Isocitrate Dehydrogenase 1/2 Wildtype Adult Astrocytoma with WHO Grade 2/3 Histological Features: Molecular Re-Classification, Prognostic Factors, Clinical Outcomes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Imaging Technique and Analysis
2.2. Treatment
2.3. Statistics
3. Results
3.1. Patient Characteristics
3.2. Tissue Results
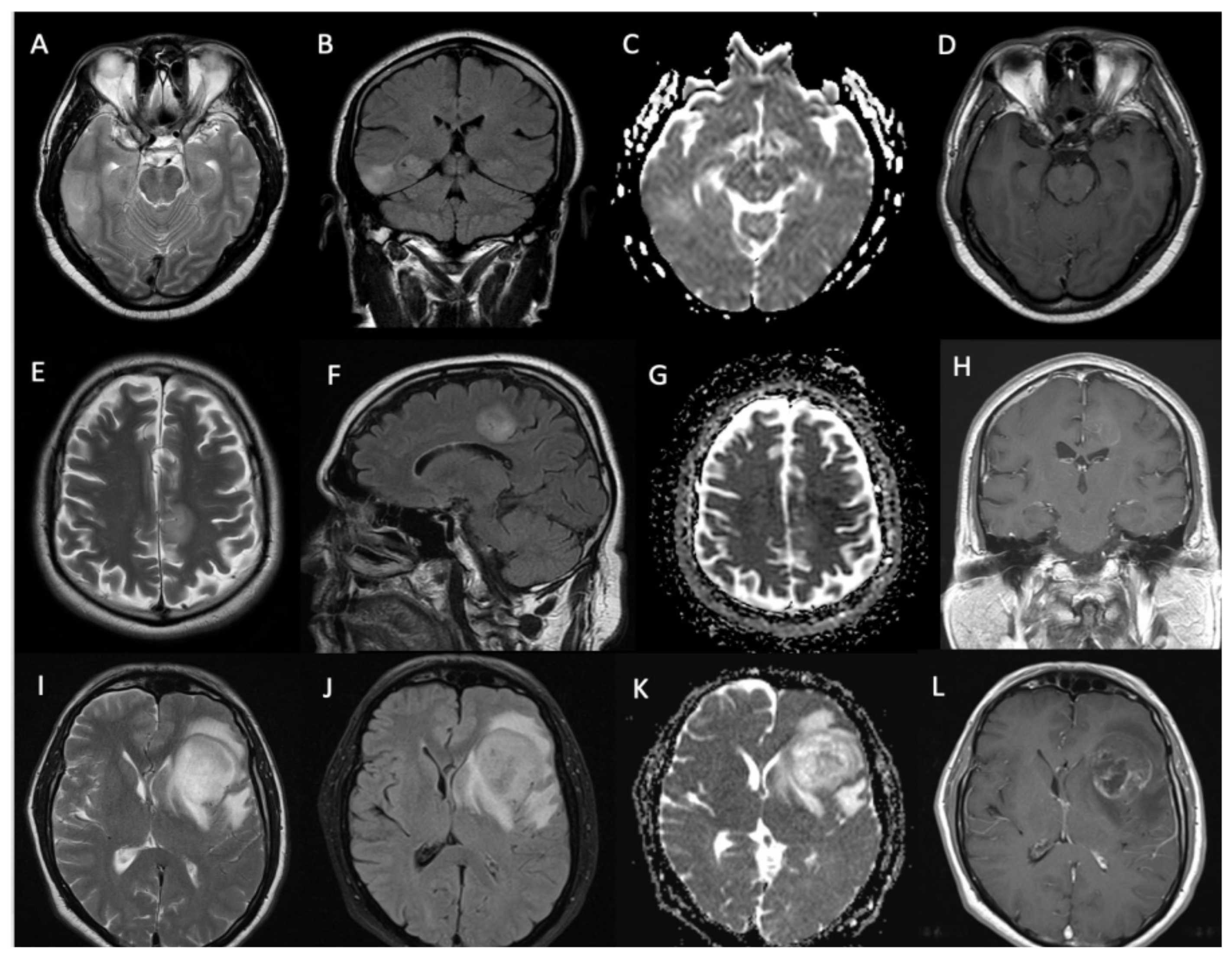
3.3. Imaging Characteristics
3.4. Treatment Characteristics
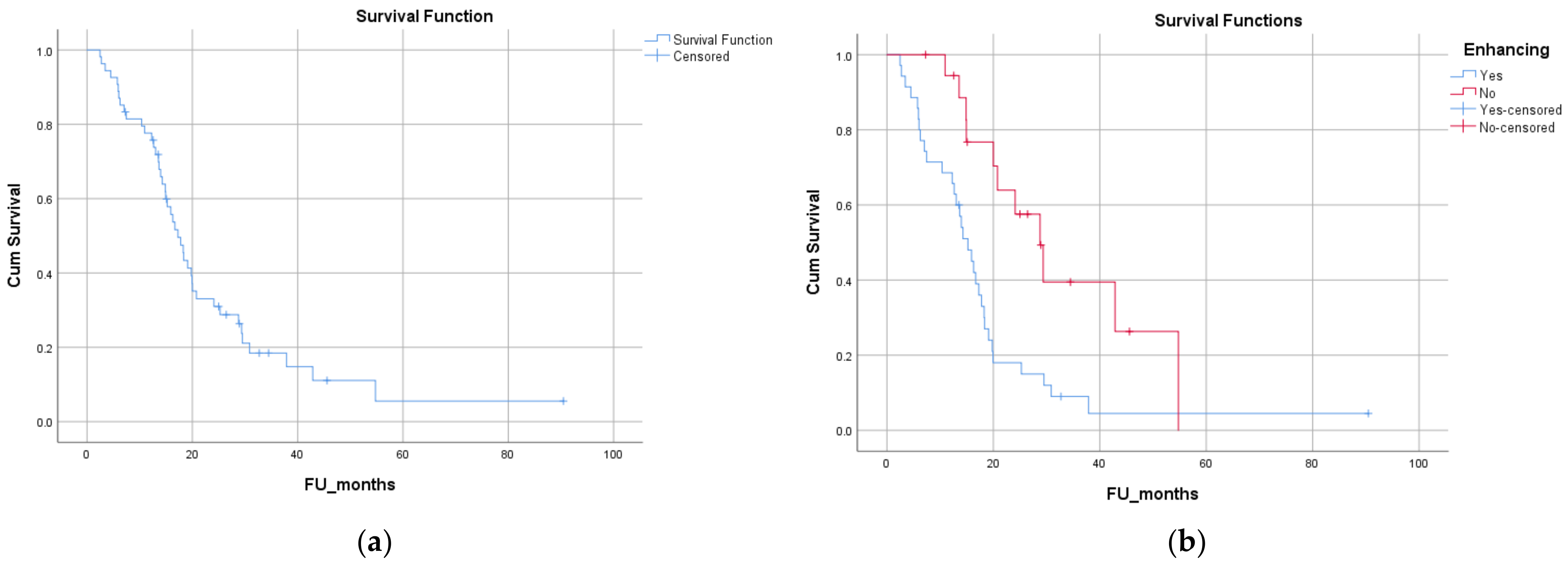
3.5. Survival Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ostrom, Q.T.; Cioffi, G.; Gittleman, H.; Patil, N.; Waite, K.; Kruchko, C.; Barnholtz-Sloan, J.S. CBTRUS Statistical Report: Primary Brain and Other Central Nervous System Tumors Diagnosed in the United States in 2012–2016. Neuro-Oncol. 2019, 21 (Suppl. 5), v1–v100. [Google Scholar]
- Louis, D.N.; Ohgaki, H.; Wiestler, O.D.; Cavenee, W.K.; Burger, P.C.; Jouvet, A.; Scheithauer, B.W. The 2007 WHO classification of tumors of the central nervous system. Acta Neuropathol. 2007, 114, 97–109. [Google Scholar] [CrossRef] [PubMed]
- Cancer Genome Atlas Research Network. Comprehensive, Integrative Genomic Analysis of Diffuse Lower-Grade Gliomas. N. Engl. J. Med. 2015, 372, 2481–2498. [Google Scholar] [CrossRef] [PubMed]
- Louis, D.N.; Perry, A.; Wesseling, P.; Brat, D.J.; Cree, I.A.; Figarella-Branger, D.; Hawkins, C.; Ng, H.K.; Pfister, S.M.; Reifenberger, G.; et al. The 2021 WHO Classification of Tumors of the Central Nervous System: A summary. Neuro-Oncol. 2021, 23, 1231–1251. [Google Scholar] [CrossRef] [PubMed]
- Brat, D.J.; Aldape, K.; Colman, H.; Holland, E.C.; Louis, D.N.; Jenkins, R.B.; Kleinschmidt-DeMasters, B.K.; Perry, A.; Reifenberger, G.; Stupp, R.; et al. cIMPACT-NOW update 3: Recommended diagnostic criteria for “Diffuse astrocytic glioma, IDH-wildtype, with molecular features of glioblastoma, WHO grade IV”. Acta Neuropathol. 2018, 136, 805–810. [Google Scholar] [CrossRef] [PubMed]
- Stichel, D.; Ebrahimi, A.; Reuss, D.; Schrimpf, D.; Ono, T.; Shirahata, M.; Reifenberger, G.; Weller, M.; Hänggi, D.; Wick, W.; et al. Distribution of EGFR amplification, combined chromosome 7 gain and chromosome 10 loss, and TERT promoter mutation in brain tumors and their potential for the reclassification of IDHwt astrocytoma to glioblastoma. Acta Neuropathol. 2018, 136, 793–803. [Google Scholar] [CrossRef] [PubMed]
- Reuss, D.E.; Kratz, A.; Sahm, F.; Capper, D.; Schrimpf, D.; Koelsche, C.; Hovestadt, V.; Bewerunge-Hudler, M.; Jones, D.T.; Schittenhelm, J.; et al. Adult IDH wild type astrocytomas biologically and clinically resolve into other tumor entities. Acta Neuropathol. 2015, 130, 407–417. [Google Scholar] [CrossRef] [PubMed]
- Stupp, R.; Mason, W.P.; van den Bent, M.J.; Weller, M.; Fisher, B.; Taphoorn, M.J.B.; Belanger, K.; Brandes, A.A.; Marosi, C.; Bogdahn, U.; et al. Radiotherapy plus Concomitant and Adjuvant Temozolomide for Glioblastoma. N. Engl. J. Med. 2005, 352, 987–996. [Google Scholar] [CrossRef]
- Ostrom, Q.T.; Patil, N.; Cioffi, G.; Waite, K.; Kruchko, C.; Barnholtz-Sloan, J.S. CBTRUS statistical report: Primary brain and other central nervous system tumors diagnosed in the United States in 2013–2017. Neuro-Oncol. 2020, 22 (Suppl. 1), iv1–iv96. [Google Scholar]
- Ringel, F.; Pape, H.; Sabel, M.; Krex, D.; Bock, H.C.; Misch, M.; Weyerbrock, A.; Westermaier, T.; Senft, C.; Schucht, P.; et al. Clinical benefit from resection of recurrent glioblastomas: Results of a multicenter study including 503 patients with recurrent glioblastomas undergoing surgical resection. Neuro-Oncol. 2016, 18, 96–104. [Google Scholar] [CrossRef] [PubMed]
- Schäfer, N.; Gielen, G.H.; Rauschenbach, L.; Kebir, S.; Till, A.; Reinartz, R.; Simon, M.; Niehusmann, P.; Kleinschnitz, C.; Herrlinger, U.; et al. Longitudinal heterogeneity in glioblastoma: Moving targets in recurrent versus primary tumors. J. Transl. Med. 2019, 17, 96. [Google Scholar] [CrossRef] [PubMed]
- Reuss, D.E.; Mamatjan, Y.; Schrimpf, D.; Capper, D.; Hovestadt, V.; Kratz, A.; Sahm, F.; Koelsche, C.; Korshunov, A.; Olar, A.; et al. IDH mutant diffuse and anaplastic astrocytomas have similar age at presentation and little difference in survival: A grading problem for WHO. Acta Neuropathol. 2015, 129, 867–873. [Google Scholar] [CrossRef] [PubMed]
- Maynard, J.; Okuchi, S.; Wastling, S.; Busaidi, A.A.; Almossawi, O.; Mbatha, W.; Brandner, S.; Jaunmuktane, Z.; Koc, A.M.; Mancini, L.; et al. World Health Organization Grade II/III Glioma Molecular Status: Prediction by MRI Morphologic Features and Apparent Diffusion Coefficient. Radiology 2020, 296, 111–121. [Google Scholar] [CrossRef] [PubMed]
- Thust, S.; Maynard, J.; Benenati, M.; Wastling, S.; Mancini, L.; Jaunmuktane, Z.; Brandner, S.; Jäger, H. Regional and Volumetric Parameters for Diffusion-Weighted WHO Grade II and III Glioma Genotyping: A Method Comparison. Am. J. Neuroradiol. 2021, 42, 441–447. [Google Scholar] [CrossRef] [PubMed]
- Berzero, G.; Di Stefano, A.L.; Ronchi, S.; Bielle, F.; Villa, C.; Guillerm, E.; Capelle, L.; Mathon, B.; Laurenge, A.; Giry, M.; et al. IDH-wildtype lower-grade diffuse gliomas: The importance of histological grade and molecular assessment for prognostic stratification. Neuro-Oncol. 2021, 23, 955–966. [Google Scholar] [CrossRef] [PubMed]
- Nakasu, S.; Deguchi, S.; Nakasu, Y. IDH wild-type lower-grade gliomas with glioblastoma molecular features: A systematic review and meta-analysis. Brain Tumor Pathol. 2023, 40, 143–157. [Google Scholar] [CrossRef] [PubMed]
- Hempel, J.-M.; Brendle, C.; Bender, B.; Bier, G.; Skardelly, M.; Gepfner-Tuma, I.; Eckert, F.; Ernemann, U.; Schittenhelm, J. Contrast enhancement predicting survival in integrated molecular subtypes of diffuse glioma: An observational cohort study. J. Neurooncol. 2018, 139, 373–381. [Google Scholar] [CrossRef] [PubMed]
- Brown, R.; Zlatescu, M.; Sijben, A.; Roldan, G.; Easaw, J.; Forsyth, P.; Parney, I.; Sevick, R.; Yan, E.; Demetrick, D.; et al. The use of magnetic resonance imaging to noninvasively detect genetic signatures in oligodendroglioma. Clin. Cancer Res. 2008, 14, 2357–2362. [Google Scholar] [CrossRef] [PubMed]
- Knopp, E.A.; Cha, S.; Johnson, G.; Mazumdar, A.; Golfinos, J.G.; Zagzag, D.; Miller, D.C.; Kelly, P.J.; Kricheff, I.I. Glial neoplasms: Dynamic contrast-enhanced T2*-weighted MR imaging. Radiology 1999, 211, 791–798. [Google Scholar] [CrossRef] [PubMed]
- Hirai, T.; Murakami, R.; Nakamura, H.; Kitajima, M.; Fukuoka, H.; Sasao, A.; Akter, M.; Hayashida, Y.; Toya, R.; Oya, N.; et al. Prognostic value of perfusion MR imaging of high-grade astrocytomas: Long-term follow-up study. Am. J. Neuroradiol. 2008, 29, 1505–1510. [Google Scholar] [CrossRef] [PubMed]
- van den Bent, M.J.; Tesileanu, C.M.S.; Wick, W.; Sanson, M.; Brandes, A.A.; Clement, P.M.; Erridge, S.; A Vogelbaum, M.; Nowak, A.K.; Baurain, J.F.; et al. Adjuvant and concurrent temozolomide for 1p/19q non-co-deleted anaplastic glioma (CATNON; EORTC study 26053–22054): Second interim analysis of a randomised, open-label, phase 3 study. Lancet Oncol. 2021, 22, 813–823. [Google Scholar] [CrossRef] [PubMed]
- Darvishi, P.; Batchala, P.; Patrie, J.; Poisson, L.; Lopes, M.-B.; Jain, R.; Fadul, C.; Schiff, D.; Patel, S. Prognostic Value of Preoperative MRI Metrics for Diffuse Lower-Grade Glioma Molecular Subtypes. AJNR Am. J. Neuroradiol. 2020, 41, 815–821. [Google Scholar] [CrossRef] [PubMed]
- Palpan Flores, A.; Vivancos Sanchez, C.; Roda, J.M.; Cerdán, S.; Barrios, A.J.; Utrilla, C.; Royo, A.; Gandía González, M.L. Assessment of Pre-operative Measurements of Tumor Size by MRI Methods as Survival Predictors in Wild Type IDH Glioblastoma. Front. Oncol. 2020, 10, 1662. [Google Scholar] [CrossRef] [PubMed]
- Burth, S.; Kickingereder, P.; Eidel, O.; Tichy, D.; Bonekamp, D.; Weberling, L.; Wick, A.; Löw, S.; Hertenstein, A.; Nowosielski, M.; et al. Clinical parameters outweigh diffusion- and perfusion-derived MRI parameters in predicting survival in newly diagnosed glioblastoma. Neuro-Oncol. 2016, 18, 1673–1679. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.-C.; Jain, R.; Radmanesh, A.; Poisson, L.; Guo, W.-Y.; Zagzag, D.; Snuderl, M.; Placantonakis, D.; Golfinos, J.; Chi, A. Predicting Genotype and Survival in Glioma Using Standard Clinical MR Imaging Apparent Diffusion Coefficient Images: A Pilot Study from The Cancer Genome Atlas. AJNR Am. J. Neuroradiol. 2018, 39, 1814–1820. [Google Scholar] [CrossRef]
- Ellingson, B.M.; Wen, P.Y.; Cloughesy, T.F. Evidence and context of use for contrast enhancement as a surrogate of disease burden and treatment response in malignant glioma. Neuro-Oncol. 2017, 20, 457–471. [Google Scholar] [CrossRef] [PubMed]

| Characteristic | Numbers (Percentage) |
|---|---|
| Tumor grade | |
| Grade 2 | 17 (32%) |
| Grade 3 | 37 (68%) |
| MGMT promoter methylation | |
| Unmethylated | 32 (60%) |
| Low to moderate methylation | 18 (35%) |
| Hypermethylated | 3 (5%) |
| EGFR amplification (n = 54) | |
| Amplified | 21 (39%) |
| Unamplified | 33 (61%) |
| TERT mutation (n = 21) | |
| Mutated | 17 (81%) |
| Non-mutated | 4 (19%) |
| Radiological characteristics | Numbers (percentage) |
| Location (n = 54) | |
| Lobar | 46 (85%) |
| Deep-seated | 8 (15%) |
| Contrast enhancement | |
| Solid pattern | 18 (33%) |
| Rim enhancement | 17 (31%) |
| Non-enhancing | 19 (36%) |
| Treatment characteristics | Numbers (percentage) |
| Surgical procedure | |
| Biopsy | 38 (67%) |
| Debulking | 6 (11%) |
| Complete excision | 10 (19%) |
| Radiotherapy dose | |
| ≥54 Gy (1.8–2 Gy/fraction) | 39 (72%) |
| 40 Gy (2.67 Gy/fraction) | 2 (4%) |
| 36 Gy (6 Gy/fraction) | 13 (24%) |
| Chemotherapy | |
| Concurrent | 25 (46%) |
| Concurrent and adjuvant | 19 (35%) |
| None | 10 (19%) |
| Test Variable | Overall Survival [Median (CI)] | p-Value |
|---|---|---|
| Age at diagnosis (dichotomized at median—≤57 years vs. >57) | 20 (12.5 to 27.5) vs. 14 (9.8 to 18.8) months | p = 0.013 |
| Sex (female vs. male) | 17.3 (14.5 to 20) vs. 17.8 (12.3 to 23.2) months | p = 0.36 |
| Performance status (0 vs. 1 vs. 2) | 24 (0 to 60) vs. 18.3 (14 to 22.6) vs. 14.3 (0.7 to 27.8) months | p = 0.004 |
| Contrast enhancement on diagnostic MRI (present vs. absent) | 15.2 (12.1 to 18.3) vs. 28.8 (21.3 to 36.2) months | p = 0.003 |
| Location of the lesion on MRI (superficial vs. deep) | 18.3 (15.3 to 21.2) vs. 14.9 (12.7 to 17) months | p = 0.32 |
| Pattern of gadolinium enhancement (solid/patchy versus rim-enhancing with central necrosis) | 15.9 (12.5 to 19.3) vs. 14 (11.7 to 16.2) months | p = 0.57 |
| Pre-operative tumor volume on MRI (dichotomized at median—66.5 cc) | 20 (15.6 to 24.2) vs. 15 (12.3 to 17.6) months | p = 0.20 |
| ADC value (dichotomized at median—1.45) | 15.2 (11.4 to 19) vs. 19.8 (17.3 to 22.3) months | p = 0.43 |
| Type of surgery (biopsy vs. maximal safe resection) | 17.8 (15.5 to 20) vs. 14 (11.3 to 16.6) months | p = 0.91 |
| Complete resection vs. incomplete resection | 14.9 (7 to 22.9) vs. 17.3 (14.5 to 20) months | p = 0.28 |
| Time to surgery (from diagnostic MRI—<1 week vs. >1 week) | 15.2 (12.2 vs. 18.2) 18.3 (15 to 21.5) | p = 0.72 |
| Radical vs. palliative radiotherapy | 18.2 (14.2 to 22.16) vs. 16 (12.7 to 19.19) months | p = 0.20 |
| Dose of radiation (high dose vs. low dose) | 18.2 (14.2 to 22.16) vs. 16 (12.7 to 19.19) months | p = 0.20 |
| Concurrent chemotherapy | 19.8 (15.7 to 23.9) vs. 16 (11.9 to 19.9) months | p = 0.39 |
| MGMT status (methylated vs. unmethylated) | 16.67 (13.2 to 20.14) vs. 18.3 (12 to 24.65) months | p = 0.40 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gupta, M.; Anjari, M.; Brandner, S.; Fersht, N.; Wilson, E.; Thust, S.; Kosmin, M. Isocitrate Dehydrogenase 1/2 Wildtype Adult Astrocytoma with WHO Grade 2/3 Histological Features: Molecular Re-Classification, Prognostic Factors, Clinical Outcomes. Biomedicines 2024, 12, 901. https://doi.org/10.3390/biomedicines12040901
Gupta M, Anjari M, Brandner S, Fersht N, Wilson E, Thust S, Kosmin M. Isocitrate Dehydrogenase 1/2 Wildtype Adult Astrocytoma with WHO Grade 2/3 Histological Features: Molecular Re-Classification, Prognostic Factors, Clinical Outcomes. Biomedicines. 2024; 12(4):901. https://doi.org/10.3390/biomedicines12040901
Chicago/Turabian StyleGupta, Meetakshi, Mustafa Anjari, Sebastian Brandner, Naomi Fersht, Elena Wilson, Steffi Thust, and Michael Kosmin. 2024. "Isocitrate Dehydrogenase 1/2 Wildtype Adult Astrocytoma with WHO Grade 2/3 Histological Features: Molecular Re-Classification, Prognostic Factors, Clinical Outcomes" Biomedicines 12, no. 4: 901. https://doi.org/10.3390/biomedicines12040901





