Revisiting Treatment of Metastatic Urothelial Cancer: Where Do Cisplatin and Platinum Ineligibility Criteria Stand?
Abstract
:1. Introduction
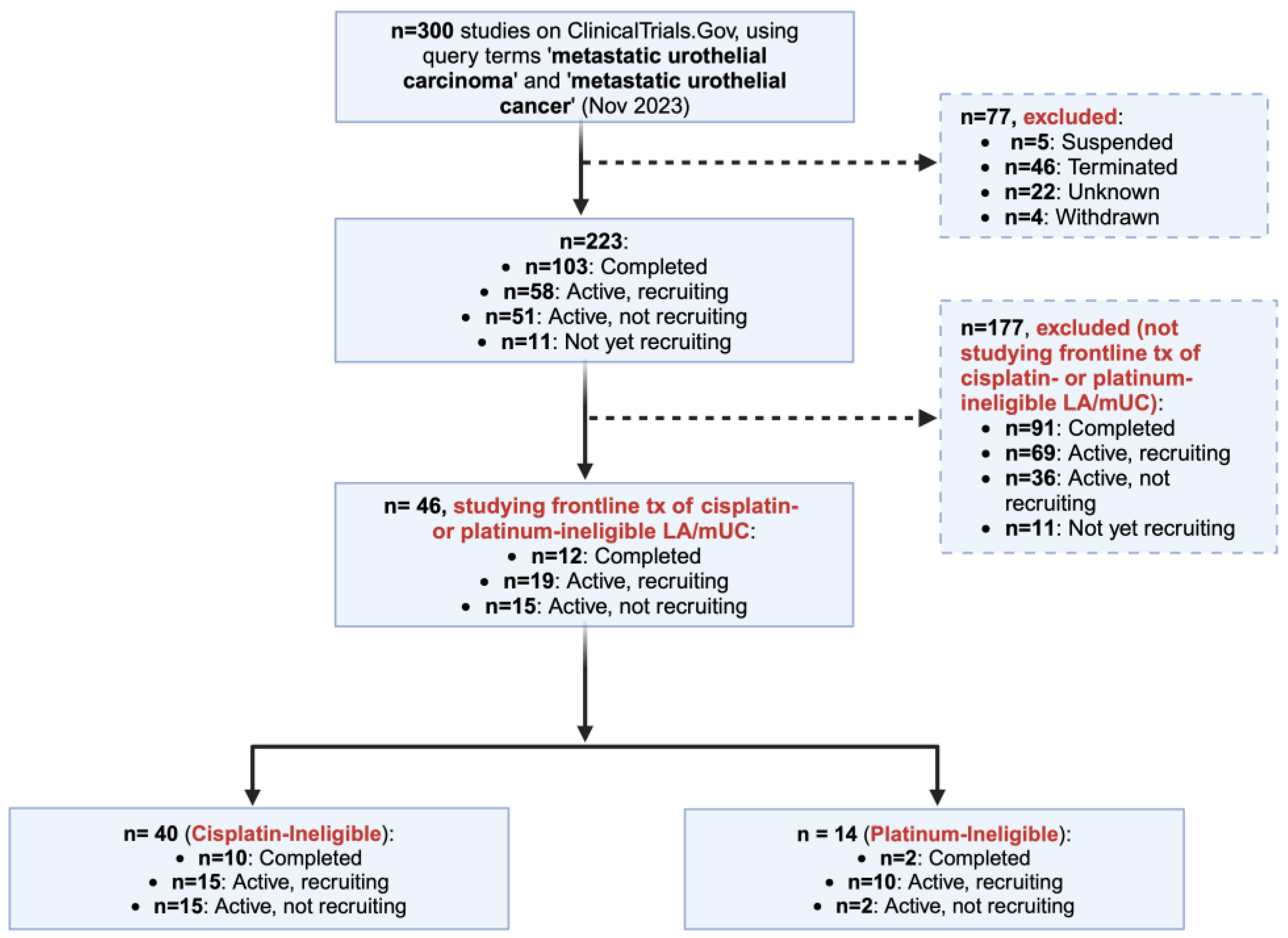
2. Materials and Methods
3. Definitions of Cisplatin Ineligibility and Platinum Ineligibility
3.1. Definition of Cisplatin Ineligibility
3.2. Definition of Platinum Ineligibility
4. Treatment of Cisplatin-Ineligible Metastatic Urothelial Carcinoma
4.1. Previous Standard of Care
4.2. The New Standard of Care
5. Investigational Regimens in Cisplatin-Ineligible Metastatic Urothelial Carcinoma
5.1. Antibody-Drug Conjugates with or without Immune Checkpoint Inhibitors (ICIs)
5.2. FGFR-Targeted Therapies with or without Immune Checkpoint Inhibitors
5.3. Single-Agent or Combined Immune Checkpoint Inhibitors
5.4. Chemotherapy with or without Other Drug Classes
5.4.1. Chemotherapy + Immunotherapy
5.4.2. Chemotherapy + Kinase Inhibitors
5.4.3. Other Non-Platinum Chemotherapy
5.5. Tyrosine Kinase Inhibitors (TKIs) with or without Immune Checkpoint Inhibitors
5.6. Bicycle Toxin Conjugates (BTCs) with Immune Checkpoint Inhibitors
5.7. Other Drugs with Immune Checkpoint Inhibitors
5.8. Radiation Therapy and Immune Checkpoint Inhibitors
6. Treatment of Platinum-Ineligible Metastatic Urothelial Carcinoma
6.1. Previous Standard of Care
6.2. The New Standard of Care
7. Investigational Regimens in Platinum-Ineligible Metastatic Urothelial Carcinoma
7.1. FGFR Inhibitors with Immune Checkpoint Inhibitors
7.2. Targeted Therapies Alone or with Immune Checkpoint Inhibitors
| NCT Number + Title (If Available) | Phase + Enrollment | Study Status | Cohorts/Arms of Interest for Treatment-Naïve Platinum-Ineligible mUC | Biomarker | Primary Endpoints |
|---|---|---|---|---|---|
| NCT02573259 | Phase 1 (147) | Completed | 5 arms with same treatment (PF-06801591) but increasing concentrations and different doses of administration | PD-L1 | Parameters related to adverse events in Part 1, ORR in Part 2 [76] |
| NCT04601857 | Phase 2 (46) | Active, recruiting | Cohort A: Futibatinib and Pembrolizumab, for patients with a FGFR3 mutation or FGFR1-4 fusion/rearrangement. | Cohort A: FGFR3 mutation or FGFR1-4 fusion/rearrangement. | ORR [77] |
| Cohort B: Same treatment, but for all other patients than in Cohort A with UC (including patients with other FGFR or non-FGFR genetic aberrations and patients with wild type [non-mutated] tumors). | Cohort B: other FGFR or non-FGFR genetic aberrations | ||||
| NCT04486781 | Phase 2 (38) | Active, recruiting | Combination therapy for all | Ephrin B2 | ORR [78] |
| NCT05645692 | Phase 2 (240) | Active, recruiting | Arm A (Atezolizumab) Q3W, Arm B (IV RO7247669) Q3W and Arm C (IV RO7247669 and tiragolumab) Q3W | PD-L1 | ORR [79] |
| NCT03854474 | Phase 1|Phase 2 (30) | Active, recruiting | Experimental: Treatment (tazemetostat, pembrolizumab) | EZH2 and H3K27me3 chromatin methylation | RP2D [80] |
| NCT03898180 [LEAP-011] | Phase 3 (487) | Active, not recruiting | Experimental: Pembrolizumab + Levantinib; Active Comparator: Pembrolizumab + Placebo; Experimental: Pembrolizumab monotherapy | N/A | PFS and OS [81] |
| NCT03288545 [EV-103] | Phase 1|Phase 2 (348) | Active, not recruiting | Cohort K: Enfortumab Vedotin + Pembrolizumab | N/A | ORR (Cohort K only) [82] |
7.3. Bispecific Antibodies with Immune Checkpoint Inhibitors
7.4. Recombinant Fusion Proteins with or without Immune Checkpoint Inhibitors
8. Discussion
8.1. What’s Next for Cisplatin and Platinum Ineligibility Criteria?
8.2. The Value of Biomarkers
8.3. Practical Implications for Healthcare Professionals
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Von der Maase, H.; Hansen, S.W.; Roberts, J.T.; Dogliotti, L.; Oliver, T.; Moore, M.J.; Bodrogi, I.; Albers, P.; Knuth, A.; Lippert, C.M.; et al. Gemcitabine and cisplatin versus methotrexate, vinblastine, doxorubicin, and cisplatin in advanced or metastatic bladder cancer: Results of a large, randomized, multinational, multicenter, phase III study. J. Clin. Oncol. 2000, 18, 3068–3077. [Google Scholar] [CrossRef] [PubMed]
- Powles, T.B.; Valderrama, B.P.; Gupta, S.; Bedke, J.; Kikuchi, E.; Hoffman-Censits, J.; Iyer, G.; Vulsteke, C.; Park, S.H.; Shin, S.J.; et al. LBA6 EV-302/KEYNOTE-A39: Open-label, randomized phase III study of enfortumab vedotin in combination with pembrolizumab (EV+P) vs chemotherapy (Chemo) in previously untreated locally advanced metastatic urothelial carcinoma (la/mUC). Ann. Oncol. 2023, 34, S1340. [Google Scholar] [CrossRef]
- FDA Grants Accelerated Approval to Enfortumab Vedotin-Ejfv with Pembrolizumab for Locally Advanced or Metastatic Urothelial Carcinoma|FDA. Available online: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-grants-accelerated-approval-enfortumab-vedotin-ejfv-pembrolizumab-locally-advanced-or-metastatic (accessed on 24 November 2023).
- FDA Approves Enfortumab Vedotin-Ejfv with Pembrolizumab for Locally Advanced or Metastatic Urothelial Cancer|FDA. Available online: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-enfortumab-vedotin-ejfv-pembrolizumab-locally-advanced-or-metastatic-urothelial-cancer (accessed on 19 December 2023).
- Thompson, R.H.; Boorjian, S.A.; Kim, S.P.; Cheville, J.C.; Thapa, P.; Tarrel, R.; Dronca, R.; Costello, B.; Frank, I. Eligibility for neoadjuvant/adjuvant cisplatin-based chemotherapy among radical cystectomy patients. BJU Int. 2014, 113, E17–E21. [Google Scholar] [CrossRef]
- Parikh, R.B.; Feld, E.K.; Galsky, M.D.; Adamson, B.J.; Cohen, A.B.; Baxi, S.S.; Ben Boursi, S.; Christodouleas, J.P.; Vaughn, D.J.; Meropol, N.J.; et al. First-line immune checkpoint inhibitor use in cisplatin-eligible patients with advanced urothelial carcinoma: A secular trend analysis. Futur. Oncol. 2020, 16, 4341–4345. [Google Scholar] [CrossRef] [PubMed]
- Galsky, M.D.; Hahn, N.M.; Rosenberg, J.E.; Sonpavde, G.; Hutson, T.; Oh, W.K.; Dreicer, R.; Vogelzang, N.J.; Sternberg, C.N.; Bajorin, D.F.; et al. Treatment of patients with metastatic urothelial cancer “Unfit” for cisplatin-based chemotherapy. J. Clin. Oncol. 2011, 29, 2432–2438. [Google Scholar] [CrossRef]
- Defining Platinum Ineligibility in Metastatic Urothelial Carcinoma Patients|PracticeUpdate. Available online: https://www.practiceupdate.com/content/defining-platinum-ineligibility-in-metastatic-urothelial-carcinoma-patients/79816 (accessed on 12 November 2023).
- De Santis, M.; Bellmunt, J.; Mead, G.; Kerst, J.M.; Leahy, M.; Maroto, P.; Gil, T.; Marreaud, S.; Daugaard, G.; Skoneczna, I.; et al. Randomized phase II/III trial assessing gemcitabine/carboplatin and methotrexate/carboplatin/vinblastine in patients with advanced urothelial cancer who are unfit for cisplatin-based chemotherapy: EORTC study 30986. J. Clin. Oncol. 2012, 30, 191–199. [Google Scholar] [CrossRef]
- Gupta, S.; Sonpavde, G.; Grivas, P.; Apolo, A.B.; Plimack, E.R.; Flaig, T.W.; Hahn, N.M.; Balar, A.V.; Bajorin, D.F.; Galsky, M.D. Defining “platinum-ineligible” patients with metastatic urothelial cancer (mUC). J. Clin. Oncol. 2019, 37 (Suppl. S7), 451. [Google Scholar] [CrossRef]
- Gupta, S.; Bellmunt, J.; Plimack, E.R.; Sonpavde, G.P.; Grivas, P.; Apolo, A.B.; Pal, S.K.; Siefker-Radtke, A.O.; Flaig, T.W.; Galsky, M.D. Defining “platinum-ineligible” patients with metastatic urothelial cancer (mUC). J. Clin. Oncol. 2022, 40 (Suppl. S16), 4577. [Google Scholar] [CrossRef]
- Ascione, C.M.; Napolitano, F.; Esposito, D.; Servetto, A.; Belli, S.; Santaniello, A.; Scagliarini, S.; Crocetto, F.; Bianco, R.; Formisano, L. Role of FGFR3 in bladder cancer: Treatment landscape and future challenges. Cancer Treat. Rev. 2023, 115, 102530. [Google Scholar] [CrossRef]
- Nadal, R.; Valderrama, B.P.; Bellmunt, J. Progress in systemic therapy for advanced-stage urothelial carcinoma. Nat. Rev. Clin. Oncol. 2023, 21, 8–27. [Google Scholar] [CrossRef]
- Crona, D.J.; Faso, A.; Nishijima, T.F.; McGraw, K.A.; Galsky, M.D.; Milowsky, M.I. A Systematic Review of Strategies to Prevent Cisplatin-Induced Nephrotoxicity. Oncologist 2017, 22, 609–619. [Google Scholar] [CrossRef]
- MMorales-Barrera, R.; Bellmunt, J.; Suárez, C.; Valverde, C.; Guix, M.; Serrano, C.; Gallén, M.; Carles, J. Cisplatin and gemcitabine administered every two weeks in patients with locally advanced or metastatic urothelial carcinoma and impaired renal function. Eur. J. Cancer 2012, 48, 1816–1821. [Google Scholar] [CrossRef] [PubMed]
- FDA Limits the Use of Tecentriq and Keytruda for Some Urothelial Cancer Patients|FDA. Available online: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-limits-use-tecentriq-and-keytruda-some-urothelial-cancer-patients (accessed on 3 November 2023).
- Genentech: Statements|Genentech Provides Update on Tecentriq U.S. Indication for Previously Untreated Metastatic Bladder Cancer. Available online: https://www.gene.com/media/statements/ps_112822 (accessed on 13 November 2023).
- Loehrer, P.J.; Einhorn, L.H.; Elson, P.J.; Crawford, E.D.; Kuebler, P.; Tannock, I.; Raghavan, D.; Stuart-Harris, R.; Sarosdy, M.F.; Lowe, B.A. A randomized comparison of cisplatin alone or in combination with methotrexate, vinblastine, and doxorubicin in patients with metastatic urothelial carcinoma: A cooperative group study. J. Clin. Oncol. 2016, 10, 1066–1073. [Google Scholar] [CrossRef] [PubMed]
- Sternberg, C.N.; de Mulder, P.H.; Schornagel, J.H.; Théodore, C.; Fossa, S.D.; van Oosterom, A.T.; Witjes, F.; Spina, M.; van Groeningen, C.J.; de Balincourt, C.; et al. Randomized phase III trial of high-dose-intensity methotrexate, vinblastine, doxorubicin, and cisplatin (MVAC) chemotherapy and recombinant human granulocyte colony-stimulating factor versus classic MVAC in advanced urothelial tract tumors: European organization for research and treatment of cancer protocol no. 30924. J. Clin. Oncol. 2001, 19, 2638–2646. [Google Scholar]
- Powles, T.; Park, S.H.; Voog, E.; Caserta, C.; Valderrama, B.P.; Gurney, H.; Kalofonos, H.; Radulović, S.; Demey, W.; Ullén, A.; et al. Avelumab Maintenance Therapy for Advanced or Metastatic Urothelial Carcinoma. N. Engl. J. Med. 2020, 383, 1218–1230. [Google Scholar] [CrossRef]
- Grivas, P.; Park, S.H.; Voog, E.; Caserta, C.; Gurney, H.; Bellmunt, J.; Kalofonos, H.; Ullén, A.; Loriot, Y.; Sridhar, S.S.; et al. Avelumab First-line Maintenance Therapy for Advanced Urothelial Carcinoma: Comprehensive Clinical Subgroup Analyses from the JAVELIN Bladder 100 Phase 3 Trial. Eur. Urol. 2023, 84, 95–108. [Google Scholar] [CrossRef]
- Calabrò, F.; Lorusso, V.; Rosati, G.; Manzione, L.; Frassineti, L.; Sava, T.; Di Paula, E.D.; Alonso, S.; Sternberg, C.N. Gemcitabine and paclitaxel every 2 weeks in patients with previously untreated urothelial carcinoma. Cancer 2009, 115, 2652–2659. [Google Scholar] [CrossRef] [PubMed]
- Ardavanis, A.; Tryfonopoulos, D.; Alexopoulos, A.; Kandylis, C.; Lainakis, G.; Rigatos, G. Gemcitabine and docetaxel as first-line treatment for advanced urothelial carcinoma: A phase II study. Br. J. Cancer 2005, 92, 645–650. [Google Scholar] [CrossRef] [PubMed]
- Mantia, C.M.; Sonpavde, G. Enfortumab vedotin-ejfv for the treatment of advanced urothelial carcinoma. Expert Rev. Anticancer Ther. 2022, 22, 449–455. [Google Scholar] [CrossRef]
- Klute, K.; Nackos, E.; Tasaki, S.; Nguyen, D.P.; Bander, N.H.; Tagawa, S.T. OncoTargets and Therapy Dovepress Microtubule inhibitor-based antibody-drug conjugates for cancer therapy. Onco Targets Ther. 2014, 7, 2227–2236. [Google Scholar]
- Rosenberg, J.; Milowsky, M.; Ramamurthy, C.; Mar, N.; McKay, R.; Friedlander, T.; Ferrario, C.; Bracarda, S.; George, S.; Moon, H.; et al. LBA73 Study EV-103 Cohort K: Antitumor activity of enfortumab vedotin (EV) monotherapy or in combination with pembrolizumab (P) in previously untreated cisplatin-ineligible patients (pts) with locally advanced or metastatic urothelial cancer (la/mUC). Ann. Oncol. 2022, 33, S1441. [Google Scholar] [CrossRef]
- O’Donnell, P.H.; Milowsky, M.I.; Petrylak, D.P.; Hoimes, C.J.; Flaig, T.W.; Mar, N.; Moon, H.H.; Friedlander, T.W.; McKay, R.R.; Bilen, M.A.; et al. Enfortumab Vedotin With or Without Pembrolizumab in Cisplatin-Ineligible Patients With Previously Untreated Locally Advanced or Metastatic Urothelial Cancer. J. Clin. Oncol. 2023, 41, 4107–4117. [Google Scholar] [CrossRef] [PubMed]
- Bajorin, D.F.; Dodd, P.M.; Mazumdar, M.; Fazzari, M.; McCaffrey, J.A.; Scher, H.I.; Herr, H.; Higgins, G.; Boyle, M.G. Long-term survival in metastatic transitional-cell carcinoma and prognostic factors predicting outcome of therapy. J. Clin. Oncol. 1999, 17, 3173–3181. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Rosenberg, J.E.; McKay, R.R.; Flaig, T.W.; Petrylak, D.P.; Hoimes, C.J.; Friedlander, T.W.; Bilen, M.A.; Srinivas, S.; Burgess, E.F.; et al. Study EV-103 dose escalation/cohort A: Long-term outcome of enfortumab vedotin + pembrolizumab in first-line (1L) cisplatin-ineligible locally advanced or metastatic urothelial carcinoma (la/mUC) with nearly 4 years of follow-up. J. Clin. Oncol. 2023, 41 (Suppl. S16), 4505. [Google Scholar] [CrossRef]
- FDA Grants Regular Approval to Enfortumab Vedotin-Ejfv for Locally Advanced or Metastatic Urothelial Cancer|FDA. Available online: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-grants-regular-approval-enfortumab-vedotin-ejfv-locally-advanced-or-metastatic-urothelial-cancer (accessed on 13 November 2023).
- FDA Center for Drug Evaluation and Research. Highlights of Prescribing Information. Available online: www.fda.gov/medwatch (accessed on 24 November 2023).
- Thomas, V.M.; Tripathi, N.; Agarwal, N.; Swami, U. Current and emerging role of sacituzumab govitecan in the management of urothelial carcinoma. Expert Rev. Anticancer Ther. 2022, 22, 335–341. [Google Scholar] [CrossRef] [PubMed]
- FDA Grants Accelerated Approval to Sacituzumab Govitecan for Advanced Urothelial Cancer|FDA. Available online: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-grants-accelerated-approval-sacituzumab-govitecan-advanced-urothelial-cancer (accessed on 27 November 2023).
- Jain, R.K.; Yang, Y.; Chadha, J.; Chatwal, M.S.; Kish, J.A.; Raymond, S.; Rembisz, J.; Jameel, G.; Mustasam, A.; Poehlman, T. Phase I/II study of ipilimumab plus nivolumab combined with sacituzumab govitecan in patients with metastatic cisplatin-ineligible urothelial carcinoma. J. Clin. Oncol. 2023, 41 (Suppl. S6), 521. [Google Scholar] [CrossRef]
- Kacew, A.; Sweis, R.F. FGFR3 Alterations in the Era of Immunotherapy for Urothelial Bladder Cancer. Available online: www.frontiersin.org (accessed on 27 November 2023).
- FDA Grants Accelerated Approval to Erdafitinib for Metastatic Urothelial Carcinoma|FDA. Available online: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-grants-accelerated-approval-erdafitinib-metastatic-urothelial-carcinoma (accessed on 27 November 2023).
- Benjamin, D.J.; Mar, N.; Rezazadeh Kalebasty, A. Immunotherapy with Checkpoint Inhibitors in FGFR-Altered Urothelial Carcinoma. Clin. Med. Insights Oncol. 2022, 16, 11795549221126252. [Google Scholar] [CrossRef]
- Loriot, Y.; Matsubara, N.; Park, S.H.; Huddart, R.A.; Burgess, E.F.; Houede, N.; Banek, S.; Laguerre, B.; Guadalupi, V.; Ku, J.H.; et al. Phase 3 THOR study: Results of erdafitinib (erda) versus chemotherapy (chemo) in patients (pts) with advanced or metastatic urothelial cancer (mUC) with select fibroblast growth factor receptor alterations (FGFRalt). J. Clin. Oncol. 2023, 41 (Suppl. S17), LBA4619. [Google Scholar] [CrossRef]
- Siefker-Radtke, A.O.; Powles, T.; Moreno, V.; Kang, T.W.; Cicin, I.; Girvin, A.; Akapame, S.; Triantos, S.; O’Hagan, A.; Zhu, W.; et al. Erdafitinib (ERDA) vs ERDA plus cetrelimab (ERDA+CET) for patients (pts) with metastatic urothelial carcinoma (mUC) and fibroblast growth factor receptor alterations (FGFRa): Final results from the phase 2 Norse study. J. Clin. Oncol. 2023, 41 (Suppl. S16), 4504. [Google Scholar] [CrossRef]
- Grünewald, S.; Politz, O.; Bender, S.; Héroult, M.; Lustig, K.; Thuss, U.; Kneip, C.; Kopitz, C.; Zopf, D.; Collin, M.; et al. Rogaratinib: A potent and selective pan-FGFR inhibitor with broad antitumor activity in FGFR-overexpressing preclinical cancer models. Int. J. Cancer 2019, 145, 1346–1357. [Google Scholar] [CrossRef]
- Rosenberg, J.E.; Gajate, P.; Morales-Barrera, R.; Lee, J.-L.; Necchi, A.; Penel, N.; Zagonel, V.; Sierecki, M.R.; Bao, W.; Zhou, Y.; et al. Safety and efficacy of rogaratinib in combination with atezolizumab in cisplatin-ineligible patients (pts) with locally advanced or metastatic urothelial cancer (UC) and FGFR mRNA overexpression in the phase Ib/II FORT-2 study. J. Clin. Oncol. 2021, 39 (Suppl. S15), 4521. [Google Scholar] [CrossRef]
- Sternberg, C.N.; Petrylak, D.P.; Bellmunt, J.; Nishiyama, H.; Necchi, A.; Gurney, H.; Lee, J.L.; van der Heijden, M.S.; Rosenbaum, E.; Penel, N.; et al. FORT-1: Phase II/III Study of Rogaratinib Versus Chemotherapy in Patients with Locally Advanced or Metastatic Urothelial Carcinoma Selected Based on FGFR1/3 mRNA Expression. J. Clin. Oncol. 2023, 41, 629–639. [Google Scholar] [CrossRef]
- Necchi, A.; Todenhöfer, T.; Deville, J.-L.; Häckl, M.; Marszewska, M.; McKernan, P.; Saulay, M.; Engelhardt, M.; De Santis, M. Efficacy and safety of derazantinib (DZB) in patients with metastatic urothelial carcinoma (mUC) with activating FGFR1/2/3 genetic aberrations (GA): Results from the phase 1b/2 FIDES-02 study. J. Clin. Oncol. 2023, 41 (Suppl. S6), 501. [Google Scholar] [CrossRef]
- Galsky, M.D.; Powles, T.; Dreicer, R.; Kitamura, H.; Asatiani, E.; Howe, J.; Zhen, H.; Oliveira, N.; Necchi, A. FIGHT-205: Phase II study of first-line pemigatinib (PEMI) plus pembrolizumab (PEMBRO) versus PEMI alone versus standard of care (SOC) for cisplatin (CIS)—Ineligible urothelial carcinoma (UC) with FGFR3 mutation or rearrangement. J. Clin. Oncol. 2020, 38 (Suppl. S6), TPS592. [Google Scholar] [CrossRef]
- Iacovelli, R.; Ciccarese, C.; Brunelli, M.; Battelli, N.; Buttigliero, C.; Caserta, C.; Buti, S.; Santini, D.; Naglieri, E.; Galli, L.; et al. First line avelumab in PD-L1+ve metastatic or locally advanced urothelial cancer (aUC) patients unfit for cisplatin (cis): The ARIES trial. J. Clin. Oncol. 2022, 40 (Suppl. S6), 439. [Google Scholar] [CrossRef]
- Powles, T.; Castellano, D.; Loriot, Y.; Ogawa, O.; Park, S.H.; De Giorgi, U.; Bögemann, M.; Bamias, A.; Gurney, H.; Fradet, Y.; et al. Durvalumab alone and durvalumab plus tremelimumab versus chemotherapy in previously untreated patients with unresectable, locally advanced or metastatic urothelial carcinoma (DANUBE): A randomised, open-label, multicentre, phase 3 trial. Lancet Oncol. 2020, 21, 1574–1588. [Google Scholar] [CrossRef]
- Bristol Myers Squibb–Bristol Myers Squibb Provides Update on CheckMate-901 Trial Evaluating Opdivo (Nivolumab) Plus Yervoy (Ipilimumab) as First-Line Treatment for Patients with Unresectable or Metastatic Urothelial Carcinoma. Available online: https://news.bms.com/news/details/2022/Bristol-Myers-Squibb-Provides-Update-on-CheckMate--901-Trial-Evaluating-Opdivo-nivolumab-Plus-Yervoy-ipilimumab-as-First-Line-Treatment-for-Patients-with-Unresectable-or-Metastatic-Urothelial-Carcinoma/default.aspx (accessed on 3 November 2023).
- van der Heijden, M.S.; Sonpavde, G.; Powles, T.; Necchi, A.; Burotto, M.; Schenker, M.; Sade, J.P.; Bamias, A.; Beuzeboc, P.; Bedke, J.; et al. Nivolumab plus Gemcitabine–Cisplatin in Advanced Urothelial Carcinoma. N. Engl. J. Med. 2023, 389, 1778–1789. [Google Scholar] [CrossRef]
- Balar, A.V.; Castellano, D.; O’Donnell, P.H.; Grivas, P.; Vuky, J.; Powles, T.; Plimack, E.R.; Hahn, N.M.; de Wit, R.; Pang, L.; et al. First-line pembrolizumab in cisplatin-ineligible patients with locally advanced and unresectable or metastatic urothelial cancer (KEYNOTE-052): A multicentre, single-arm, phase 2 study. Lancet Oncol. 2017, 18, 1483–1492. [Google Scholar] [CrossRef]
- Vuky, J.; Balar, A.V.; Castellano, D.; O’donnell, P.H.; Grivas, P.; Bellmunt, J.; Powles, T.; Bajorin, D.; Hahn, N.M.; Savage, M.J.; et al. Long-term outcomes in KEYNOTE-052: Phase II study investigating first-line pembrolizumab in cisplatin-ineligible patients with locally advanced or metastatic urothelial cancer. J. Clin. Oncol. 2020, 38, 2658–2666. [Google Scholar] [CrossRef] [PubMed]
- Powles, T.; Matsubara, N.; Cheng, S.Y.-S.; Fradet, Y.; Oudard, S.; Vulsteke, C.; Barrera, R.M.; Gunduz, S.; Loriot, Y.; Rodriguez-Vida, A.; et al. Pembrolizumab alone or combined with chemotherapy versus chemotherapy as first-line therapy for advanced urothelial carcinoma (KEYNOTE-361): A randomised, open-label, phase 3 trial. Lancet Oncol. 2021, 22, 931–945. [Google Scholar] [CrossRef]
- Balar, A.V.; Galsky, M.D.; Rosenberg, J.E.; Powles, T.; Petrylak, D.P.; Bellmunt, J.; Loriot, Y.; Necchi, A.; Hoffman-Censits, J.; Perez-Gracia, J.L.; et al. Atezolizumab as first-line treatment in cisplatin-ineligible patients with locally advanced and metastatic urothelial carcinoma: A single-arm, multicentre, phase 2 trial. Lancet 2017, 389, 67–76. [Google Scholar] [CrossRef]
- Galsky, M.D.; Arija, J.Á.A.; Bamias, A.; Davis, I.D.; De Santis, M.; Kikuchi, E.; Garcia-Del-Muro, X.; De Giorgi, U.; Mencinger, M.; Izumi, K.; et al. Atezolizumab with or without chemotherapy in metastatic urothelial cancer (IMvigor130): A multicentre, randomised, placebo-controlled phase 3 trial. Lancet 2020, 395, 1547–1557. [Google Scholar] [CrossRef]
- Galsky, M.D.; Necchi, A.; Sridhar, S.S.; Ogawa, O.; Angra, N.; Hois, S.; Xiao, F.; Goluboff, E.; Bellmunt, J. A phase III, randomized, open-label, multicenter, global study of first-line durvalumab plus standard of care (SoC) chemotherapy and durvalumab plus tremelimumab, and SoC chemotherapy versus SoC chemotherapy alone in unresectable locally advanced or metastatic urothelial cancer (NILE). J. Clin. Oncol. 2021, 39 (Suppl. S6), TPS504. [Google Scholar] [CrossRef]
- ESMO Virtual Congress 2020|OncologyPRO. Available online: https://oncologypro.esmo.org/meeting-resources/esmo-virtual-congress-2020/phase-ii-multicenter-randomized-study-to-evaluate-efficacy-and-safety-of-avelumab-with-gemcitabine-carboplatin-cg-vs-cg-alone-in-patients-with-u (accessed on 27 November 2023).
- Ferrarotto, R.; Anderson, I.; Medgyasszay, B.; García-Campelo, M.R.; Edenfield, W.; Feinstein, T.M.; Johnson, J.M.; Kalmadi, S.; Lammers, P.E.; Sanchez-Hernandez, A.; et al. Trilaciclib prior to chemotherapy reduces the usage of supportive care interventions for chemotherapy-induced myelosuppression in patients with small cell lung cancer: Pooled analysis of three randomized phase 2 trials. Cancer Med. 2021, 10, 5748–5756. [Google Scholar] [CrossRef] [PubMed]
- Balar, A.V.; Apolo, A.B.; Ostrovnaya, I.; Mironov, S.; Iasonos, A.; Trout, A.; Regazzi, A.M.; Garcia-Grossman, I.R.; Gallagher, D.J.; Milowsky, M.I.; et al. Phase II study of gemcitabine, carboplatin, and bevacizumab in patients with advanced unresectable or metastatic urothelial cancer. J. Clin. Oncol. 2013, 31, 724–730. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, J.E.; Ballman, K.A.; Halabi, S.; Atherton, P.J.; Mortazavi, A.; Sweeney, C.; Stadler, W.M.; Teply, B.A.; Picus, J.; Tagawa, S.T.; et al. Randomized Phase III Trial of Gemcitabine and Cisplatin With Bevacizumab or Placebo in Patients With Advanced Urothelial Carcinoma: Results of CALGB 90601 (Alliance). J. Clin. Oncol. 2021, 39, 2486–2496. [Google Scholar] [CrossRef]
- Gerullis, H.; Wawroschek, F.; Köhne, C.H.; Ecke, T.H. Vinflunine in the treatment of advanced urothelial cancer: Clinical evidence and experience. Ther. Adv. Urol. 2016, 9, 28–35. [Google Scholar] [CrossRef]
- Holmsten, K.; Jensen, N.V.; Mouritsen, L.S.; Jonsson, E.; Mellnert, C.; Agerbæk, M.; Nilsson, C.; Moe, M.; Carus, A.; Öfverholm, E.; et al. Vinflunine/gemcitabine versus carboplatin/gemcitabine as first-line treatment in cisplatin-ineligible patients with advanced urothelial carcinoma: A randomised phase II trial (VINGEM). Eur. J. Cancer 2020, 127, 173–182. [Google Scholar] [CrossRef]
- Garje, R.; Packiam, V.T.; Koski, A.; Milhem, M.M.; O’Donnell, M.A.; Zakharia, Y. Phase Ib study of avelumab and taxane based chemotherapy in platinum-refractory or ineligible metastatic urothelial cancer (AVETAX study). J. Clin. Oncol. 2022, 40 (Suppl. S6), 503. [Google Scholar] [CrossRef]
- Kwilas, A.R.; Ardiani, A.; Donahue, R.N.; Aftab, D.; Hodge, J.W. Effects of Cabozantinib, a small molecule tyrosine kinase inhibitor, on the immune permissiveness of the tumor microenvironment and immune-mediated killing of tumor cells. J. Immunother. Cancer 2014, 2, P185. [Google Scholar] [CrossRef]
- Pal, S.K.; Agarwal, N.; Singh, P.; Necchi, A.; McGregor, B.A.; Hauke, R.J.; Powles, T.; Suárez, C.; Van Herpen, C.M.; Vaishampayan, U.N.; et al. Cabozantinib (C) in combination with atezolizumab (A) in urothelial carcinoma (UC): Results from Cohorts 3, 4, 5 of the COSMIC-021 study. J. Clin. Oncol. 2022, 40 (Suppl. S16), 4504. [Google Scholar] [CrossRef]
- Loriot, Y.; Grivas, P.; De Wit, R.; Balar, A.V.; Siefker-Radtke, A.O.; Zolnierek, J.; Csoszi, T.; Shin, S.J.; Park, S.H.; Atduev, V.; et al. First-line pembrolizumab (pembro) with or without lenvatinib (lenva) in patients with advanced urothelial carcinoma (LEAP-011): A phase 3, randomized, double-blind study. J. Clin. Oncol. 2022, 40 (Suppl. S6), 432. [Google Scholar] [CrossRef]
- Rhodes, C.A.; Pei, D. Bicyclic Peptides as Next-Generation Therapeutics. Chem. A Eur. J. 2017, 23, 12690–12703. [Google Scholar] [CrossRef] [PubMed]
- Baldini, C.; Goldschmidt, V.; Brana, I.; Doger, B.; Italiano, A.; Cousin, S.; Falchook, G.S.; Necchi, A.; Reig, O.; Carter, L.; et al. BT8009-100: A phase I/II study of novel bicyclic peptide and MMAE conjugate BT8009 in patients (pts) with advanced malignancies associated with nectin-4 expression, including urothelial cancer (UC). J. Clin. Oncol. 2023, 41 (Suppl. S6), 498. [Google Scholar] [CrossRef]
- Rigby, M.; Bennett, G.; Chen, L.; Mudd, G.E.; Harrison, H.; Beswick, P.J.; Van Rietschoten, K.; Watcham, S.M.; Scott, H.S.; Brown, A.N.; et al. BT8009; A Nectin-4 Targeting Bicycle Toxin Conjugate for Treatment of Solid Tumors. Mol. Cancer Ther. 2022, 21, 1747–1756. [Google Scholar] [CrossRef] [PubMed]
- Bicycle Therapeutics Announces Interim BT8009 Phase I Clinical Trial Results at the 2022 AACR Annual Meeting|Bicycle Therapeutics. Available online: https://investors.bicycletherapeutics.com/news-releases/news-release-details/bicycle-therapeutics-announces-interim-bt8009-phase-i-clinical-0 (accessed on 27 November 2023).
- Sadeghi, S.; Quinn, D.; Dorff, T.; Pal, S.; Groshen, S.; Tsao-Wei, D.; Parikh, R.; Devitt, M.; Parikh, M.; Jackovich, A.; et al. EphrinB2 Inhibition and Pembrolizumab in Metastatic Urothelial Carcinoma. J. Clin. Oncol. 2023, 41, 640–650. [Google Scholar] [CrossRef] [PubMed]
- Perera, J.; Hoskin, P. The role of radiotherapy in metastatic bladder cancer. J. Cancer Metastasis Treat. 2022, 8, 4. [Google Scholar] [CrossRef]
- Ukleja, J.; Kusaka, E.; Miyamoto, D.T. Immunotherapy Combined With Radiation Therapy for Genitourinary Malignancies. Front. Oncol. 2021, 11, 663852. [Google Scholar] [CrossRef] [PubMed]
- Kundu, P.; Lee, A.; Drakaki, A.; Liu, S.; Shen, J.; Chin, A.I.; Chamie, K.; Chang, A. Safety lead-in of phase II SBRT and durvalumab with or without tremelimumab for unresectable and cisplatin-ineligible, locally advanced or metastatic bladder cancer. J. Clin. Oncol. 2022, 40 (Suppl. S6), 517. [Google Scholar] [CrossRef]
- Koshkin, V.S.; Sonpavde, G.P.; Hwang, C.; Mellado, B.; Tomlinson, G.; Shimura, M.; Chisamore, M.J.; Gil, M.; Loriot, Y. Futibatinib plus pembrolizumab in patients (pts) with advanced or metastatic urothelial carcinoma (mUC): Preliminary safety results from a phase 2 study. J. Clin. Oncol. 2022, 40 (Suppl. S6), 501. [Google Scholar] [CrossRef]
- Taylor, M.H.; Schmidt, E.V.; Dutcus, C.; Pinheiro, E.M.; Funahashi, Y.; Lubiniecki, G.; Rasco, D. The LEAP program: Lenvatinib plus pembrolizumab for the treatment of advanced solid tumors. Future Oncol. 2021, 17, 637–647. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, J.E.; Park, S.H.; Dao, T.V.; Castellano, D.E.; Li, J.-R.; Mukherjee, S.; Howells, K.; Dry, H.; Lanasa, M.C.; Stewart, R.; et al. BAYOU: A phase II, randomized, multicenter, double-blind, study of durvalumab (D) in combination with olaparib (O) for the first-line treatment of platinum-ineligible patients with unresectable, stage IV urothelial carcinoma (UC). J. Clin. Oncol. 2022, 40 (Suppl. S6), 437. [Google Scholar] [CrossRef]
- A Dose Escalation Study of PF-06801591 in Melanoma, Head And Neck Cancer (SCCHN), Ovarian, Sarcoma, Non-Small Cell Lung Cancer, Urothelial Carcinoma or Other Solid Tumors–Full Text View–ClinicalTrials.gov. Available online: https://classic.clinicaltrials.gov/ct2/show/NCT02573259 (accessed on 3 November 2023).
- Futibatinib and Pembrolizumab Combination in the Treatment of Advanced or Metastatic Urothelial Carcinoma–Full Text View –ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/show/NCT04601857 (accessed on 12 June 2023).
- A Study of Pembrolizumab+ sEphB4 in Metastatic Urothelial Carcinoma–Full Text View–ClinicalTrials.gov. Available online: https://www.clinicaltrials.gov/ct2/show/NCT04486781 (accessed on 12 June 2023).
- A Study of RO7247669 Alone or in Combination with Tiragolumab vs. Atezolizumab in Participants with Untreated Locally Advanced or Metastatic Urothelial Cancer–Full Text View–ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/show/NCT05645692 (accessed on 12 June 2023).
- Testing the Addition of Tazemetostat to the Immunotherapy Drug, Pembrolizumab (MK-3475), in Advanced Urothelial Carcinoma–Full Text View—ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/show/NCT03854474 (accessed on 12 June 2023).
- Study of First-line Pembrolizumab (MK-3475) with Lenvatinib (MK-7902/E7080) in Urothelial Carcinoma Cisplatin-ineligible Participants Whose Tumors Express Programmed Cell Death-Ligand 1 and in Participants Ineligible for Platinum-containing Chemotherapy (MK-7902-011/E7080-G000-317/LEAP-011)–Full Text View—ClinicalTrials.gov. Available online: https://classic.clinicaltrials.gov/ct2/show/NCT03898180 (accessed on 12 June 2023).
- A Study of Enfortumab Vedotin Alone or with Other Therapies for Treatment of Urothelial Cancer–Full Text View—ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/show/NCT03288545 (accessed on 12 June 2023).
- Duan, R.; Du, W.; Guo, W. EZH2: A novel target for cancer treatment. J. Hematol. Oncol. 2020, 13, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Esfandiari, A.; Cassidy, S.; Webster, R.M. Bispecific antibodies in oncology. Nat. Rev. Drug Discov. 2022, 21, 411–412. [Google Scholar] [CrossRef]
- NCI Thesaurus. Available online: https://ncit.nci.nih.gov/ncitbrowser/ConceptReport.jsp?dictionary=NCI%20Thesaurus&code=C173539 (accessed on 27 November 2023).
- Definition of Anti-LAG-3/Anti-PD-L1 Bispecific Antibody IBI323–NCI Drug Dictionary–NCI. Available online: https://www.cancer.gov/publications/dictionaries/cancer-drug/def/anti-lag-3-anti-pd-l1-bispecific-antibody-ibi323# (accessed on 27 November 2023).
- Tao, H.y.; Wang, R.q.; Sheng, W.j.; Zhen, Y.s. The development of human serum albumin-based drugs and relevant fusion proteins for cancer therapy. Int. J. Biol. Macromol. 2021, 187, 24–34. [Google Scholar] [CrossRef] [PubMed]
- Kertesz, N.; Krasnoperov, V.; Reddy, R.; Leshanski, L.; Kumar, S.R.; Zozulya, S.; Gill, P.S. The soluble extracellular domain of EphB4 (sEphB4) antagonizes EphB4-EphrinB2 interaction, modulates angiogenesis, and inhibits tumor growth. Blood 2006, 107, 2330–2338. [Google Scholar] [CrossRef]
- Siefker-Radtke, A.O.; Cauley, D.; Alhalabi, O. Combinations, Sequencing, and the Contribution of Components: New Frontline Standards for Metastatic Urothelial Carcinoma. J. Clin. Oncol. 2023, 41, 4084–4086. [Google Scholar] [CrossRef]
- Challita-Eid, P.M.; Satpayev, D.; Yang, P.; An, Z.; Morrison, K.; Shostak, Y.; Raitano, A.; Nadell, R.; Liu, W.; Lortie, D.R.; et al. Enfortumab vedotin antibody-drug conjugate targeting nectin-4 is a highly potent therapeutic agent in multiple preclinical cancer models. Cancer Res. 2016, 76, 3003–3013. [Google Scholar] [CrossRef]
- Faltas, B.; Goldenberg, D.M.; Ocean, A.J.; Govindan, S.V.; Wilhelm, F.; Sharkey, R.M.; Hajdenberg, J.; Hodes, G.; Nanus, D.M.; Tagawa, S.T. Sacituzumab Govitecan, a Novel Antibody–Drug Conjugate, in Patients with Metastatic Platinum-Resistant Urothelial Carcinoma. Clin. Genitourin. Cancer 2016, 14, e75–e79. [Google Scholar] [CrossRef]
- Tomlinson, D.C.; Baldo, O.; Hamden, P.; Knowles, M.A. FGFR3 protein expression and its relationship to mutation status and prognostic variables in bladder cancer. J. Pathol. 2007, 213, 91–98. [Google Scholar] [CrossRef]
- Li, X.; Choi, W.W.; Yan, R.; Yu, H.; Krasnoperov, V.; Kumar, S.R.; Schuckman, A.; Klumpp, D.J.; Pan, C.-X.; Quinn, D.; et al. The Differential Expression of EphB2 and EphB4 Receptor Kinases in Normal Bladder and in Transitional Cell Carcinoma of the Bladder. PLoS ONE 2014, 9, e105326. [Google Scholar] [CrossRef]
- Chen, J.; Tang, Y.; Liu, H.; Sun, G.; Liu, H.; Zhao, J.; Wang, Z.; Zhang, Y.; Lou, F.; Cao, S.; et al. The mutational pattern of homologous recombination repair genes in urothelial carcinoma and its correlation with immunotherapeutic response. Cancer Med. 2023, 12, 22370. [Google Scholar] [CrossRef]
- Scherrer, E.; Kang, A.; Bloudek, L.M.; Koshkin, V.S. HER2 expression in urothelial carcinoma, a systematic literature review. Front Oncol. 2022, 12, 1011885. [Google Scholar] [CrossRef]
- Alhalabi, O.; Chen, J.; Zhang, Y.; Lu, Y.; Wang, Q.; Ramachandran, S.; Tidwell, R.S.; Han, G.; Yan, X.; Meng, J.; et al. MTAP deficiency creates an exploitable target for antifolate therapy in 9p21-loss cancers. Nat. Commun. 2022, 13, 1797. [Google Scholar] [CrossRef]
- Butler, K.; Banday, A.R. APOBEC3-mediated mutagenesis in cancer: Causes, clinical significance and therapeutic potential. J. Hematol. Oncol. 2023, 16, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Chandran, E.; Iannantuono, G.M.; Akbulut, D.; Atiq, S.O.; Gurram, S.; Teo, M.Y.; Coleman, J.; Sinaii, N.; Apolo, A.B. Mismatch repair deficiency and microsatellite instability-high in urothelial carcinoma: A systematic review and meta-analysis. J. Clin. Oncol. 2023, 41 (Suppl. S16), 4570. [Google Scholar] [CrossRef]
- Voutsadakis, I.A. Urothelial Bladder Carcinomas with High Tumor Mutation Burden Have a Better Prognosis and Targetable Molecular Defects beyond Immunotherapies. Curr. Oncol. 2022, 29, 1390–1407. [Google Scholar] [CrossRef] [PubMed]
- Vaghjiani, R.G.; Skitzki, J.J. Tertiary Lymphoid Structures as Mediators of Immunotherapy Response. Cancers 2022, 14, 3748. [Google Scholar] [CrossRef] [PubMed]
- Meeks, J.J.; Black, P.C.; Galsky, M.; Grivas, P.; Hahn, N.M.; Hussain, S.A.; Milowsky, M.I.; Steinberg, G.D.; Svatek, R.S.; Rosenberg, J.E. Checkpoint Inhibitors in Urothelial Carcinoma—Future Directions and Biomarker Selection. Eur. Urol. 2023, 84, 473–483. [Google Scholar] [CrossRef] [PubMed]
- Andreev-Drakhlin, A.; Shah, A.Y.; Adriazola, A.C.; Shaw, L.; Lopez, L.; James, M.; Matin, S.F.; Alhalabi, O.; Gao, J.; Siefker-Radtke, A.O.; et al. Efficacy of immune checkpoint blockade in patients with advanced upper tract urothelial cancer and mismatch repair deficiency or microsatellite instability (MSI). J. Clin. Oncol. 2021, 39 (Suppl. S6), 487. [Google Scholar] [CrossRef]
- Sharma, P.; Retz, M.; Siefker-Radtke, A.; Baron, A.; Necchi, A.; Bedke, J.; Plimack, E.R.; Vaena, D.; Grimm, M.-O.; Bracarda, S.; et al. Nivolumab in metastatic urothelial carcinoma after platinum therapy (CheckMate 275): A multicentre, single-arm, phase 2 trial. Lancet Oncol. 2017, 18, 312–322. [Google Scholar] [CrossRef]
- Gao, J.; Navai, N.; Alhalabi, O.; Siefker-Radtke, A.; Campbell, M.T.; Tidwell, R.S.; Guo, C.C.; Kamat, A.M.; Matin, S.F.; Araujo, J.C.; et al. Neoadjuvant PD-L1 plus CTLA-4 blockade in patients with cisplatin-ineligible operable high-risk urothelial carcinoma. Nat. Med. 2020, 26, 1845–1851. [Google Scholar] [CrossRef]
- Litchfield, K.; Reading, J.L.; Puttick, C.; Thakkar, K.; Abbosh, C.; Bentham, R.; Watkins, T.B.K.; Rosenthal, R.; Biswas, D.; Rowan, A.; et al. Meta-analysis of tumor- and T cell-intrinsic mechanisms of sensitization to checkpoint inhibition. Cell 2021, 184, 596–614.e14. [Google Scholar] [CrossRef]
- Alhalabi, O.; Zhu, Y.; Hamza, A.; Qiao, W.; Lin, Y.; Wang, R.M.; Shah, A.Y.; Campbell, M.T.; Holla, V.; Kamat, A.; et al. Integrative Clinical and Genomic Characterization of MTAP-deficient Metastatic Urothelial Cancer. Eur. Urol. Oncol. 2023, 6, 228–232. [Google Scholar] [CrossRef]
- Gjuka, D.; Adib, E.; Garrison, K.; Chen, J.; Zhang, Y.; Li, W.; Boutz, D.; Lamb, C.; Tanno, Y.; Nassar, A.; et al. Enzyme-mediated depletion of methylthioadenosine restores T cell function in MTAP-deficient tumors and reverses immunotherapy resistance. Cancer Cell 2023, 41, 1774–1787.e9. [Google Scholar] [CrossRef]
- Liu, W.; Newhall, K.P.; Khani, F.; Barlow, L.; Nguyen, D.; Gu, L.; Eng, K.; Bhinder, B.; Uppal, M.; Récapet, C.; et al. The Cytidine Deaminase APOBEC3G Contributes to Cancer Mutagenesis and Clonal Evolution in Bladder Cancer. Cancer Res. 2023, 83, 506–520. [Google Scholar] [CrossRef] [PubMed]
- Faltas, B.M.; Prandi, D.; Tagawa, S.T.; Molina, A.M.; Nanus, D.M.; Sternberg, C.; Rosenberg, J.; Mosquera, J.M.; Robinson, B.; Elemento, O.; et al. Clonal evolution of chemotherapy-resistant urothelial carcinoma. Nat. Genet. 2016, 48, 1490–1499. [Google Scholar] [CrossRef] [PubMed]
- Mitra, A.P.; Kokorovic, A.; Miest, T.; Narayan, V.M.; Sundi, D.; Lim, A.; Mokkapati, S.; Dinney, C.P. Characterization of FOXF1 as a novel regulator of nodal metastasis in bladder cancer. J. Clin. Oncol. 2021, 39 (Suppl. S6), 480. [Google Scholar] [CrossRef]
- Conde, M.; Frew, I.J. Therapeutic significance of ARID1A mutation in bladder cancer. Neoplasia 2022, 31, 100814. [Google Scholar] [CrossRef] [PubMed]
- Qiu, H.; Makarov, V.; Bolzenius, J.K.; Halstead, A.; Parker, Y.; Wang, A.; Iyer, G.V.; Wise, H.; Kim, D.; Thayaparan, V.; et al. KDM6A Loss Triggers an Epigenetic Switch That Disrupts Urothelial Differentiation and Drives Cell Proliferation in Bladder Cancer. Cancer Res. 2023, 83, 814–829. [Google Scholar] [CrossRef] [PubMed]
- Ler, L.D.; Ghosh, S.; Chai, X.; Thike, A.A.; Heng, H.L.; Siew, E.Y.; Dey, S.; Koh, L.K.; Lim, J.Q.; Lim, W.K.; et al. Loss of tumor suppressor KDM6A amplifies PRC2-regulated transcriptional repression in bladder cancer and can be targeted through inhibition of EZH2. Sci. Transl. Med. 2017, 9, aai8312. [Google Scholar] [CrossRef]
- Society of Urologic Oncology—Arid1a Deficiency Primes an Invasive Transformation in Metastatic Bladder Cancer. Available online: https://suo-abstracts.secure-platform.com/a/gallery/rounds/18/details/2845 (accessed on 10 February 2024).
- Jindal, T.; Zhang, L.; Jiang, C.; Kilari, D.; Alhalabi, O.; Nizam, A.; Basu, A.; Bilen, M.A.; Zakharia, Y.; Milowsky, M.I.; et al. Independent biomarkers predictive of outcomes with enfortumab vedotin (EV) in patients (pts) with advanced urothelial carcinoma (aUC): Analysis of the UNITE study. J. Clin. Oncol. 2023, 41 (Suppl. S16), 4573. [Google Scholar] [CrossRef]
- Hoffman-Censits, J.H.; Lombardo, K.A.; Parimi, V.; Kamanda, S.; Choi, W.; Hahn, N.M.; McConkey, D.J.; McGuire, B.M.; Bivalacqua, T.J.; Kates, M.; et al. Expression of Nectin-4 in Bladder Urothelial Carcinoma, in Morphologic Variants, and Nonurothelial Histotypes. Appl. Immunohistochem. Mol. Morphol. 2021, 29, 619–625. [Google Scholar] [CrossRef]
- Society of Urologic Oncology—Mechanisms and Strategies to Overcome Resistance to Enfortumab Vedotin in Bladder Cancer. Available online: https://suo-abstracts.secure-platform.com/a/gallery/rounds/18/details/2870 (accessed on 4 January 2024).
- Nizam, A.; Zhang, L.; Jindal, T.; Nguyen, C.; Alhalabi, O.; Basu, A.; Evans, S.; Hoimes, C.; Zakharia, Y.; Milowsky, M.; et al. 2394P Biomarkers of treatment (Tx)-related toxicity in advanced urothelial carcinoma (aUC) pts treated with enfortumab vedotin (EV): Analysis of UNITE study. Ann. Oncol. 2023, 34, S1219. [Google Scholar] [CrossRef]
- Study Details|A Study of Enfortumab Vedotin Alone or with Other Therapies for Treatment of Urothelial Cancer|ClinicalTrials.gov. Available online: https://www.clinicaltrials.gov/study/NCT03288545#contacts-and-locations (accessed on 20 December 2023).
- Klümper, N.; Ralser, D.J.; Ellinger, J.; Roghmann, F.; Albrecht, J.; Below, E.; Alajati, A.; Sikic, D.; Breyer, J.; Bolenz, C.; et al. Membranous NECTIN-4 Expression Frequently Decreases during Metastatic Spread of Urothelial Carcinoma and Is Associated with Enfortumab Vedotin Resistance. Clin. Cancer Res. 2023, 29, 1496–1505. [Google Scholar] [CrossRef] [PubMed]
- Chang, E.; Weinstock, C.; Zhang, L.; Charlab, R.; Dorff, S.E.; Gong, Y.; Hsu, V.; Li, F.; Ricks, T.K.; Song, P.; et al. FDA Approval Summary: Enfortumab Vedotin for Locally Advanced or Metastatic Urothelial Carcinoma. Clin. Cancer Res. 2021, 27, 922–927. [Google Scholar] [CrossRef] [PubMed]
- Aggen, D.H.; Chu, C.E.; Rosenberg, J.E. Scratching the Surface: NECTIN-4 as a Surrogate for Enfortumab Vedotin Resistance. Clin. Cancer Res. 2023, 29, 1377–1380. [Google Scholar] [CrossRef]
- Tufano, A.; Cordua, N.; Nardone, V.; Ranavolo, R.; Flammia, R.S.; D’antonio, F.; Borea, F.; Anceschi, U.; Leonardo, C.; Morrione, A.; et al. Prognostic Significance of Organ-Specific Metastases in Patients with Metastatic Upper Tract Urothelial Carcinoma. J. Clin. Med. 2022, 11, 5310. [Google Scholar] [CrossRef] [PubMed]
- Bajorin, D.F.; Witjes, J.A.; Gschwend, J.E.; Schenker, M.; Valderrama, B.P.; Tomita, Y.; Bamias, A.; Lebret, T.; Shariat, S.F.; Park, S.H.; et al. Adjuvant Nivolumab versus Placebo in Muscle-Invasive Urothelial Carcinoma. N. Engl. J. Med. 2021, 384, 2102–2114. [Google Scholar] [CrossRef]
- Christensen, E.; Birkenkamp-Demtröder, K.; Sethi, H.; Shchegrova, S.; Salari, R.; Nordentoft, I.; Wu, H.T.; Knudsen, M.; Lamy, P.; Lindskrog, S.V.; et al. Early detection of metastatic relapse and monitoring of therapeutic efficacy by ultra-deep sequencing of plasma cell-free DNA in patients with urothelial bladder carcinoma. J. Clin. Oncol. 2019, 37, 1547–1557. [Google Scholar] [CrossRef]
- Powles, T.; Assaf, Z.J.; Davarpanah, N.; Banchereau, R.; Szabados, B.E.; Yuen, K.C.; Grivas, P.; Hussain, M.; Oudard, S.; Gschwend, J.E.; et al. ctDNA guiding adjuvant immunotherapy in urothelial carcinoma. Nature 2021, 595, 7867. [Google Scholar] [CrossRef] [PubMed]
- Bratman, S.V.; Yang, S.Y.C.; Iafolla, M.A.J.; Liu, Z.; Hansen, A.R.; Bedard, P.L.; Lheureux, S.; Spreafico, A.; Razak, A.A.; Shchegrova, S.; et al. Personalized circulating tumor DNA analysis as a predictive biomarker in solid tumor patients treated with pembrolizumab. Nat. Cancer 2020, 1, 873–881. [Google Scholar] [CrossRef] [PubMed]


| Parameters | Cisplatin Ineligibility (Galsky et al., 2011) [7] | Platinum Ineligibility (Gupta et al., 2022) [11] |
|---|---|---|
| ECOG PS | ≥2, or KPS of ≤60%–70% | ≥3 |
| CrCl | <60 mL/min | <30 mL/min |
| NYHA Heart Failure Class | ≥3 | >3 |
| Peripheral neuropathy | Grade ≥ 2 (i.e., sensory alteration or paresthesia, including tingling, but not interfering with activities of daily living) | Grade ≥ 2 |
| Different parameters | Hearing loss (measured at audiometry) of 25 dB at 2 contiguous frequencies | ECOG PS of 2 and CrCl < 30 mL/min |
| NCT Number | Study Name | Most Current Study Status † | FGFRi + ICI Combination? | Target of FGFR Inhibitor | Phase | Primary Outcome Measures |
|---|---|---|---|---|---|---|
| NCT03473743 | NORSE | Active, not recruiting | Erdafitinib + cetrelimab | FGFR1-4 | Ib/II | DLTs (Phase I), ORR and AEs (Phase II) |
| NCT04045613 | FIDES-02 (Cohort 3) | Completed | Derazantinib + atezolizumab | Pan-FGFR | Ib/II | ORR, Safety, and tolerability of derazantinib alone and with atezolizumab |
| NCT03473756 | FORT-2 | Active, not recruiting | Rogaratinib + atezolizumab | FGFR1-4 | Ib/II | DLTs, Number of subjects with TEAEs, drug related TEAEs, and TESAEs |
| NCT04003610 | FIGHT-205 | Terminated (business decision) | Pemigatinib + pembrolizumab | FGFR1-3 | II | PFS |
| Biomarkers | Expression in UC as Clinical Interest | Alteration in UC as Clinical Interest | Details about Frequency of Expression or Alteration |
|---|---|---|---|
| Nectin-4 | X | Frequently expressed in 83% [90] | |
| Trop-2 | X | Frequently expressed in ≤83% [91] | |
| FGFR | X | X | FGFR3 most frequently expressed FGF receptor in normal urothelium [92]; FGFR1-4 alterations in 33% [12] |
| Ephb-2/ Ephb4 | X | Extremely low Ephb2 expression (~nil) and high Ephb4 expression in 94% [93] | |
| HRR genes (BRCA1, BRCA2, ATM, CDK12, etc.) | X | HRR mutations identified in 31.4% of a TCGA cohort (n = 822) and 34.1% of a retrospective single-center cohort (n = 343) [94] | |
| HER2 | X | X | Expression in 6–37% Alteration in 12% [95] |
| MTAP | X | Loss in 28% [96] | |
| APOBEC | X | Mutation signature in 80% [97] | |
| MSS/MMR | X | Prevalence of dMMR in 6% of UC and 2% in BC; Prevalence of MSI-H in 3% of UC and 1% in BC [98] | |
| TMB | X | High TMB in 26% of Stage II-Stage IV BC, low TMB in 74% of cases [99] | |
| TLS | X | ~25% of NMIBC and ~75% of MIBC [100] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moussa, M.J.; Campbell, M.T.; Alhalabi, O. Revisiting Treatment of Metastatic Urothelial Cancer: Where Do Cisplatin and Platinum Ineligibility Criteria Stand? Biomedicines 2024, 12, 519. https://doi.org/10.3390/biomedicines12030519
Moussa MJ, Campbell MT, Alhalabi O. Revisiting Treatment of Metastatic Urothelial Cancer: Where Do Cisplatin and Platinum Ineligibility Criteria Stand? Biomedicines. 2024; 12(3):519. https://doi.org/10.3390/biomedicines12030519
Chicago/Turabian StyleMoussa, Mohammad Jad, Matthew T. Campbell, and Omar Alhalabi. 2024. "Revisiting Treatment of Metastatic Urothelial Cancer: Where Do Cisplatin and Platinum Ineligibility Criteria Stand?" Biomedicines 12, no. 3: 519. https://doi.org/10.3390/biomedicines12030519







