Development of a Cell Culture Chamber for Investigating the Therapeutic Effects of Electrical Stimulation on Neural Growth
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. MTT Assay
2.3. Electrical Properties
2.4. Design of the ES Chamber
2.5. ES of Cells
3. Results
3.1. Cytotixicity of Materials
3.2. Verification of Electrical Properties of Stimulation Chamber
3.3. Validation Experiments
3.4. Observations
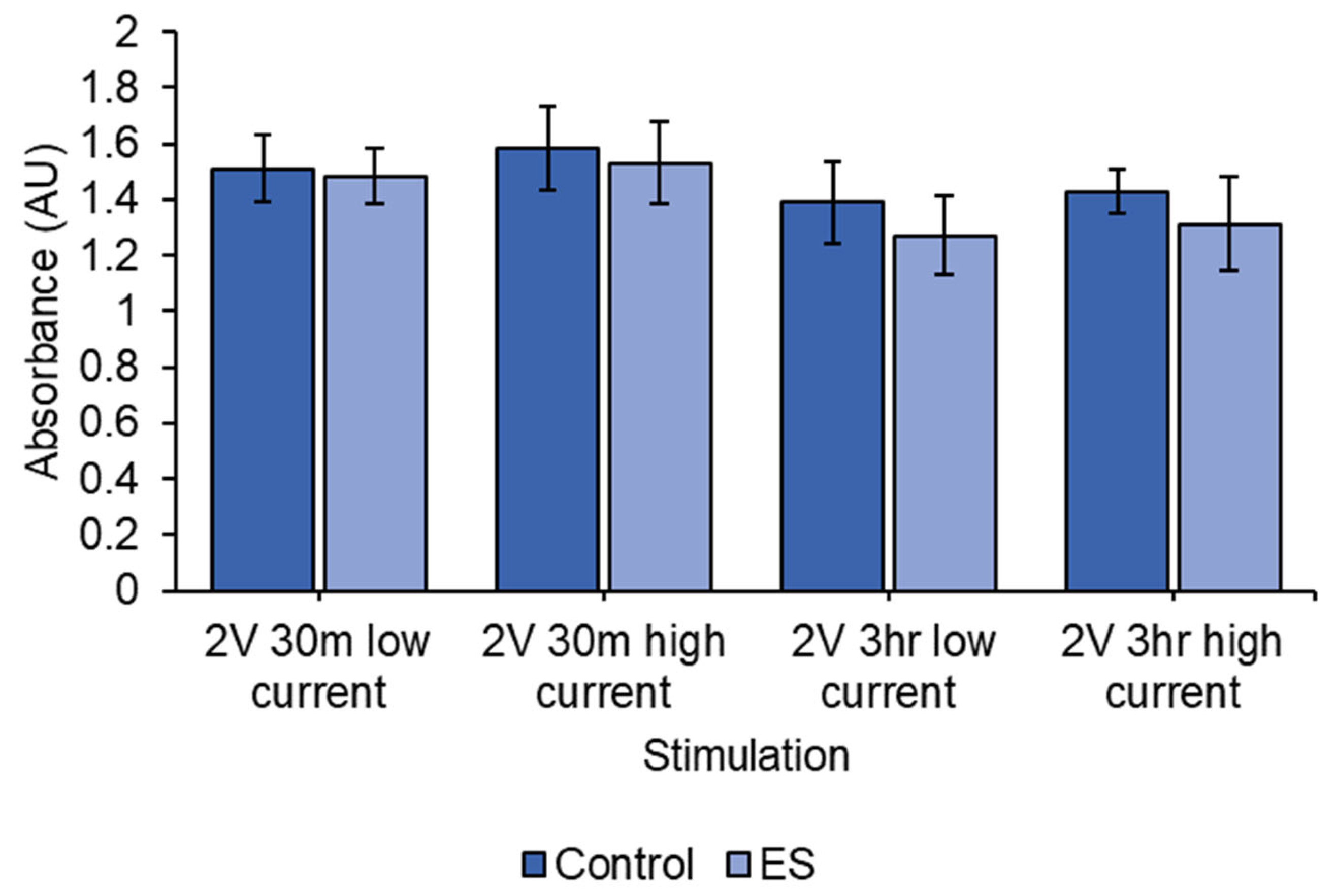
3.5. Cell Viability of ES Using 2 V DC EF on SH-SY5Y Cells
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shi, R.; Borgens, R.B. Three-dimensional gradients of voltage during development of the nervous system as invisible coordinates for the establishment of embryonic pattern. Dev. Dyn. 1995, 202, 101–114. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Mehta, A.S.; Zhao, M. Biomedical applications of electrical stimulation. Cell. Mol. Life Sci. 2020, 77, 2681–2699. [Google Scholar] [CrossRef] [PubMed]
- Reid, B.; Song, B.; Zhao, M. Electric currents in Xenopus tadpole tail regeneration. Dev. Biol. 2009, 335, 198–207. [Google Scholar] [CrossRef] [PubMed]
- Lv, Q.; Du, A.; Wei, W.; Li, Y.; Liu, G.; Wang, X.P. Deep Brain Stimulation: A Potential Treatment for Dementia in Alzheimer’s Disease (AD) and Parkinson’s Disease Dementia (PDD). Front. Neurosci. 2018, 12, 360. [Google Scholar] [CrossRef] [PubMed]
- Dhanasingh, A.; Jolly, C. An overview of cochlear implant electrode array designs. Hear. Res. 2017, 356, 93–103. [Google Scholar] [CrossRef] [PubMed]
- Merabet, L.B. Building the bionic eye: An emerging reality and opportunity. Prog. Brain Res. 2011, 192, 3–15. [Google Scholar] [CrossRef]
- Ngan, C.G.Y.; Kapsa, R.M.I.; Choong, P.F.M. Strategies for neural control of prosthetic limbs: From electrode interfacing to 3D printing. Materials 2019, 12, 1927. [Google Scholar] [CrossRef]
- Cooper, M.S.; Keller, R.E. Perpendicular orientation and directional migration of amphibian neural crest cells in dc electrical fields. Proc. Natl. Acad. Sci. USA 1984, 81, 160–164. [Google Scholar] [CrossRef]
- Bunn, S.J.; Lai, A.; Li, J. DC Electric Fields Induce Perpendicular Alignment and Enhanced Migration in Schwann Cell Cultures. Ann. Biomed. Eng. 2019, 47, 1584–1595. [Google Scholar] [CrossRef]
- Banks, T.A.; Luckman, P.S.B.; Frith, J.E.; Cooper-White, J.J. Effects of electric fields on human mesenchymal stem cell behaviour and morphology using a novel multichannel device. Integr. Biol. 2015, 7, 693–712. [Google Scholar] [CrossRef]
- Lang, M.; Bunn, S.J.; Gopalakrishnan, B.; Li, J. Use of weak DC electric fields to rapidly align mammalian cells. J. Neural Eng. 2021, 18, 054002. [Google Scholar] [CrossRef]
- Song, S.; Amores, D.; Chen, C.; McConnell, K.; Oh, B.; Poon, A.; George, P.M. Controlling properties of human neural progenitor cells using 2D and 3D conductive polymer scaffolds. Sci. Rep. 2019, 9, 19565. [Google Scholar] [CrossRef] [PubMed]
- Sordini, L.; Garrudo, F.F.F.; Rodrigues, C.A.V.; Linhardt, R.J.; Cabral, J.M.S.; Ferreira, F.C.; Morgado, J. Effect of Electrical Stimulation Conditions on Neural Stem Cells Differentiation on Cross-Linked PEDOT:PSS Films. Front. Bioeng. Biotechnol. 2021, 9, 591838. [Google Scholar] [CrossRef] [PubMed]
- Park, H.J.; Rouabhia, M.; Lavertu, D.; Zhang, Z. Electrical Stimulation Modulates the Expression of Multiple Wound Healing Genes in Primary Human Dermal Fibroblasts. Tissue Eng. Part A 2015, 21, 1982–1990. [Google Scholar] [CrossRef] [PubMed]
- Meng, S.; Rouabhia, M.; Zhang, Z. Electrical stimulation modulates osteoblast proliferation and bone protein production through heparin-bioactivated conductive scaffolds. Bioelectromagnetics 2013, 34, 189–199. [Google Scholar] [CrossRef]
- Zhu, R.; Sun, Z.; Li, C.; Ramakrishna, S.; Chiu, K.; He, L. Electrical stimulation affects neural stem cell fate and function in vitro. Exp. Neurol. 2019, 319, 112963. [Google Scholar] [CrossRef] [PubMed]
- Staehlke, S.; Bielfeldt, M.; Zimmermann, J.; Gruening, M.; Barke, I.; Freitag, T.; Speller, S.; Van Rienen, U.; Nebe, B. Pulsed Electrical Stimulation Affects Osteoblast Adhesion and Calcium Ion Signaling. Cells 2022, 11, 2650. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.H.; McCullen, S.D.; Piedrahita, J.A.; Loboa, E.G.; Olby, N.J. Alternating current electric fields of varying frequencies: Effects on proliferation and differentiation of porcine neural progenitor cells. Cell Reprogram. 2013, 15, 405–412. [Google Scholar] [CrossRef] [PubMed]
- Fridman, G.Y.; Della Santina, C.C. Safe direct current stimulator 2: Concept and design. Annu. Int. Conf. IEEE Eng. Med. Biol. Soc. 2013, 2013, 3126–3129. [Google Scholar] [CrossRef]
- Chen, C.; Bai, X.; Ding, Y.; Lee, I. Electrical stimulation as a novel tool for regulating cell behavior in tissue engineering. Biomater. Res. 2019, 23, 25. [Google Scholar] [CrossRef]
- Xiong, G.M.; Do, A.T.; Wang, J.K.; Yeoh, C.L.; Yeo, K.S.; Choong, C. Development of a miniaturized stimulation device for electrical stimulation of cells. J. Biol. Eng. 2015, 9, 14. [Google Scholar] [CrossRef] [PubMed]
- Mobini, S.; Leppik, L.; Barker, J.H. Direct current electrical stimulation chamber for treating cells in vitro. BioTechniques 2016, 60, 95–98. [Google Scholar] [CrossRef] [PubMed]
- Khaw, J.S.; Xue, R.; Cassidy, N.J.; Cartmell, S.H. Electrical stimulation of titanium to promote stem cell orientation, elongation and osteogenesis. Acta Biomater. 2022, 139, 204–217. [Google Scholar] [CrossRef]
- Guo, B.; Ma, P.X. Conducting Polymers for Tissue Engineering. Biomacromolecules 2018, 19, 1764–1782. [Google Scholar] [CrossRef]
- Fu, C.; Pan, S.; Ma, Y.; Kong, W.; Qi, Z.; Yang, X. Effect of electrical stimulation combined with graphene-oxide-based membranes on neural stem cell proliferation and differentiation. Artif. Cells Nanomed. Biotechnol. 2019, 47, 1867–1876. [Google Scholar] [CrossRef] [PubMed]
- Jun, I.; Han, H.-S.; Edwards, J.R.; Jeon, H. Electrospun fibrous scaffolds for tissue engineering: Viewpoints on architecture and fabrication. Int. J. Mol. Sci. 2018, 19, 745. [Google Scholar] [CrossRef]
- Xu, J.; Tsai, Y.L.; Hsu, S.H. Design Strategies of Conductive Hydrogel for Biomedical Applications. Molecules 2020, 25, 5296. [Google Scholar] [CrossRef]
- Huynh, Q.-S.; Holsinger, R.M.D. Fiber and Electrical Field Alignment Increases BDNF Expression in SH-SY5Y Cells following Electrical Stimulation. Pharmaceuticals 2023, 16, 138. [Google Scholar] [CrossRef]
- Lang, Q.; Wu, Y.; Ren, Y.; Tao, Y.; Lei, L.; Jiang, H. AC Electrothermal Circulatory Pumping Chip for Cell Culture. ACS Appl. Mater. Interfaces 2015, 7, 26792–26801. [Google Scholar] [CrossRef]
- BioSolve. PBS Buffer 10X (Sterile) Molecular Biology. Available online: https://shop.biosolve-chemicals.eu/detail.php?id=2093 (accessed on 25 April 2023).
- Fisher Bioreagents. Phosphate Buffered Saline 1X Solution (PBS). Available online: https://www.fishersci.ie/webfiles/uk/web-docs/UKLS_1257.PDF (accessed on 25 April 2023).
- Meng, S.; Rouabhia, M.; Zhang, Z. Electrical Stimulation and Cellular Behaviors in Electric Field in Biomedical Research. Materials 2021, 15, 165. [Google Scholar] [CrossRef]
- Tef-Gel Pty Ltd. Tef-GelTM Technical Information. Available online: https://www.tefgel.com.au/technical-information/ (accessed on 25 April 2023).
- Park, J.-Y.; Lee, J.-H.; Kim, C.-H.; Kim, Y.-J. Fabrication of polytetrafluoroethylene nanofibrous membranes for guided bone regeneration. RSC Adv. 2018, 8, 34359–34369. [Google Scholar] [CrossRef]
- Pascual, G.; Sotomayor, S.; Rodríguez, M.; Pérez-Köhler, B.; Kühnhardt, A.; Fernández-Gutiérrez, M.; San Román, J.; Bellón, J.M. Cytotoxicity of Cyanoacrylate-Based Tissue Adhesives and Short-Term Preclinical In Vivo Biocompatibility in Abdominal Hernia Repair. PLoS ONE 2016, 11, e0157920. [Google Scholar] [CrossRef] [PubMed]
- Landegren, T.; Risling, M.; Persson, J.K.; Sondén, A. Cyanoacrylate in nerve repair: Transient cytotoxic effect. Int. J. Oral Maxillofac. Surg. 2010, 39, 705–712. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M. Electrical fields in wound healing—An overriding signal that directs cell migration. Semin. Cell Dev. Biol. 2009, 20, 674–682. [Google Scholar] [CrossRef] [PubMed]
- Ghasemi-Mobarakeh, L.; Prabhakaran, M.P.; Morshed, M.; Nasr-Esfahani, M.H.; Ramakrishna, S. Electrical Stimulation of Nerve Cells Using Conductive Nanofibrous Scaffolds for Nerve Tissue Engineering. Tissue Eng. Part A 2009, 15, 3605–3619. [Google Scholar] [CrossRef]
- Koppes, A.N.; Nordberg, A.L.; Paolillo, G.M.; Goodsell, N.M.; Darwish, H.A.; Zhang, L.; Thompson, D.M. Electrical stimulation of schwann cells promotes sustained increases in neurite outgrowth. Tissue Eng. Part A 2014, 20, 494–506. [Google Scholar] [CrossRef]
- Ganeson, S.; Abdul Jamil, M.M.; Mamman, H.B.; Abd Rahman, N.A. Correlation between Electrical Field Strength and Pulse Width Analysis on Cell Viability. J. Phys. Conf. Ser. 2018, 1019, 012012. [Google Scholar] [CrossRef]
- Lyte, M.; Gannon, J.E.; O’Clock, G.D., Jr. Effects of in vitro electrical stimulation on enhancement and suppression of malignant lymphoma cell proliferation. J. Natl. Cancer Inst. 1991, 83, 116–119. [Google Scholar] [CrossRef]
- Hartshorn, D.O.; Miller, J.M.; Altschuler, R.A. Protective effect of electrical stimulation in the deafened guinea pig cochlea. Otolaryngol. Head Neck Surg. 1991, 104, 311–319. [Google Scholar] [CrossRef]





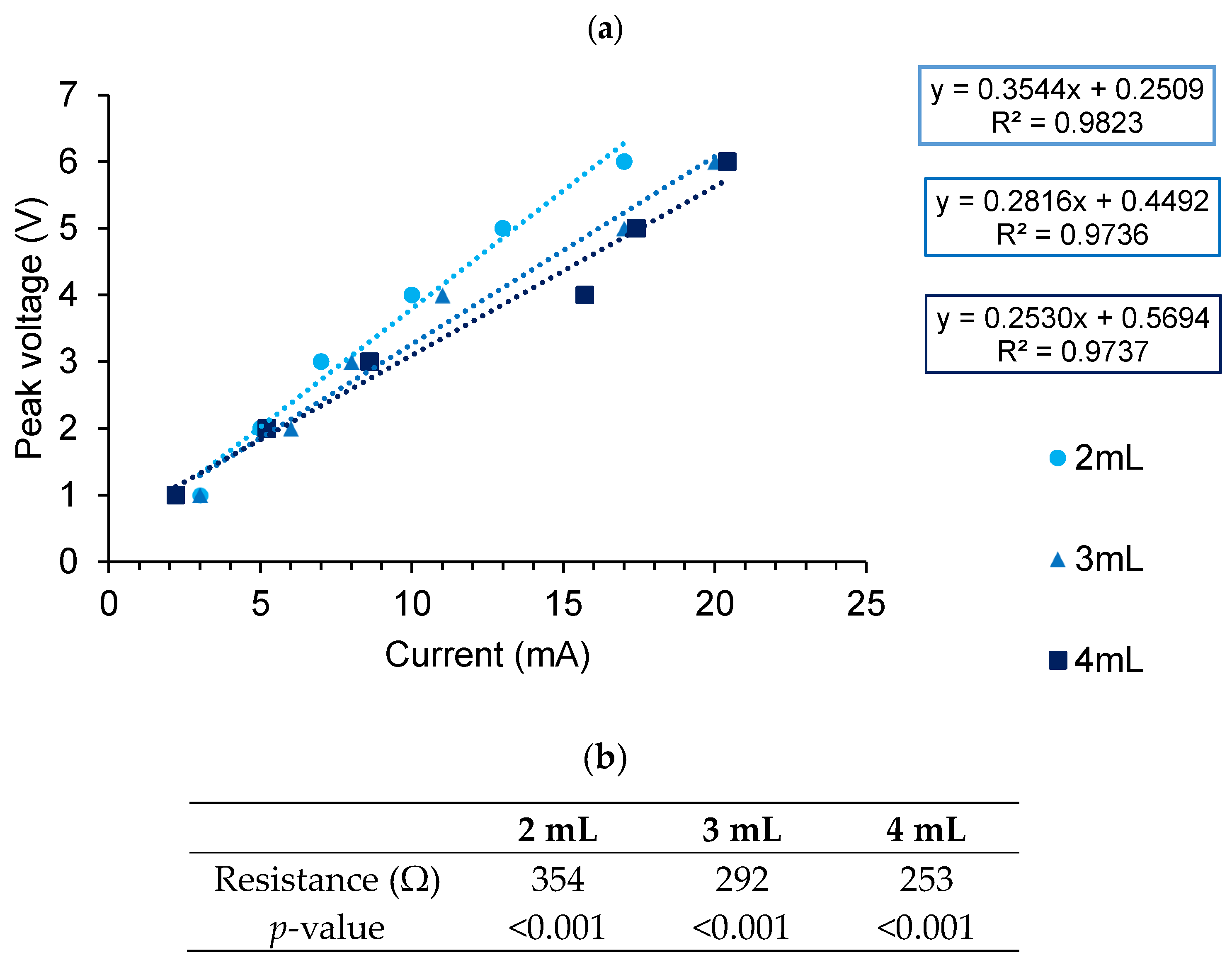

| Peak Voltage (V) | Measured Current (µA) 2 mL | Measured Current (µA) 3 mL | Measured Current (µA) 4 mL |
|---|---|---|---|
| 1 | 25 | 24 | 23 |
| 2 | 24 | 25 | 24 |
| 3 | 26 | 24 | 26 |
| 4 | 22 | 26 | 25 |
| 5 | 22 | 22 | 26 |
| 6 | 23 | 23 | 24 |
| Average | 23.7 | 24.0 | 24.7 |
| Predicted | 21.5 | 20.0 | 19.8 |
| p-value for gradient | <0.001 | <0.001 | <0.001 |
| %error | 10.23% | 20.00% | 24.75% |
| Electrical Conductivity (σ) (Sm−1) | Calculation of Resistivity | Resistivity (ρ) (Ω · m) | Reference |
|---|---|---|---|
| Fresh media—1.68 | 0.595 | [29] | |
| PBS—1.4 | 0.71 | [30,31] | |
| PBS—2.0 | 0.50 | [30,31] |
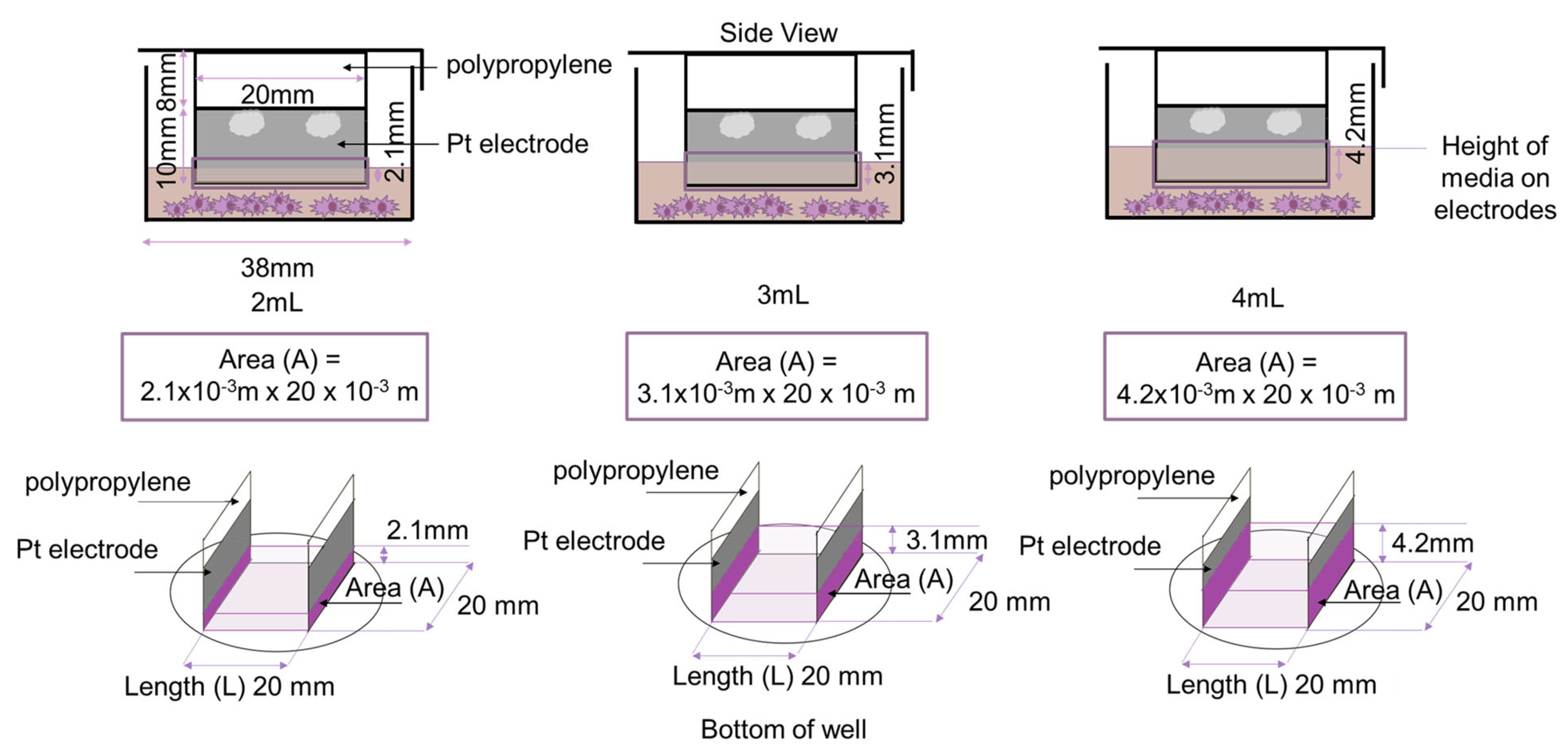
| (Ω · m) | Area (A) (m2) | Volume of Media (mL) | Calculation |
|---|---|---|---|
| 0.595 | 4.2 × 10−5 | 2 | |
| 6.2 × 10−5 | 3 | ||
| 8.4 × 10−5 | 4 | ||
| 0.71 | 4.2 × 10−5 | 2 | |
| 6.2 × 10−5 | 3 | ||
| 8.4 × 10−5 | 4 | ||
| 0.50 | 4.2 × 10−5 | 2 | |
| 6.2 × 10−5 | 3 | ||
| 8.4 × 10−5 | 4 |
| 2 mL | 3 mL | 4 mL | |
|---|---|---|---|
| Resistance (R) (Ω) | 354 | 292 | 253 |
| R calculated from [29] (Ω) | 283 | 190 | 142 |
| % error | 25.1 | 53.7 | 78.2 |
| R from PBS (1.4 Sm−1) (Ω) | 340 | 230 | 170 |
| % error | 4.08 | 26.7 | 48.8 |
| R from PBS (2 Sm−1) (Ω) | 238 | 161 | 119 |
| % error | 48.7 | 81.0 | 113 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huynh, Q.-S.; Holsinger, R.M.D. Development of a Cell Culture Chamber for Investigating the Therapeutic Effects of Electrical Stimulation on Neural Growth. Biomedicines 2024, 12, 289. https://doi.org/10.3390/biomedicines12020289
Huynh Q-S, Holsinger RMD. Development of a Cell Culture Chamber for Investigating the Therapeutic Effects of Electrical Stimulation on Neural Growth. Biomedicines. 2024; 12(2):289. https://doi.org/10.3390/biomedicines12020289
Chicago/Turabian StyleHuynh, Quy-Susan, and R. M. Damian Holsinger. 2024. "Development of a Cell Culture Chamber for Investigating the Therapeutic Effects of Electrical Stimulation on Neural Growth" Biomedicines 12, no. 2: 289. https://doi.org/10.3390/biomedicines12020289





