Serum Chemerin Is Decreased by Roux-en-Y Gastric Bypass and Low Calorie-Formula Diet in Obese Individuals
Abstract
:1. Introduction
- -
- Regulation of circulating chemerin in severe obesity and its correlation with anthropometric, inflammatory, and metabolic parameters;
- -
- Comparative investigation of weight loss-associated effects on chemerin driven by either bariatric surgery (RYGB) or conservative therapy (LCD);
- -
- Elucidation of chemerin as a biomarker of metabolic disorders and as a predictor of an ameliorated metabolic-inflammatory state as a consequence of weight loss.
2. Materials and Methods
2.1. ROBS (Research in Obesity and Bariatric Surgery) Study Cohort
2.2. Data Collection
2.3. Quantification of Circulating Chemerin Concentrations
2.4. Statistical Analysis
3. Results
3.1. Study Cohort Characteristics
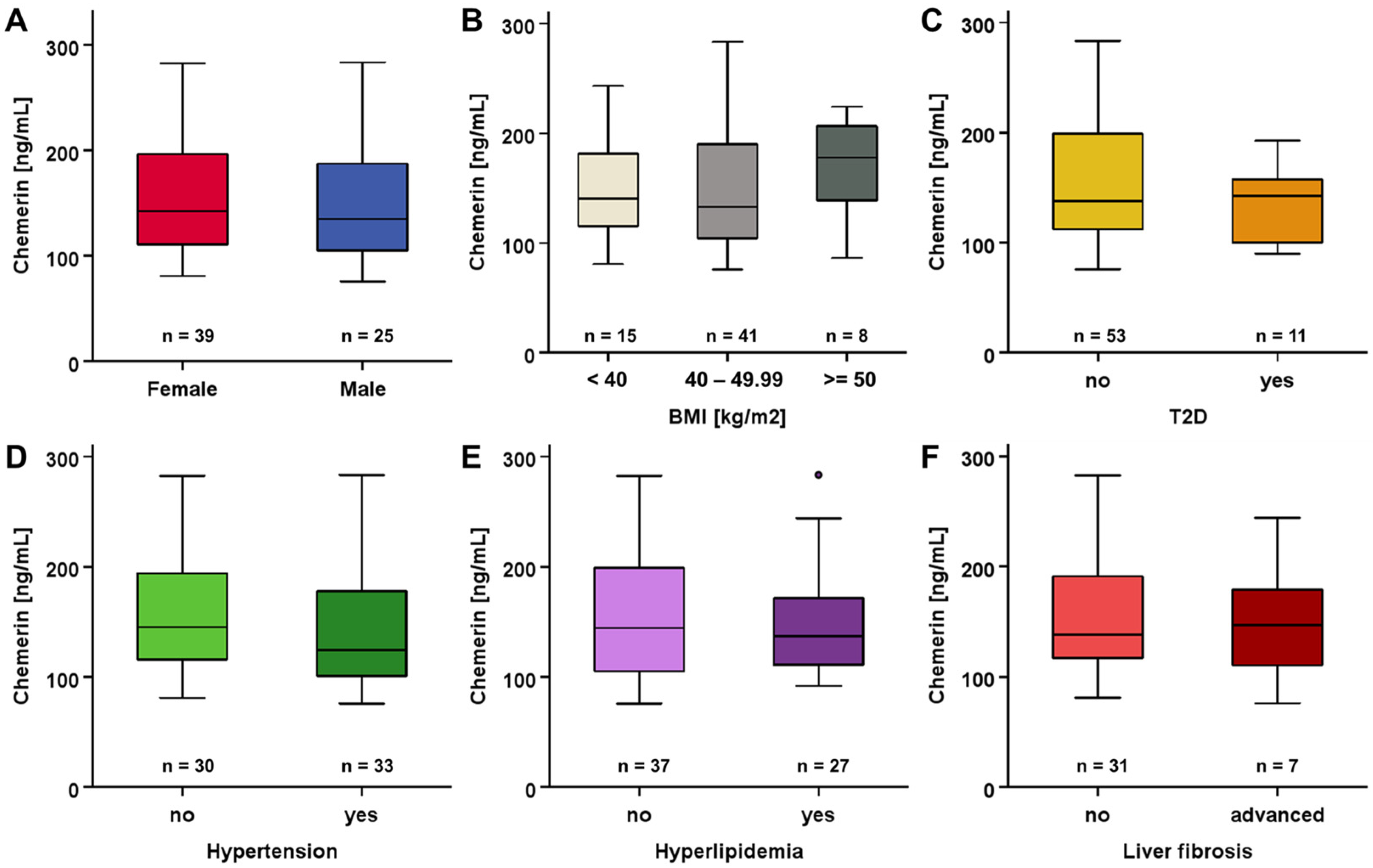
3.2. Systemic Chemerin Levels at Study Baseline
3.3. Changes in Chemerin Levels during Weight Loss
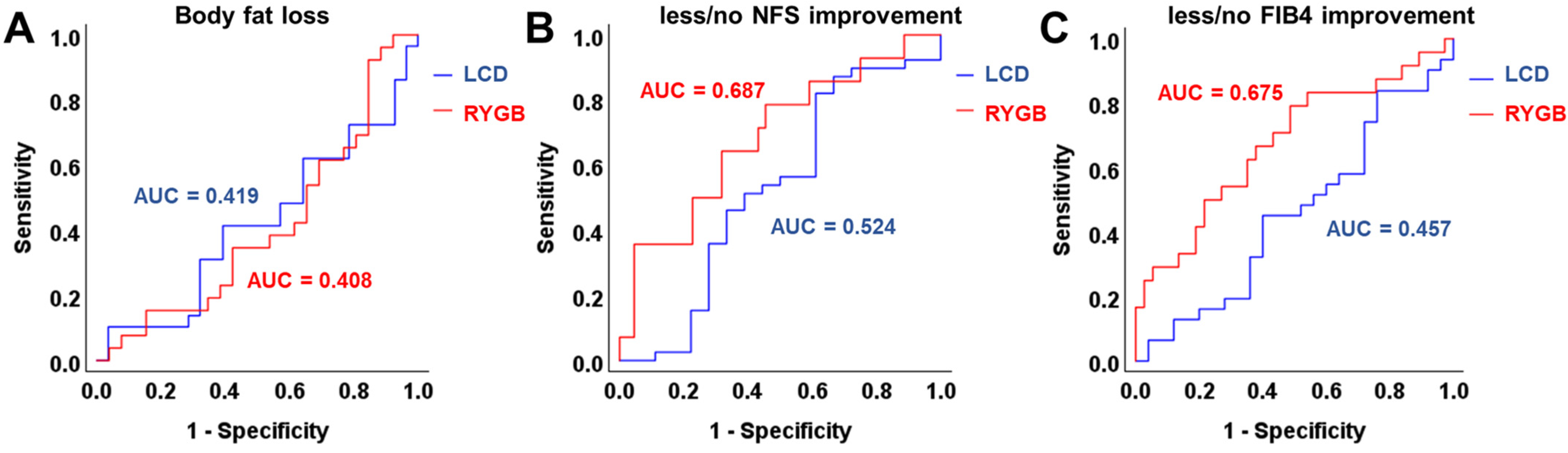
3.4. ROC and Correlation Analysis of Chemerin Levels and Improvement of Metabolic Parameters
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Saklayen, M.G. The Global Epidemic of the Metabolic Syndrome. Curr. Hypertens. Rep. 2018, 20, 12. [Google Scholar] [CrossRef]
- Hotamisligil, G.S. Inflammation, metaflammation and immunometabolic disorders. Nature 2017, 542, 177–185. [Google Scholar] [CrossRef] [PubMed]
- Paik, J.M.; Henry, L.; Younossi, Y.; Ong, J.; Alqahtani, S.; Younossi, Z.M. The burden of nonalcoholic fatty liver disease (NAFLD) is rapidly growing in every region of the world from 1990 to 2019. Hepatol. Commun. 2023, 7, e0251. [Google Scholar] [CrossRef] [PubMed]
- Rinella, M.E.; Lazarus, J.V.; Ratziu, V.; Francque, S.M.; Sanyal, A.J.; Kanwal, F.; Romero, D.; Abdelmalek, M.F.; Anstee, Q.M.; Arab, J.P.; et al. A multi-society Delphi consensus statement on new fatty liver disease nomenclature. J. Hepatol. 2023, 79, 1542–1556. [Google Scholar] [CrossRef]
- Polyzos, S.A.; Kountouras, J.; Mantzoros, C.S. Obesity and nonalcoholic fatty liver disease: From pathophysiology to therapeutics. Metabolism 2019, 92, 82–97. [Google Scholar] [CrossRef] [PubMed]
- Strychar, I. Diet in the management of weight loss. Can. Med. Assoc. J. 2006, 174, 56–63. [Google Scholar] [CrossRef]
- Buchwald, H.; Avidor, Y.; Braunwald, E.; Jensen, M.D.; Pories, W.; Fahrbach, K.; Schoelles, K. Bariatric surgery: A systematic review and meta-analysis. J. Am. Med. Assoc. 2004, 292, 1724–1737. [Google Scholar] [CrossRef]
- Bray, G.A.; Ryan, D.H. Evidence-based weight loss interventions: Individualized treatment options to maximize patient outcomes. Diabetes, Obes. Metab. 2021, 23, 50–62. [Google Scholar] [CrossRef]
- Brock, J.; Schmid, A.; Karrasch, T.; Pfefferle, P.; Schlegel, J.; Busse, I.; Hauenschild, A.; Schmidt, B.; Koukou, M.; Arapogianni, E.; et al. Progranulin serum levels and gene expression in subcutaneous vs visceral adipose tissue of severely obese patients undergoing bariatric surgery. Clin. Endocrinol. 2019, 91, 400–410. [Google Scholar] [CrossRef]
- Schmid, A.; Gehl, J.; Thomalla, M.; Hochberg, A.; Kreiß, A.; Patz, M.; Karrasch, T.; Schäffler, A. Downregulation of ctrp-3 by weight loss in vivo and by bile acids and incretins in adipocytes in vitro. Int. J. Mol. Sci. 2020, 21, 8168. [Google Scholar] [CrossRef]
- Unamuno, X.; Gómez-Ambrosi, J.; Rodríguez, A.; Becerril, S.; Frühbeck, G.; Catalán, V. Adipokine dysregulation and adipose tissue inflammation in human obesity. Eur. J. Clin. Investig. 2018, 48, e12997. [Google Scholar] [CrossRef] [PubMed]
- Helfer, G.; Wu, Q.F. Chemerin: A multifaceted adipokine involved in metabolic disorders. J. Endocrinol. 2018, 238, R79–R94. [Google Scholar] [CrossRef] [PubMed]
- Jialal, I. Chemerin levels in metabolic syndrome: A promising biomarker. Arch. Physiol. Biochem. 2021, 129, 1009–1011. [Google Scholar] [CrossRef] [PubMed]
- Goralski, K.B.; McCarthy, T.C.; Hanniman, E.A.; Zabel, B.A.; Butcher, E.C.; Parlee, S.D.; Muruganandan, S.; Sinal, C.J. Chemerin, a novel adipokine that regulates adipogenesis and adipocyte metabolism. J. Biol. Chem. 2007, 282, 28175–28188. [Google Scholar] [CrossRef] [PubMed]
- Krautbauer, S.; Wanninger, J.; Eisinger, K.; Hader, Y.; Beck, M.; Kopp, A.; Schmid, A.; Weiss, T.S.; Dorn, C.; Buechler, C. Chemerin is highly expressed in hepatocytes and is induced in non-alcoholic steatohepatitis liver. Exp. Mol. Pathol. 2013, 95, 199–205. [Google Scholar] [CrossRef] [PubMed]
- Du, X.Y.; Leung, L.L.K. Proteolytic regulatory mechanism of chemerin bioactivity. Acta Biochim. Biophys. Sin. 2009, 41, 973–979. [Google Scholar] [CrossRef] [PubMed]
- Pohl, R.; Feder, S.; Haberl, E.M.; Rein-Fischboeck, L.; Weiss, T.S.; Spirk, M.; Bruckmann, A.; McMullen, N.; Sinal, C.J.; Buechler, C. Chemerin Overexpression in the Liver Protects against Inflammation in Experimental Non-Alcoholic Steatohepatitis. Biomedicines 2022, 10, 132. [Google Scholar] [CrossRef] [PubMed]
- Karczewska-Kupczewska, M.; Nikołajuk, A.; Stefanowicz, M.; Matulewicz, N.; Kowalska, I.; Strączkowski, M. Serum and adipose tissue chemerin is differentially related to insulin sensitivity. Endocr. Connect. 2020, 9, 360–369. [Google Scholar] [CrossRef]
- Schmid, A.; Arians, M.; Karrasch, T.; Pons-Kühnemann, J.; Schäffler, A.; Roderfeld, M.; Roeb, E. Improvement of Type 2 Diabetes Mellitus and Attenuation of NAFLD Are Associated with the Success of Obesity Therapy. J. Clin. Med. 2022, 11, 1756. [Google Scholar] [CrossRef]
- Angulo, P.; Hui, J.M.; Marchesini, G.; Bugianesi, E.; George, J.; Farrell, G.C.; Enders, F.; Saksena, S.; Burt, A.D.; Bida, J.P.; et al. The NAFLD fibrosis score: A noninvasive system that identifies liver fibrosis in patients with NAFLD. Hepatology 2007, 45, 846–854. [Google Scholar] [CrossRef]
- Sterling, R.K.; Lissen, E.; Clumeck, N.; Sola, R.; Correa, M.C.; Montaner, J.; Sulkowski, M.S.; Torriani, F.J.; Dieterich, D.T.; Thomas, D.L.; et al. Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology 2006, 43, 1317–1325. [Google Scholar] [CrossRef] [PubMed]
- Harrison, S.A.; Oliver, D.; Arnold, H.L.; Gogia, S.; Neuschwander-Tetri, B.A. Development and validation of a simple NAFLD clinical scoring system for identifying patients without advanced disease. Gut 2008, 57, 1441–1447. [Google Scholar] [CrossRef] [PubMed]
- Léniz, A.; González, M.; Besné, I.; Carr-Ugarte, H.; Gómez- García, I.; Portillo, M.P. Role of chemerin in the control of glucose homeostasis. Mol. Cell. Endocrinol. 2022, 541, 111504. [Google Scholar] [CrossRef] [PubMed]
- Bekaert, M.; Ouwens, D.M.; Hörbelt, T.; Van de Velde, F.; Fahlbusch, P.; Herzfeld de Wiza, D.; Van Nieuwenhove, Y.; Calders, P.; Praet, M.; Hoorens, A.; et al. Reduced expression of chemerin in visceral adipose tissue associates with hepatic steatosis in patients with obesity. Obesity 2016, 24, 2544–2552. [Google Scholar] [CrossRef] [PubMed]
- Ernst, M.C.; Sinal, C.J. Chemerin: At the crossroads of inflammation and obesity. Trends Endocrinol. Metab. 2010, 21, 660–667. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.; Zhao, H.; Yin, C.; Lan, X.; Wu, L.; Du, X.; Griffiths, H.R.; Gao, D. Adipokines, Hepatokines and Myokines: Focus on Their Role and Molecular Mechanisms in Adipose Tissue Inflammation. Front. Endocrinol. 2022, 13, 873699. [Google Scholar] [CrossRef] [PubMed]
- Terra, X.; Auguet, T.; Guiu-Jurado, E.; Berlanga, A.; Orellana-Gavaldà, J.M.; Hernández, M.; Sabench, F.; Porras, J.A.; Llutart, J.; Martinez, S.; et al. Long-term changes in leptin, chemerin and ghrelin levels following different bariatric surgery procedures: Roux-en-Y gastric bypass and sleeve gastrectomy. Obes. Surg. 2013, 23, 1790–1798. [Google Scholar] [CrossRef] [PubMed]
- Salman, M.A.; El-ghobary, M.; Soliman, A.; El Sherbiny, M.; Abouelregal, T.E.; Albitar, A.; Abdallah, A.; Mikhail, H.M.S.; Nafea, M.A.; Sultan, A.A.E.A.; et al. Long-Term Changes in Leptin, Chemerin, and Ghrelin Levels Following Roux-en-Y Gastric Bypass and Laparoscopic Sleeve Gastrectomy. Obes. Surg. 2020, 30, 1052–1060. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, J.; Wang, H. Correlation of blood glucose, serum chemerin and insulin resistance with NAFLD in patients with type 2 diabetes mellitus. Exp. Ther. Med. 2018, 15, 2936–2940. [Google Scholar] [CrossRef]
- Ren, Q.; Wang, H.; Zeng, Y.; Fang, X.; Wang, M.; Li, D.; Huang, W.; Xu, Y. Circulating chemerin levels in metabolic-associated fatty liver disease: A systematic review and meta-analysis. Lipids Health Dis. 2022, 21, 27. [Google Scholar] [CrossRef]
- Stanley, T.L.; Fourman, L.T.; Zheng, I.; McClure, C.M.; Feldpausch, M.N.; Torriani, M.; Corey, K.E.; Chung, R.T.; Lee, H.; Kleiner, D.E.; et al. Relationship of IGF-1 and IGF-Binding Proteins to Disease Severity and Glycemia in Nonalcoholic Fatty Liver Disease. J. Clin. Endocrinol. Metab. 2021, 106, e520–e533. [Google Scholar] [CrossRef] [PubMed]
- Arnouk, J.; Rachakonda, V.P.; Jaiyeola, D.; Behari, J. Differential Outcomes and Clinical Challenges of NAFLD With Extreme Obesity. Hepatol. Commun. 2020, 4, 1419–1429. [Google Scholar] [CrossRef] [PubMed]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schmid, A.; Roderfeld, M.; Karrasch, T.; Roeb, E.; Schäffler, A. Serum Chemerin Is Decreased by Roux-en-Y Gastric Bypass and Low Calorie-Formula Diet in Obese Individuals. Biomedicines 2024, 12, 33. https://doi.org/10.3390/biomedicines12010033
Schmid A, Roderfeld M, Karrasch T, Roeb E, Schäffler A. Serum Chemerin Is Decreased by Roux-en-Y Gastric Bypass and Low Calorie-Formula Diet in Obese Individuals. Biomedicines. 2024; 12(1):33. https://doi.org/10.3390/biomedicines12010033
Chicago/Turabian StyleSchmid, Andreas, Martin Roderfeld, Thomas Karrasch, Elke Roeb, and Andreas Schäffler. 2024. "Serum Chemerin Is Decreased by Roux-en-Y Gastric Bypass and Low Calorie-Formula Diet in Obese Individuals" Biomedicines 12, no. 1: 33. https://doi.org/10.3390/biomedicines12010033





