Diagnostic Accuracy of Hand-Held Fundus Camera and Artificial Intelligence in Diabetic Retinopathy Screening
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Patients
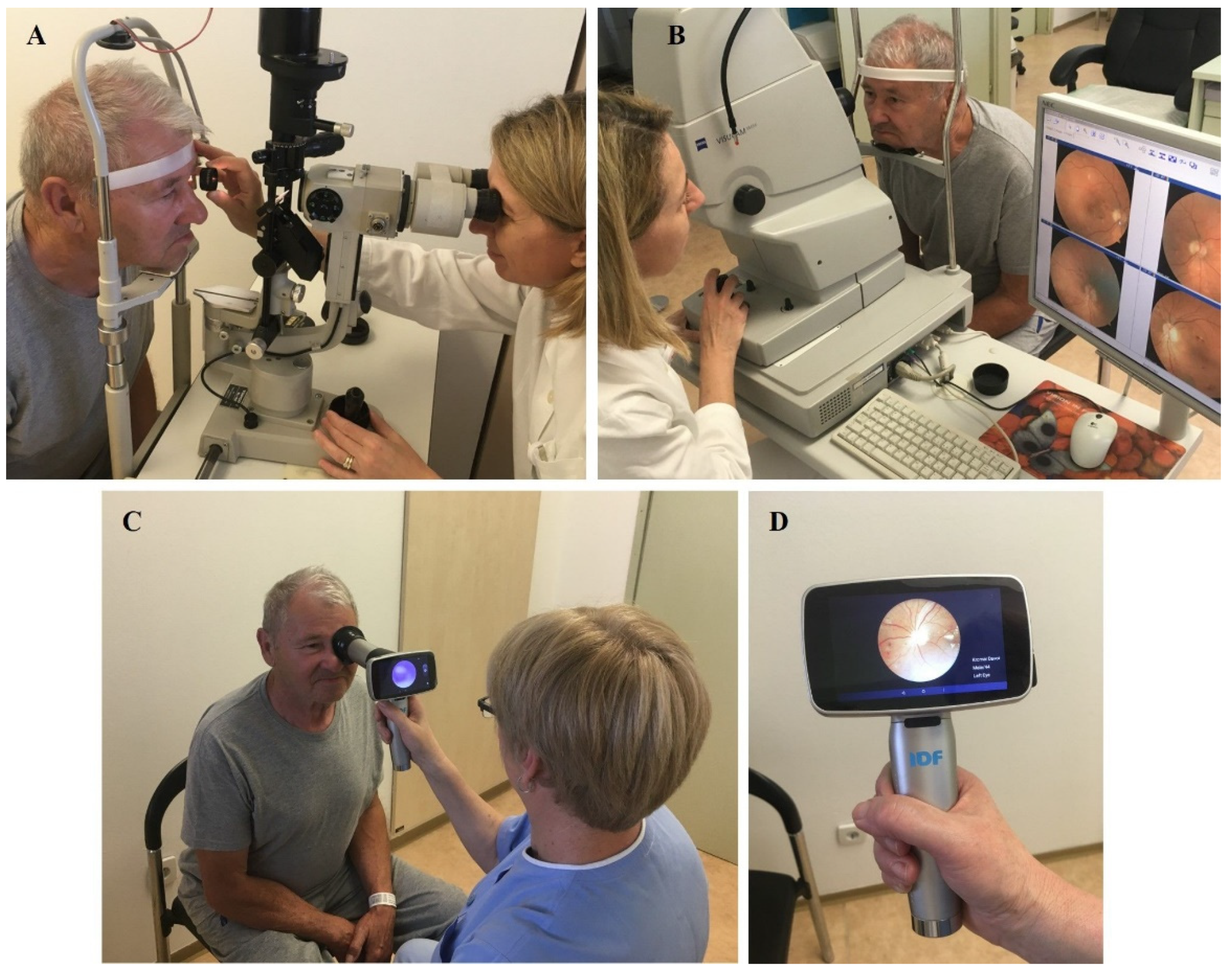
2.2. Ophthalmologic Retinal Examination and Fundus Photography
2.3. Statistical Analysis
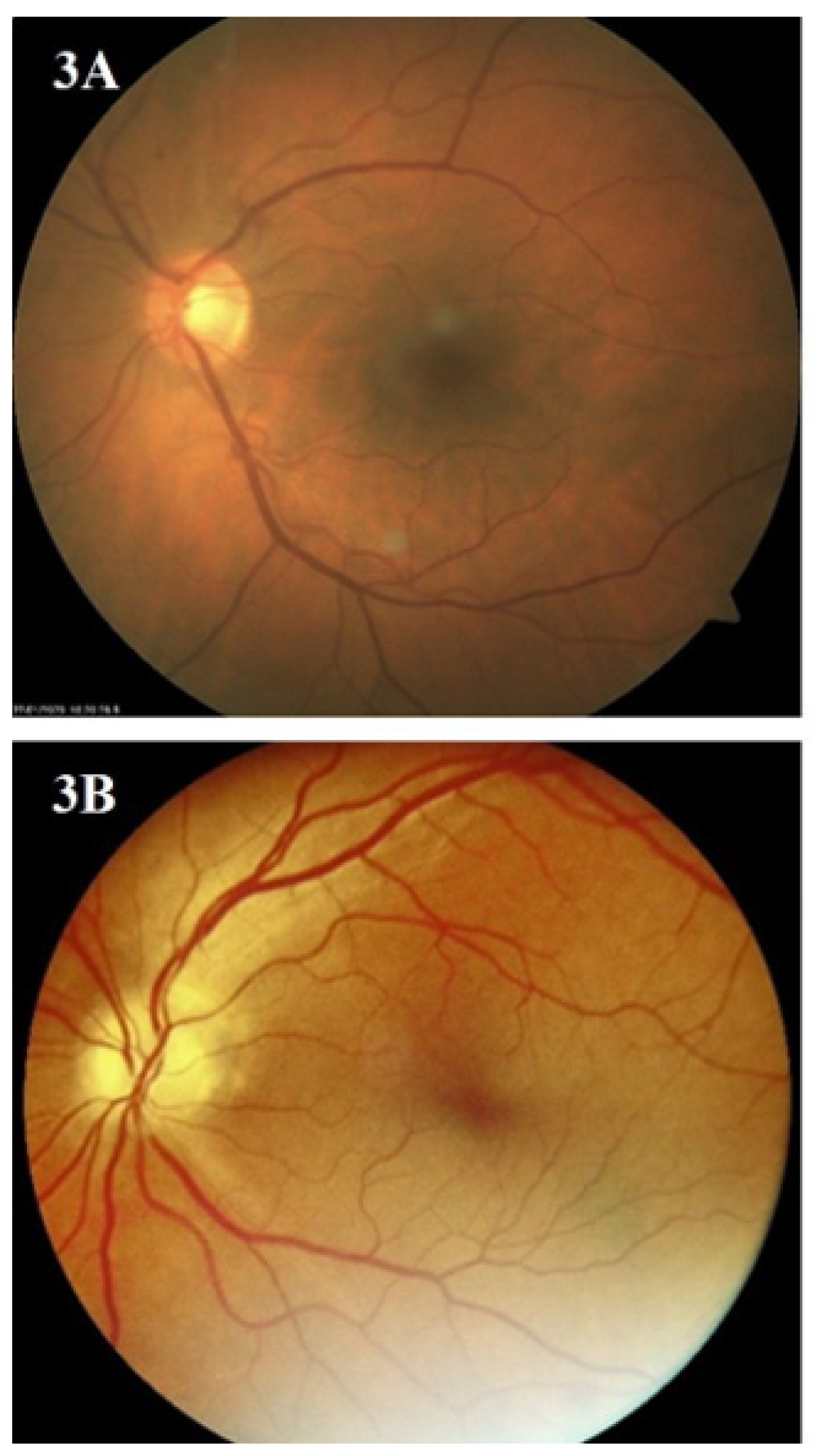
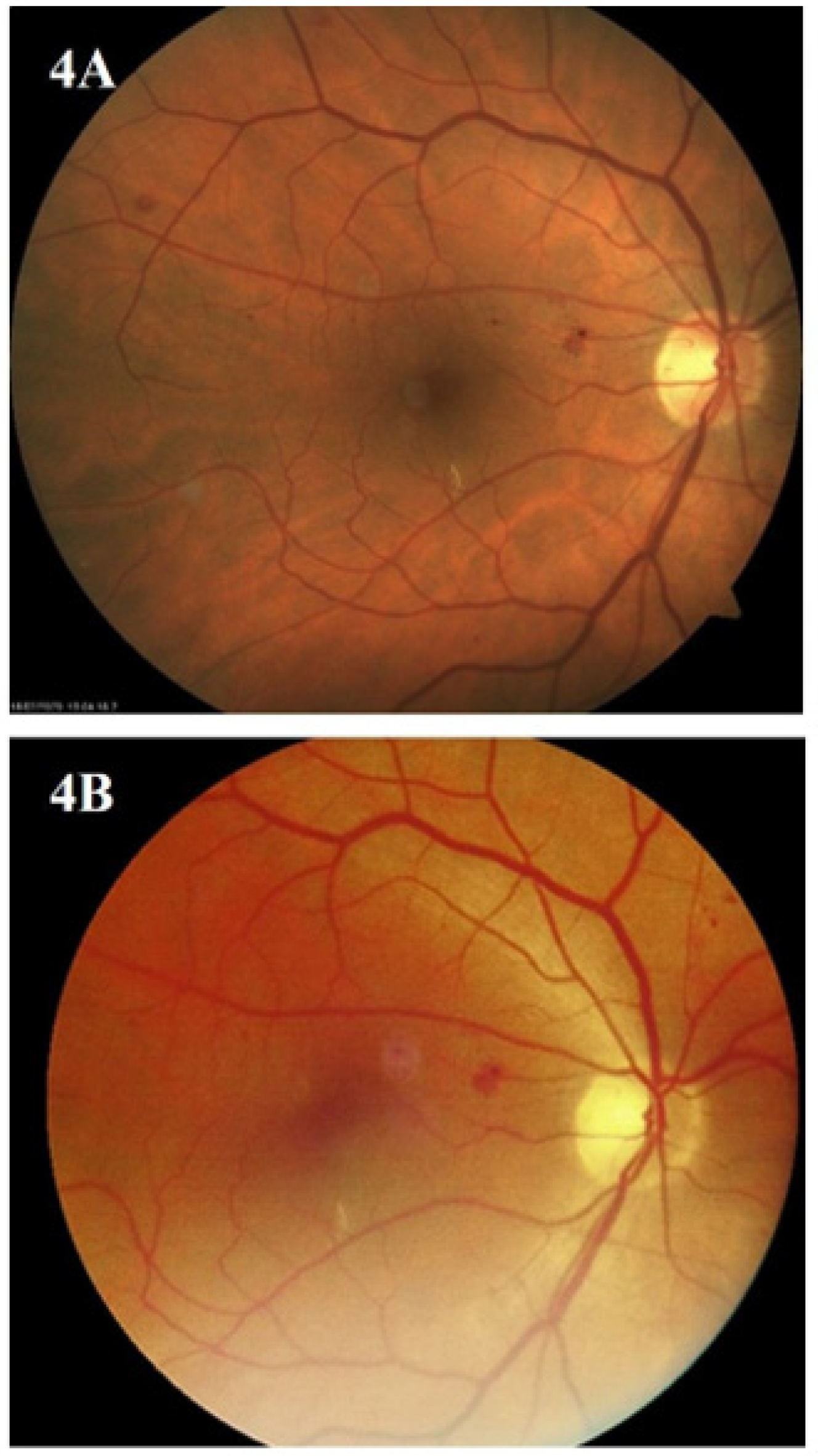
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- International Diabetes Federation. IDF Diabetes Atlas, 10th ed.; International Diabetes Federation: Brussels, Belgium, 2021; Available online: http://www.diabetesatlas.org/ (accessed on 1 October 2023).
- Flaxman, S.R.; Bourne, R.R.A.; Resnikoff, S.; Ackland, P.; Braithwaite, T.; Cicinelli, M.V.; Das, A.; Jonas, J.B.; Keeffe, J.; Kempen, J.H.; et al. Global causes of blindness and distance vision impairment 1990–2020: A systematic review and meta-analysis. Lancet Glob. Health 2017, 5, e1221–e1234. [Google Scholar] [CrossRef] [PubMed]
- Teo, Z.L.; Tham, Y.C.; Yu, M.; Chee, M.L.; Rim, T.H.; Cheung, N.; Bikbov, M.M.; Wang, Y.X.; Tang, Y.; Lu, Y.; et al. Global prevalence of diabetic retinopathy and projection of burden through 2045: Systematic review and meta-analysis. Ophthalmology 2021, 128, 1580–1591. [Google Scholar] [CrossRef] [PubMed]
- Javitt, J.C.; Aiello, L.P. Cost-effectiveness of detecting and treating diabetic retinopathy. Ann. Intern. Med. 1996, 124 Pt 2, 164–169. [Google Scholar] [CrossRef] [PubMed]
- Rohan, T.E.; Frost, C.D.; Wald, N.J. Prevention of blindness by screening for diabetic retinopathy: A quantitative assessment. BMJ 1989, 299, 1198–1201. [Google Scholar] [CrossRef] [PubMed]
- Wong, T.Y.; Sun, J.; Kawasaki, R.; Ruamviboonsuk, P.; Gupta, N.; Lansingh, V.C.; Maia, M.; Mathenge, W.; Moreker, S.; Muqit, M.M.K.; et al. Guidelines on diabetic eye care: The international council of ophthalmology recommendations for screening, follow-up, referral, and treatment based on resource settings. Ophthalmology 2018, 125, 1608–16622. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Regional Office for Europe (WHO/Europe). Diabetic Retinopathy Screening: A Short Guide: Increase Effectiveness, Maximize Benefits and Minimize Harm; World Health Organization, Regional Office for Europe: Copenhagen, Denmark, 2020; Available online: https://apps.who.int/iris/handle/10665/336660 (accessed on 1 October 2023).
- Garg, S.; Jani, P.D.; Kshirsagar, A.V.; King, B.; Chaum, E. Telemedicine and retinal imaging for improving diabetic retinopathy evaluation. Arch. Intern. Med. 2012, 172, 1677–1678. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Wu, H.; Dong, J.; Jiang, K.; Lu, X.; Shi, J. Telemedicine for detecting diabetic retinopathy: A systematic review and meta-analysis. Br. J. Ophthalmol. 2015, 99, 823–831. [Google Scholar] [CrossRef]
- Jin, K.; Lu, H.; Su, Z.; Cheng, C.; Ye, J.; Qian, D. Telemedicine screening of retinal diseases with a handheld portable non-mydriatic fundus camera. BMC. Ophthalmol. 2017, 17, 89. [Google Scholar] [CrossRef]
- Padhy, S.K.; Takkar, B.; Chawla, R.; Kumar, A. Artificial intelligence in diabetic retinopathy: A natural step to the future. Indian J. Ophthalmol. 2019, 67, 1004–1009. [Google Scholar] [CrossRef]
- US Food and Drug Administration. FDA Permits Marketing of Artificial Intelligence-Based Device to Detect Certain Diabetes-Related Eye Problems; US Food and Drug Administration: Silver Spring, MD, USA, 2018. Available online: https://www.fda.gov/news-events/press-announcements/fda-permits-marketing-artificial-intelligence-based-device-detect-certain-diabetes-related-eye (accessed on 1 October 2023).
- Bhaskaranand, M.; Ramachandra, C.; Bhat, S.; Cuadros, J.; Nittala, M.G.; Sadda, S.R.; Solanki, K. The value of automated diabetic retinopathy screening with the EyeArt system: A study of more than 100,000 consecutive encounters from people with diabetes. Diabetes Technol. Ther. 2019, 21, 635–643. [Google Scholar] [CrossRef]
- Wang, X.N.; Dai, L.; Li, S.T.; Kong, H.Y.; Sheng, B.; Wu, Q. Automatic grading system for diabetic retinopathy diagnosis using deep learning artificial intelligence software. Curr. Eye Res. 2020, 45, 1550–1555. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Shi, D.; Tan, Z.; Niu, Y.; Jiang, Y.; Xiong, R.; Peng, G.; He, M. Screening referable diabetic retinopathy using a semi-automated deep learning algorithm assisted approach. Front. Med. 2021, 8, 740987. [Google Scholar] [CrossRef] [PubMed]
- Orujov, F.; Maskeliūnas, R.; Damaševičius, R.; Wei, W. Fuzzy based image edge detection algorithm for blood vessel detection in retinal images. Appl. Soft Comput. 2020, 94, 106452. [Google Scholar] [CrossRef]
- Hu, K.; Zhang, Z.; Niu, X.; Zhang, Y.; Cao, C.; Xiao, F.; Gao, X. Retinal vessel segmentation of color fundus images using multiscale convolutional neural network with an improved cross-entropy loss function. Neurocomputing 2018, 309, 179–191. [Google Scholar] [CrossRef]
- Jiang, Z.; Yepez, J.; An, S.; Ko, S. Fast, accurate and robust retinal vessel segmentation system. Biocybern. Biomed. Eng. 2017, 37, 412–421. [Google Scholar] [CrossRef]
- Liu, H.; Wei, D.; Lu, D.; Li, Y.; Ma, K.; Wang, L.; Zheng, Y. Simultaneous Alignment and Surface Regression Using Hybrid 2D-3D Networks for 3D Coherent Layer Segmentation of Retina OCT Images. In Medical Image Computing and Computer Assisted Intervention—MICCA 2021I; Springer: Berlin/Heidelberg, Germany, 2021. [Google Scholar] [CrossRef]
- Apon, T.S.; Hasan, M.M.; Islam, A.; Alam, G.R. Demystifying Deep Learning Models for Retinal OCT Disease Classification using Explainable AI. In Proceedings of the 2021 IEEE Asia-Pacific Conference on Computer Science and Data Engineering (CSDE), Brisbane, Australia, 8–10 December 2021. [Google Scholar] [CrossRef]
- Zhelev, Z.; Peters, J.; Rogers, M.; Allen, M.; Kijauskaite, G.; Seedat, F.; Wilkinson, E.; Hyde, C. Test accuracy of artificial intelligence-based grading of fundus images in diabetic retinopathy screening: A systematic review. J. Med. Screen. 2023, 30, 97–112. [Google Scholar] [CrossRef] [PubMed]
- The Fred Hollows Foundation, International Diabetes Federation. Diabetes Eye Health: A Guide for Health Professionals; International Diabetes Federation: Brussels, Belgium, 2015; Available online: www.idf.org/eyehealth (accessed on 1 October 2023).
- Scanlon, P.H.; Malhotra, R.; Greenwood, R.H.; Aldington, S.J.; Foy, C.; Flatman, M.; Downes, S. Comparison of two reference standards in validating two field mydriatic digital photography as a method of screening for diabetic retinopathy. Br. J. Ophthalmol. 2003, 87, 1258–1263. [Google Scholar] [CrossRef]
- Boucher, M.C.; Gresset, J.A.; Angioi, K.; Olivier, S. Effectiveness and safety of screening for diabetic retinopathy with two nonmydriatic digital images compared with the seven standard stereoscopic photographic fields. Can. J. Ophthalmol. 2003, 38, 557–568. [Google Scholar] [CrossRef]
- Wilkinson, C.P.; Ferris, F.L., 3rd; Klein, R.E.; Lee, P.P.; Agardh, C.D.; Davis, M.; Dills, D.; Kampik, A.; Pararajasegaram, R.; Verdaguer, J.T.; et al. Proposed international clinical diabetic retinopathy and diabetic macular edema disease severity scales. Ophthalmology 2003, 110, 1677–1682. [Google Scholar] [CrossRef]
- Shanghai Jiao Tong University. Project 27: Screening and Auxiliary Diagnosis System of Diabetic Retinopathy based on Artificial Intelligence. Available online: https://global.sjtu.edu.cn/en/studyatSJTU/session/436 (accessed on 1 October 2023).
- Rajalakshmi, R.; Arulmalar, S.; Usha, M.; Prathiba, V.; Kareemuddin, K.S.; Anjana, R.M.; Mohan, V. Validation of smartphone-based retinal photography for diabetic retinopathy screening. PLoS ONE 2015, 10, e0138285. [Google Scholar] [CrossRef]
- Zhang, W.; Nicholas, P.; Schuman, S.G.; Allingham, M.J.; Faridi, A.; Suthar, T.; Cousins, S.W.; Prakalapakornet, S.G. Screening for diabetic retinopathy using a portable, noncontact, nonmydriatic handheld retinal camera. J. Diabetes Sci. Technol. 2017, 11, 128–134. [Google Scholar] [CrossRef]
- Tan, C.H.; Kyaw, B.M.; Smith, H.; Tan, C.S.; Tudor Car, L. Use of smartphones to detect diabetic retinopathy: Scoping review and meta-analysis of diagnostic test accuracy studies. J. Med. Internet Res. 2020, 22, e16658. [Google Scholar] [CrossRef] [PubMed]
- Gajiwala, U.R.; Pachchigar, S.; Patel, D.; Mistry, I.; Oza, Y.; Kundaria, D.; Shamanna, B.R. Non-mydriatic fundus photography as an alternative to indirect ophthalmoscopy for screening of diabetic retinopathy in community settings: A comparative pilot study in rural and tribal India. BMJ Open 2022, 12, e058485. [Google Scholar] [CrossRef] [PubMed]
- Poljičanin, T.; Buble, T. National Diabetes Registry CroDiab—Report for 2021; Croatian Institute of Public Health: Zagreb, Croatia, 2022. [Google Scholar]
- Tomić, M.; Raštegorac, P.; Vrabec, R.; Poljičanin, T.; Rahelić, D. Telemedicine for diabetic retinopathy screening in Croatia: A dream that could become a reality. Coll. Antropol. 2020, 44, 175–179. [Google Scholar] [CrossRef]
- Connolly, A.; Hosker, C. The nurse’s role in screening for diabetic retinopathy. Nurs. Times 2001, 97, 40. Available online: https://www.nursingtimes.net/clinical-archive/diabetes-clinical-archive/the-nurses-role-in-screening-for-diabetic-retinopathy-29-03-2001 (accessed on 1 October 2023). [PubMed]
- Kirkwood, B.J.; Coster, D.J.; Essex, R.W. Ophthalmic nurse practitioner led diabetic retinopathy screening. Results of a 3-month trial. Eye 2006, 20, 173–177. [Google Scholar] [CrossRef]
- Spurr, S.; Bullin, C.; Bally, J.; Trinder, K.; Khan, S. Nurse-led diabetic retinopathy screening: A pilot study to evaluate a new approach to vision care for Canadian Aboriginal peoples. Int. J. Circumpolar. Health 2018, 77, 1422670. [Google Scholar] [CrossRef]
- Boucher, M.C.; Nguyen, M.T.D.; Qian, J. Assessment of training outcomes of nurse readers for diabetic retinopathy telescreening: Validation study. JMIR Diabetes 2020, 5, e17309. [Google Scholar] [CrossRef]

| No DR | Mild/Moderate NPDR | Severe NPDR/PDR | p-Value | |
|---|---|---|---|---|
| Gender (m/f) | 50/50 | 73/27 | 80/20 | 0.058 a |
| Age (yrs.) | 65 (48–83) | 64(54–77) | 62 (45–70) | 0.408 b |
| Diabetes duration (yrs.) | 12.5 (2–33) | 14 (7–24) | 15 (2–30) | 0.161 b |
| BCVA (decimal) | 0.98 ± 0.05 | 0.98 ± 0.08 | 0.89 ± 0.27 | 0.027 c |
| IOP (mmHg) | 14.92 ± 2.29 | 15.27 ± 1.16 | 15.20 ± 0.68 | 0.776 c |
| Glaucoma (no/yes) | 94/6 | 100/0 | 93/7 | 0.612 a |
| Lens (1/2/3) | 16/68/16 | 7/80/13 | 0/87/13 | 0.447 a |
| Fundus Photography with a Hand-Held Fundus Camera | ||||
|---|---|---|---|---|
| No Retinopathy | Mild/Moderate NPDR | Severe NPDR/PDR | ||
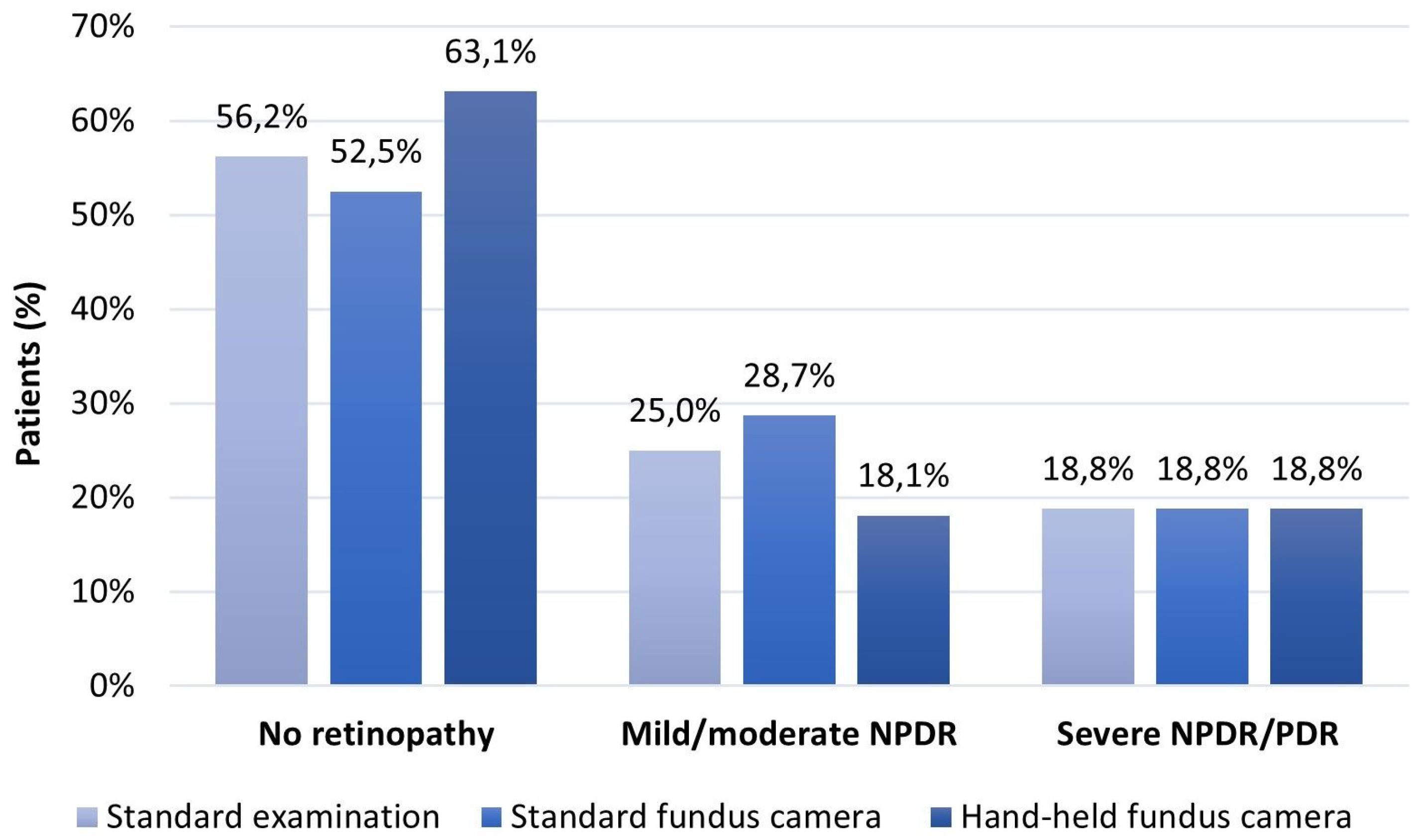
| Standardclinical examination | No retinopathy | 180 (56.2) | 0 (0) | 0 (0) |
| Mild/moderate NPDR | 22 (6.9) | 58 (18.1) | 0 (0) | |
| Severe NPDR/PDR | 0 (0) | 0 (0) | 60 (18.8) | |
| Standard fundus camera | No retinopathy | 168 (52.5) | 0 (0) | 0 (0) |
| Mild/moderate NPDR | 34 (10.6) | 58 (18.1) | 0 (0) | |
| Severe NPDR/PDR | 0 (0) | 0 (0) | 60 (18.8) | |
| Fundus Photography with a Hand-Held Fundus Camera | ||||
|---|---|---|---|---|
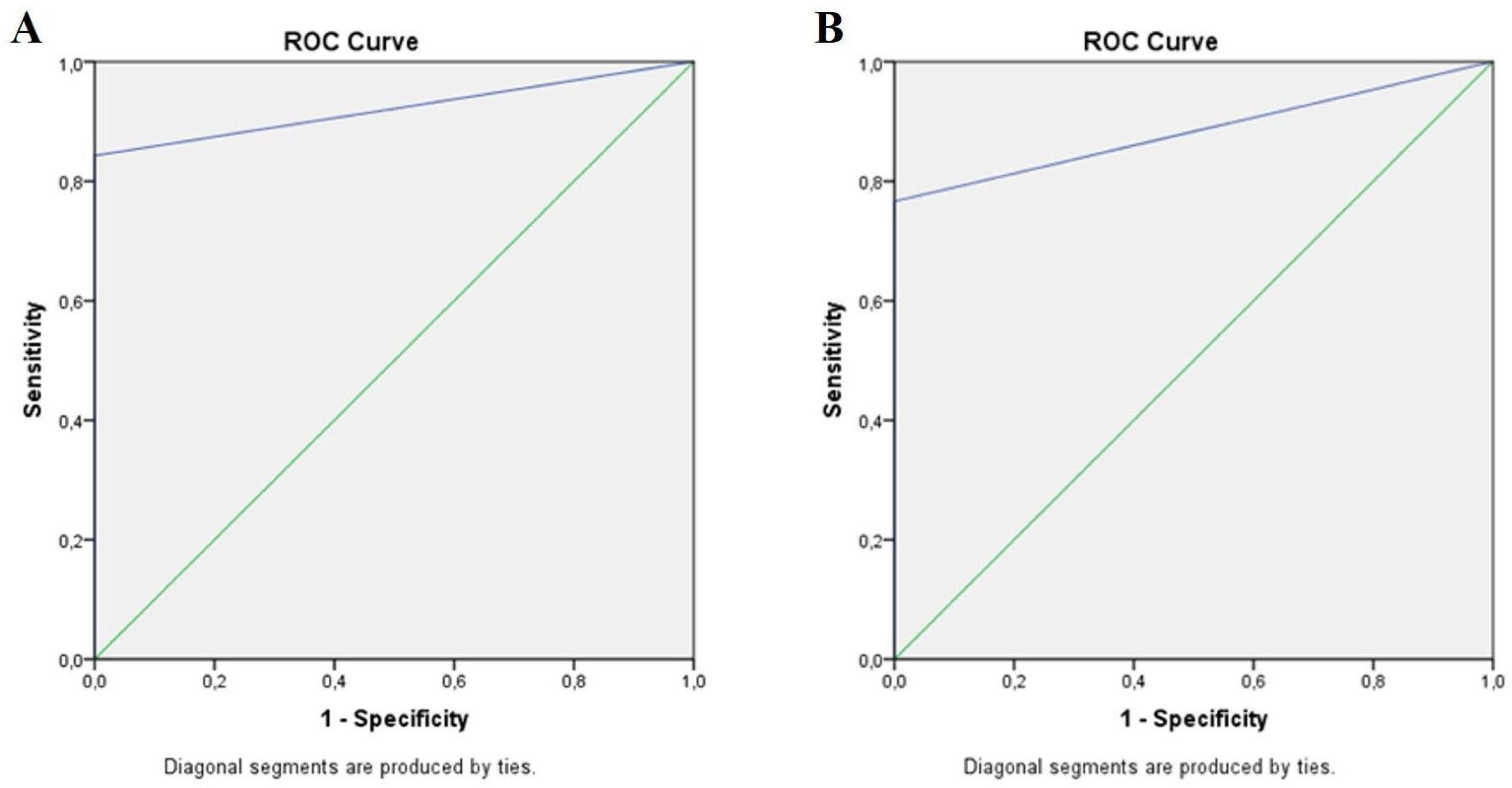
| Area | Std. Error | 95% CI | p | |
| Standard clinical examination | 0.921 | 0.026 | 0.870–0.973 | 0.000 |
| Standard fundus camera | 0.883 | 0.030 | 0.824–0.942 | 0.000 |
| Hand-Held Fundus Camera vs. | Standard Examination | Standard Fundus Camera | ||
|---|---|---|---|---|
| Estimate | 95% CI | Estimate | 95% CI | |
| Sensitivity | 89.1% | 81.3–94.4% | 83.2% | 74.4–89.9% |
| Specificity | 100% | 93.9–100% | 100% | 93.9–100% |
| PPV | 100% | 95.9–100% | 100% | 95.7–100% |
| NPV | 91.4% | 85.9–94.9% | 87.3% | 81.7–91.4% |
| LR+ | Infinity | NaN-Infinity | Infinity | NaN-Infinity |
| LR− | 0.11 | 0.06–0.19 | 0.17 | 0.11–0.26 |
| Kappa ± SE | 0.86 ± 0.04 | 0.77–0.94 | 0.78 ± 0.05 | 0.69–0.88 |
| DOR | 936.48 | 54.2–16194.6 | 574.6 | 33.8–9743.5 |
| DE | 94.9% | 90.3–97.8% | 92.2% | 86.9–95.8% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tomić, M.; Vrabec, R.; Hendelja, Đ.; Kolarić, V.; Bulum, T.; Rahelić, D. Diagnostic Accuracy of Hand-Held Fundus Camera and Artificial Intelligence in Diabetic Retinopathy Screening. Biomedicines 2024, 12, 34. https://doi.org/10.3390/biomedicines12010034
Tomić M, Vrabec R, Hendelja Đ, Kolarić V, Bulum T, Rahelić D. Diagnostic Accuracy of Hand-Held Fundus Camera and Artificial Intelligence in Diabetic Retinopathy Screening. Biomedicines. 2024; 12(1):34. https://doi.org/10.3390/biomedicines12010034
Chicago/Turabian StyleTomić, Martina, Romano Vrabec, Đurđica Hendelja, Vilma Kolarić, Tomislav Bulum, and Dario Rahelić. 2024. "Diagnostic Accuracy of Hand-Held Fundus Camera and Artificial Intelligence in Diabetic Retinopathy Screening" Biomedicines 12, no. 1: 34. https://doi.org/10.3390/biomedicines12010034





