Comparative EPR Studies on the Influence of Genistein on Free Radicals in Non-Irradiated and UV-Irradiated MCF7, T47D and MDA-MB-231 Breast Cancer Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Lines
2.2. Genistein
2.3. Cell Viability Measurement
2.4. Cell Proliferation Measurement
2.5. UV Irradiation
2.6. EPR Measurements
2.7. Statistical Analysis
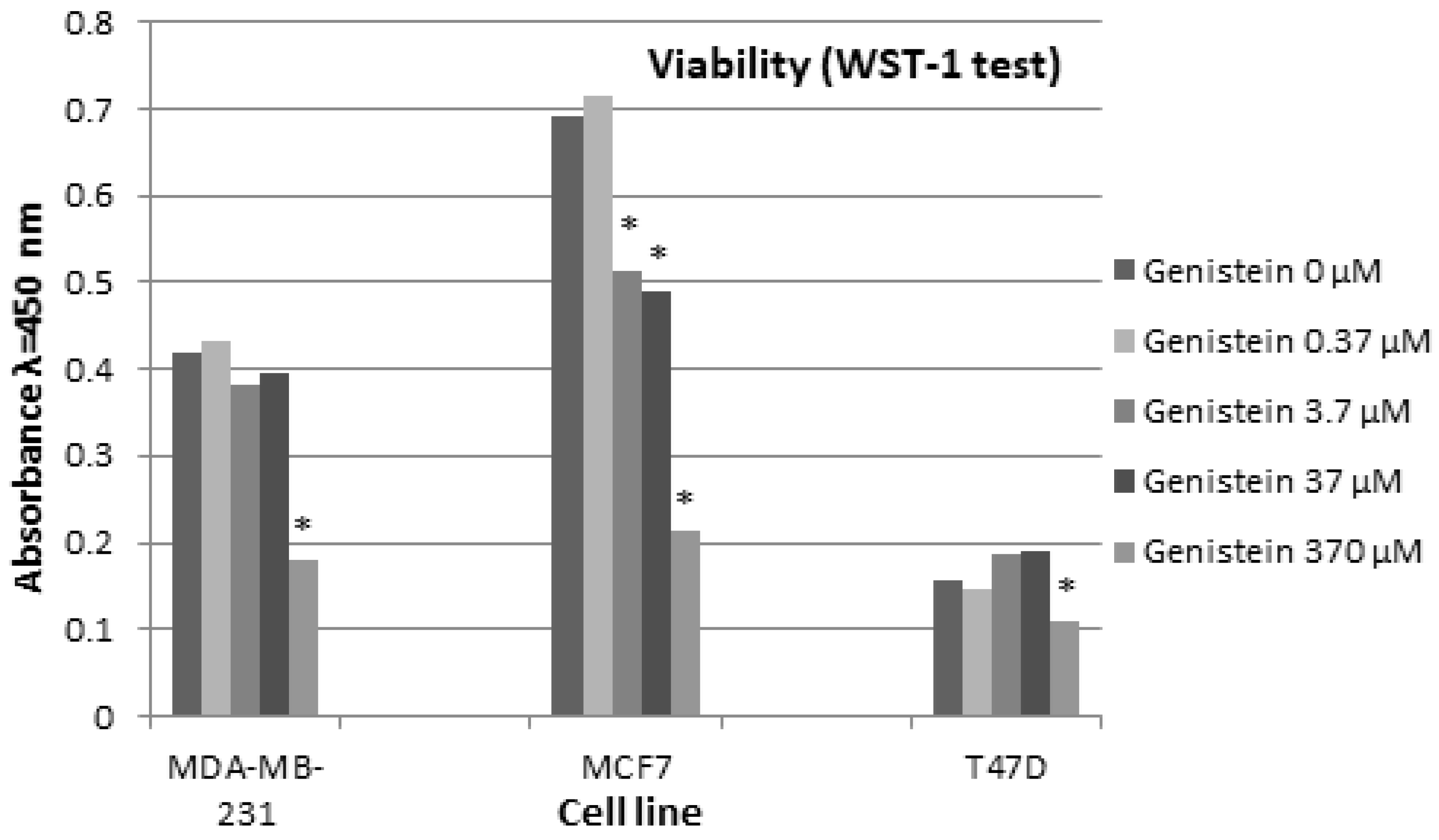
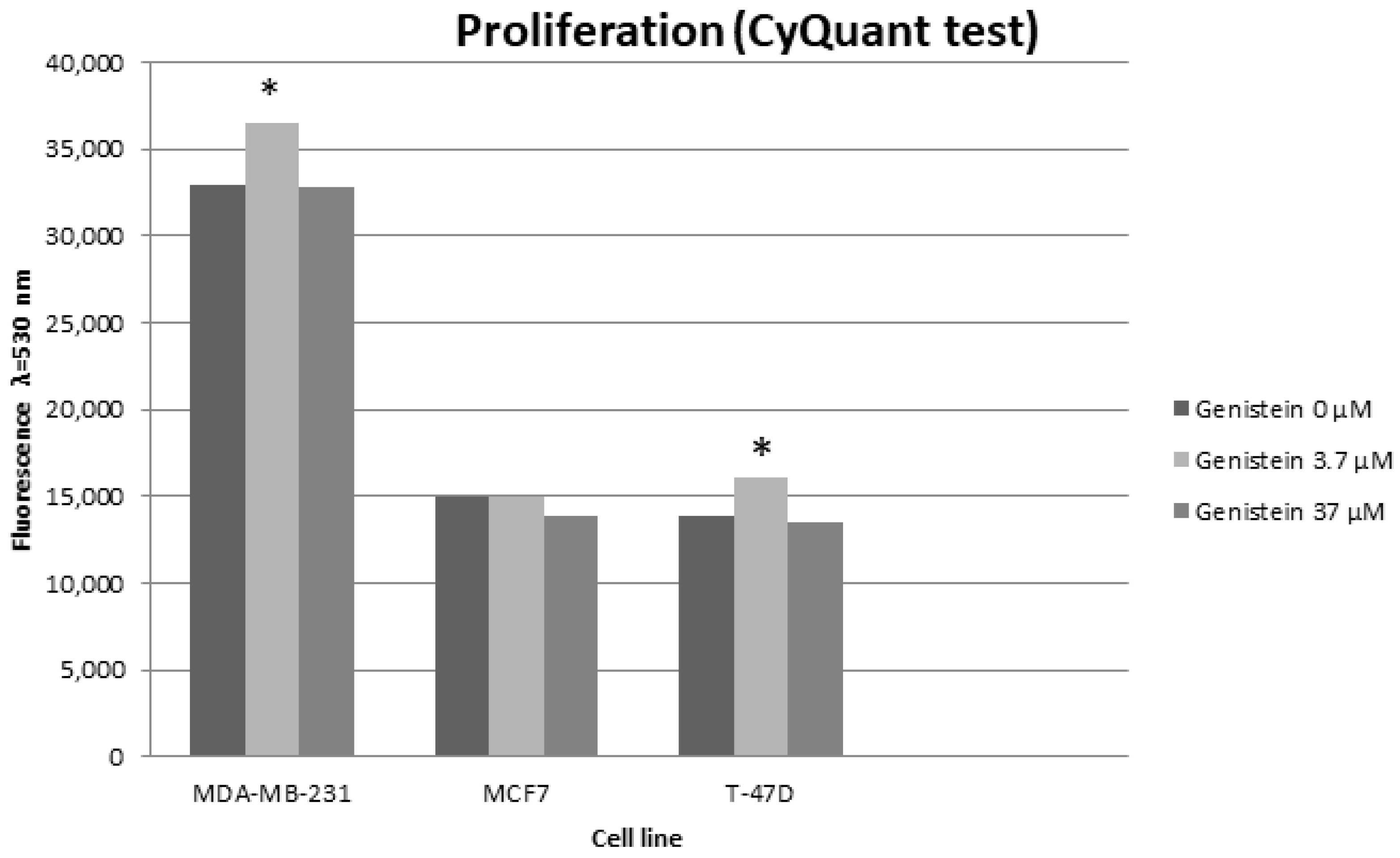
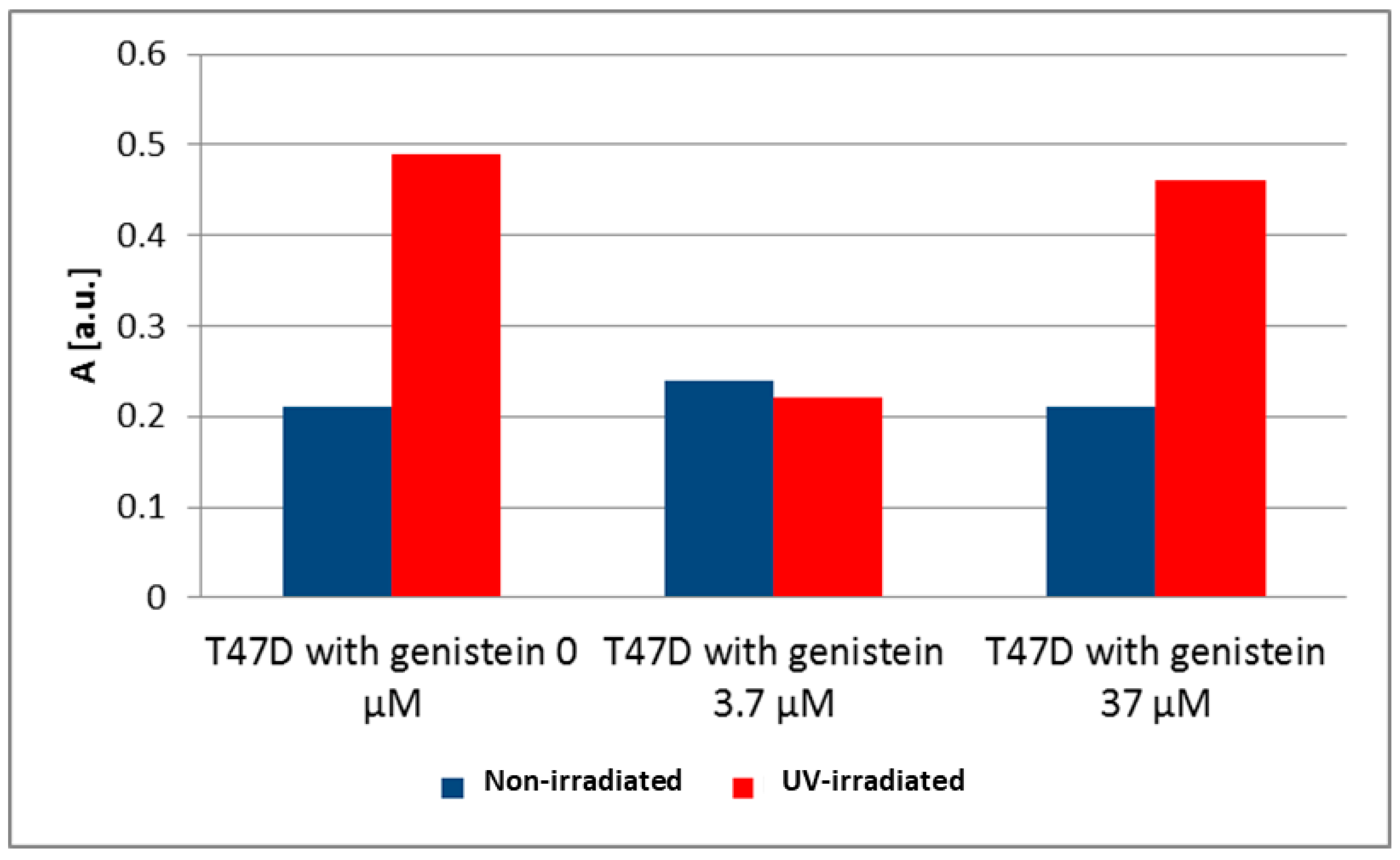
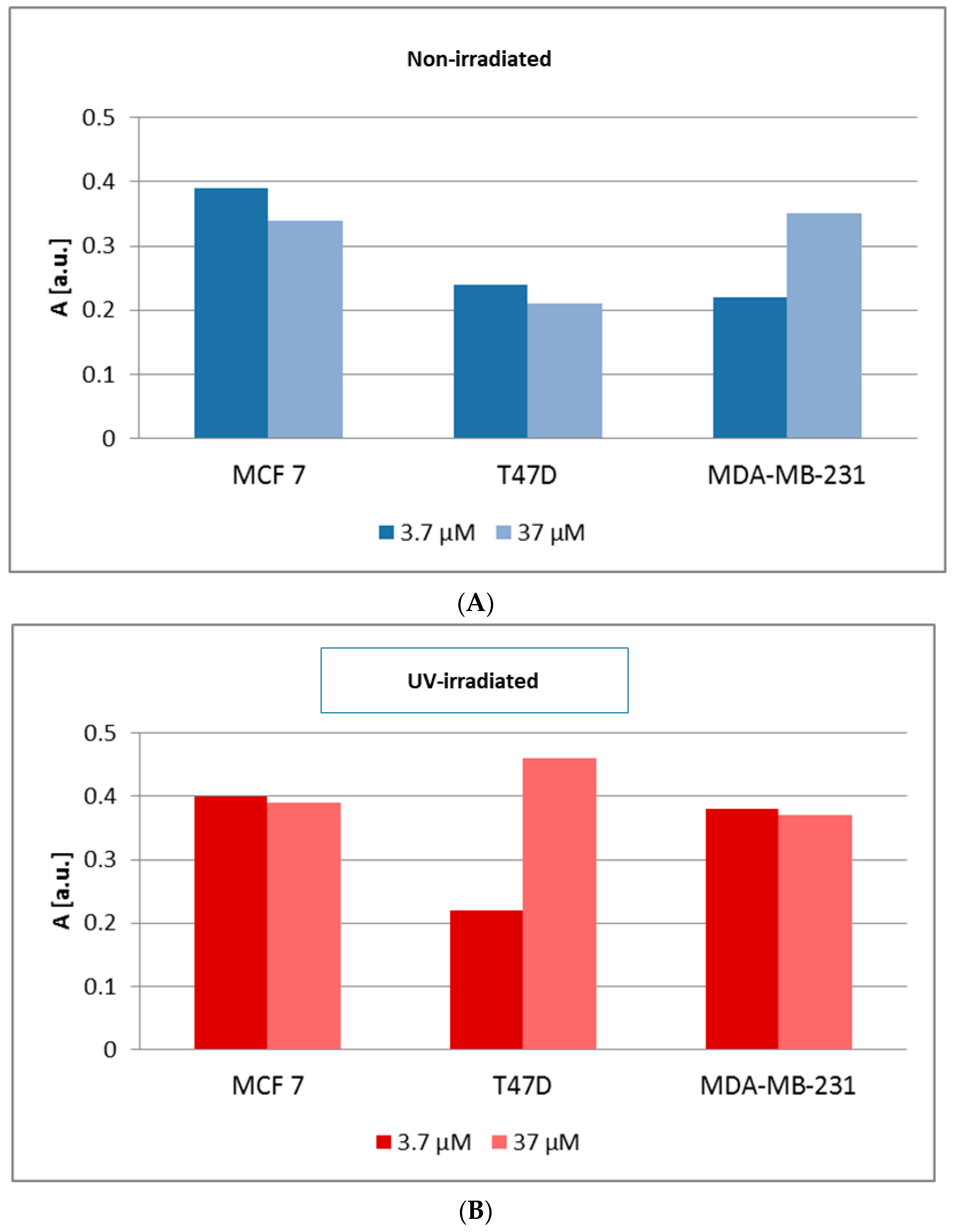
3. Results and Discussion
Cells Viability and Proliferation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Siegel, R.L.; Wagle, N.S.; Cercek, A.; Smith, R.A.; Jemal, A. Colorectal cancer statistics, 2023. CA Cancer J. Clin. 2023, 73, 233–254. [Google Scholar] [CrossRef]
- Sun, Y.S.; Zhao, Z.; Yang, Z.N.; Xu, F.; Lu, H.J.; Zhu, Z.Y.; Shi, W.; Jiang, J.; Yao, P.P.; Zhu, H.P. Risk Factors and Preventions of Breast Cancer. Int. J. Biol. Sci. 2017, 13, 1387–1397. [Google Scholar] [CrossRef]
- Habara, M.; Shimada, M. Estrogen receptor α revised: Expression, structure, function, and stability. Bioessays 2022, 44, e2200148. [Google Scholar] [CrossRef] [PubMed]
- Mal, R.; Magner, A.; David, J.; Datta, J.; Vallabhaneni, M.; Kassem, M.; Manouchehri, J.; Willingham, N.; Stover, D.; Vandeusen, J.; et al. Estrogen Receptor Beta (ERβ): A Ligand Activated Tumor Suppressor. Front. Oncol. 2020, 10, 587386. [Google Scholar] [CrossRef]
- Derakhshan, F.; Reis-Filho, J.S. Pathogenesis of Triple-Negative Breast Cancer. Annu. Rev. Pathol. 2022, 17, 181–204. [Google Scholar] [CrossRef] [PubMed]
- Malik, P.; Singh, R.; Kumar, M.; Malik, A.; Mukherjee, T.K. Understanding the Phytoestrogen Genistein Actions on Breast Cancer: Insights on Estrogen Receptor Equivalence, Pleiotropic Essence and Emerging Paradigms in Bioavailability Modulation. CTMC 2023, 23, 1395–1413. [Google Scholar] [CrossRef]
- Hu, C.; Wang, M.; Hu, M.; Ma, S.; Yang, B.; Xiao, W.; Zhou, Q.; Zhou, M.; Li, Z. Genistein Induces Endocrine Resistance in Human Breast Cancer by Suppressing H3K27 Trimethylation. Endocr. Relat. Cancer 2023, 30, e220191. [Google Scholar] [CrossRef]
- Bezerra, P.H.A.; Amaral, C.; Almeida, C.F.; Correia-da-Silva, G.; Torqueti, M.R.; Teixeira, N. In Vitro Effects of Combining Genistein with Aromatase Inhibitors: Concerns Regarding Its Consumption during Breast Cancer Treatment. Molecules 2023, 28, 4893. [Google Scholar] [CrossRef]
- Sotoca, A.M.; Gelpke, M.D.; Boeren, S.; Ström, A.; Gustafsson, J.Å.; Murk, A.J.; Rietjens, I.M.; Vervoort, J. Quantitative proteomics and transcriptomics addressing the estrogen receptor subtype-mediated effects in T47D breast cancer cells exposed to the phytoestrogen genistein. Mol. Cell Proteom. 2011, 10, M110.002170. [Google Scholar] [CrossRef] [PubMed]
- Matsumura, K.; Tanaka, T.; Kawashima, H.; Nakatani, T. Involvement of the estrogen receptor beta in genistein-induced expression of p21(waf1/cip1) in PC-3 prostate cancer cells. Anticancer. Res. 2008, 28, 709–714. [Google Scholar]
- Shrivastava, A.; Aggarwal, L.M.; Mishra, S.P.; Khanna, H.D. Free radicals and antioxidants in normal versus cancerous cells—An overview. Indian J. Biochem. Biophys. 2019, 56, 7–19. [Google Scholar]
- Jiang, H.; Zuo, J.; Li, B.; Chen, R.; Luo, K.; Xiang, X.; Lu, S.; Huang, C.; Liu, L.; Tang, J.; et al. Drug-induced oxidative stress in cancer treatments: Angel or devil? Redox Biol. 2023, 63, 102754. [Google Scholar] [CrossRef] [PubMed]
- Sharifi-Rad, J.; Quispe, C.; Imran, M.; Rauf, A.; Nadeem, M.; Gondal, T.A.; Ahmad, B.; Atif, M.; Mubarak, M.S.; Sytar, O.; et al. Genistein: An Integrative Overview of Its Mode of Action, Pharmacological Properties, and Health Benefits. Oxid. Med. Cell Longev. 2021, 2021, 3268136. [Google Scholar] [CrossRef] [PubMed]
- Dai, X.; Cheng, H.; Bai, Z.; Li, J. Breast Cancer Cell Line Classification and Its Relevance with Breast Tumor Subtyping. J. Cancer 2017, 8, 3131–3141. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Kim, T.; Yoo, K.H.; Kang, K. The T47D cell line is an ideal experimental model to elucidate the progesterone-specific effects of a luminal A subtype of breast cancer. Biochem. Biophys. Res. Commun. 2017, 486, 752–758. [Google Scholar] [CrossRef] [PubMed]
- Radde, B.N.; Ivanova, M.M.; Mai, H.X.; Salabei, J.K.; Hill, B.G.; Klinge, C.M. Bioenergetic differences between MCF-7 and T47D breast cancer cells and their regulation by oestradiol and tamoxifen. Biochem. J. 2015, 465, 49–61. [Google Scholar] [CrossRef] [PubMed]
- Ortega, M.A.; Fraile-Martínez, O.; Asúnsolo, Á.; Buján, J.; García-Honduvilla, N.; Coca, S. Signal Transduction Pathways in Breast Cancer: The Important Role of PI3K/Akt/mTOR. J. Oncol. 2020, 2020, 9258396. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://www.proteinatlas.org/humanproteome/cell+line/Breast+cancer (accessed on 6 February 2024).
- Gordon, L.A.; Mulligan, K.T.; Maxwell-Jones, H.; Adams, M.; Walker, R.A.; Jones, J.L. Breast cell invasive potential relates to the myoepithelial phenotype. Int. J. Cancer 2003, 106, 8–16. [Google Scholar] [CrossRef]
- Holliday, D.L.; Speirs, V. Choosing the right cell line for breast cancer research. Breast Cancer Res. BCR 2011, 13, 215. [Google Scholar] [CrossRef]
- Chekhun, S.; Bezdenezhnykh, N.; Shvets, J.; Lukianova, N. Expression of biomarkers related to cell adhesion, metastasis and invasion of breast cancer cell lines of different molecular subtype. Exp. Oncol. 2013, 35, 174–179. [Google Scholar]
- Eaton, G.R.; Eaton, S.S.; Salikhov, K.M. Foundations of Modern EPR; World Scientific: Singapore, 1998. [Google Scholar]
- Wertz, J.E.; Bolton, J.R. Electron Spin Resonance: Elementary Theory and Practical Applications; Chapman and Hall: London, UK, 1986. [Google Scholar]
- Yang, S.; Zhou, Q.; Yang, X. Caspase-3 status is a determinant of the differential responses to genistein between MDA-MB-231 and MCF-7 breast cancer cells. Biochim. Biophys. Acta 2007, 1773, 903–911. [Google Scholar] [CrossRef]
- Chen, W.F.; Huang, M.H.; Tzang, C.H.; Yang, M.; Wong, M.S. Inhibitory actions of genistein in human breast cancer (MCF-7) cells. Biochim. Biophys. Acta—Mol. Basis Dis. 2003, 1638, 187–196. [Google Scholar] [CrossRef]
- Jordan, C.; Munzlinger, M.; Potterfield, A.; Mayne, J. The effects of genistein on MCF-7 and MDA-MB-231 breast cancer cell proliferation. FASEB J. 2009, 23, LB19. [Google Scholar] [CrossRef]
- Kousidou, O.C.; Mitropoulou, T.N.; Roussidis, A.E.; Kletsas, D.; Theocharis, A.D.; Karamanos, N.K. Genistein suppresses the invasive potential of human breast cancer cells through transcriptional regulation of metalloproteinases and their tissue inhibitors. Int. J. Oncol. 2005, 26, 1101–1109. [Google Scholar] [CrossRef] [PubMed]
- Astuti, P.; Utami, E.G.; Nugrahani, A.W.; Sudjadi, S. Genistein abrogates G2 arrest induced by curcumin in p53 deficient T47D cells. Daru 2012, 20, 82. [Google Scholar] [CrossRef] [PubMed]
- Choi, E.J.; Jung, J.Y.; Kim, G.H. Genistein inhibits the proliferation and differentiation of MCF-7 and 3T3-L1 cells via the regulation of ERα expression and induction of apoptosis. Exp. Ther. Med. 2014, 8, 454–458. [Google Scholar] [CrossRef] [PubMed]
- De Lemos, M.L. Effects of soy phytoestrogens genistein and daidzein on breast cancer growth. Ann. Pharmacother. 2001, 35, 1118–1121. [Google Scholar] [CrossRef]
- Dampier, K.; Hudson, E.A.; Howells, L.M.; Manson, M.M.; Walker, R.A.; Gescher, A. Differences between human breast cell lines in susceptibility towards growth inhibition by genistein. Br. J. Cancer 2001, 85, 618–624. [Google Scholar] [CrossRef]
- Cappelletti, V.; Fioravanti, L.; Miodini, P.; di Fronzo, G. Genistein blocks breast cancer cells in the G2M phase of the cell cycle. J. Cell. Biochem. 2000, 79, 594–600. [Google Scholar] [CrossRef] [PubMed]
- Jurzak, M.; Ramos, P.; Pilawa, B. The influence of genistein on free radicals in normal dermal fibroblasts and keloid fibroblasts examined by EPR spectroscopy. Med. Chem. Res. 2017, 26, 1297–1305. [Google Scholar] [CrossRef]
- Schumacker, P.T. Reactive oxygen species in cancer cells: Live by the sword, die by the sword. Cancer Cell 2006, 10, 175–176. [Google Scholar] [CrossRef] [PubMed]
- Xing, G.; Romanyukha, A.; Bunger, R. Reactive Oxygen Species (ROS) in Human Breast Cancer cell lines Differing in Malignancy: An Electron Paramagnetic Resonance (EPR) Study. FASEB J. 2008, 22, 794.10. [Google Scholar] [CrossRef]
- Nogueira, V.; Hay, N. Molecular pathways: Reactive oxygen species homeostasis in cancer cells and implications for cancer therapy. Clin. Cancer Res. 2013, 19, 4309–4314. [Google Scholar] [CrossRef]
- Borrás, C.; Gambini, J.; Gómez-Cabrera, M.C.; Sastre, J.; Pallardó, F.V.; Mann, G.E.; Viña, J. Genistein, a soy isoflavone, up-regulates expression of antioxidant genes: Involvement of estrogen receptors, ERK1/2, and NFkappaB. FASEB J. 2006, 20, 2136–2138. [Google Scholar] [CrossRef] [PubMed]
- Wei, H.; Zhang, X.; Wang, Y.; Lebwohl, M. Inhibition of ultraviolet light-induced oxidative events in the skin and internal organs of hairless mice by isoflavone genistein. Cancer Lett. 2002, 185, 21–29. [Google Scholar] [CrossRef]
- Landauer, M.R.; Srinivasan, V.; Seed, T.M. Genistein treatment protects mice from ionizing radiation injury. J. Appl. Toxicol. 2003, 23, 379–385. [Google Scholar] [CrossRef]
- Sarkar, F.H.; Li, Y. Using chemopreventive agents to enhance the efficacy of cancer therapy. Cancer Res. 2006, 66, 3347–3350. [Google Scholar] [CrossRef]
- Garg, A.K.; Buchholz, T.A.; Aggarwal, B.B. Chemosensitization and radiosensitization of tumors by plant polyphenols. Antioxid. Redox Signal. 2005, 7, 1630–1647. [Google Scholar] [CrossRef]
- Nadal-Serrano, M.; Pons, D.G.; Sastre-Serra, J.; Blanquer-Rossellò, M.M.; Roca, P.; Oliver, J. Genistein modulates oxidative stress in breast cancer cell lines according to ERa/ERβ ratio: Effects on mitochondrial functionality, sirtuins, uncoupling protein 2 and antioxidant enzymes. Int. J. Biochem. Cell Biol. 2013, 45, 2045–2051. [Google Scholar] [CrossRef]
- Kim, I.G.; Kim, J.K.; Lee, J.H.; Cho, E.W. Genistein decreases cellular redox potential, partially suppresses cell growth in HL-60 leukemia cells and sensitizes cells to γ-radiation-induced cell death. Mol. Med. Rep. 2014, 10, 2786–2792. [Google Scholar] [CrossRef]
- Koss-Mikołajczyk, I.; Baranowska, M.; Namieśnik, J.; Bartoszek, A. Determination of antioxidantactivity of phytochemicals in cellular models by fluorescence/luminescence methods. Postepy Hig. Med. Dosw. 2017, 71, 602–617. [Google Scholar] [CrossRef] [PubMed]
- Almaghrabi, O.A. Molecular and biochemical investigations on the effect of quercetin on oxidative stress induced by cisplatin in rat kidney. Saudi J. Biol. Sci. 2015, 22, 227–231. [Google Scholar] [CrossRef] [PubMed]
- Sarmiento-Salinas, F.L.; Delgado-Magallón, A.; Cortés-Hernández, P.; Reyes-Leyva, J.; Herrera-Camacho, I. Breast Cancer Subtypes Present a Differential Production of Reactive Oxygen Species (ROS) and Susceptibility to Antioxidant Treatment. Front. Oncol. 2019, 9, 480. [Google Scholar] [CrossRef]
- Perillo, B.; Di Donato, M.; Pezone, A.; Di Zazzo, E.; Giovannelli, P.; Galasso, G.; Castoria, G.; Migliaccio, A. ROS in cancer therapy: The bright side of the moon. Exp. Mol. Med. 2020, 52, 192–203. [Google Scholar] [CrossRef] [PubMed]
- Luo, M.; Zhou, L.; Huang, Z.; Li, B.; Nice, E.C.; Xu, J.; Huang, C. Antioxidant Therapy in Cancer: Rationale and Progress. Antioxidants 2022, 11, 1128. [Google Scholar] [CrossRef]
- Qi, X.; Jha, S.K.; Jha, N.K.; Dewanjee, S.; Dey, A.; Deka, R.; Pritam, P.; Ramgopal, K.; Liu, W.; Hou, K. Antioxidants in brain tumors: Current therapeutic significance and future prospects. Mol. Cancer 2022, 21, 204. [Google Scholar] [CrossRef]
- Bhat, S.S.; Prasad, S.K.; Shivamallu, C.; Prasad, K.S.; Syed, A.; Reddy, P.; Cull, C.A.; Amachawadi, R.G. Genistein: A Potent Anti-Breast Cancer Agent. Curr. Issues Mol. Biol. 2021, 43, 1502–1517. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jurzak, M.; Ramos, P.; Pilawa, B.; Bednarek, I.A. Comparative EPR Studies on the Influence of Genistein on Free Radicals in Non-Irradiated and UV-Irradiated MCF7, T47D and MDA-MB-231 Breast Cancer Cells. Biomedicines 2024, 12, 518. https://doi.org/10.3390/biomedicines12030518
Jurzak M, Ramos P, Pilawa B, Bednarek IA. Comparative EPR Studies on the Influence of Genistein on Free Radicals in Non-Irradiated and UV-Irradiated MCF7, T47D and MDA-MB-231 Breast Cancer Cells. Biomedicines. 2024; 12(3):518. https://doi.org/10.3390/biomedicines12030518
Chicago/Turabian StyleJurzak, Magdalena, Paweł Ramos, Barbara Pilawa, and Ilona Anna Bednarek. 2024. "Comparative EPR Studies on the Influence of Genistein on Free Radicals in Non-Irradiated and UV-Irradiated MCF7, T47D and MDA-MB-231 Breast Cancer Cells" Biomedicines 12, no. 3: 518. https://doi.org/10.3390/biomedicines12030518






