon. 2024. "Oxidative Stress and the Pathogenesis of Aortic Aneurysms" Biomedicines 12, no. 1: 3. https://doi.org/10.3390/biomedicines12010003






